Abstract
The liver is a pioneer internal organ that orchestrates major metabolic, detoxification, and endocrine roles. Acute factors like hepatitis and drug allergy and chronic causes like metabolic dysfunction-associated fatty liver disease (MASLD) and Hepatocellular carcinoma (HCC) drive hepatic wellness imbalances. Liver fibrosis is a reversible and curable anomaly, but the limited availability of safe and higher-specificity therapeutics is a challenging quest in hepatology. This study investigates the hepato-protective effect of Phyllanthus niruri compounds against liver fibrosis targets like lysyl oxidase-like 2 (LOXL2), heat shock protein 47 (HSP47), bromodomain-containing protein 4 (BRD4) and inhibitory kappa B kinase beta (IKKβ) and compare their anti-hepatic fibrosis activity against known inhibitors. Potential plant compounds from P. niruri were retrieved from the literature repositories, and the top 35 compounds were screened based on molecular weight, Lipinski’s rule of 5, and bioavailability score. The in silico molecular docking and in silico ADMET results provide valuable insights into hit compounds of P. niruri, namely quercitrin and hinokinin, to have good binding scores (BE) below −7 kcal/mol threshold and molecular interactions with many key residues of all the four liver fibrosis targets namely the BRD4, HSP47, LOLX2, and IKKB proteins explored in this research. Quercitrin has been identified to have BE values of −8.1, −8.3, −8.2, and −9.1 kcal/mol scores against the BRD4, HSP47, LOLX2, and IKKB proteins, respectively. Similarly, hinokinin also shows BE values of −8.8, −7.4, −6.7, and −9.0 kcal/mol scores with BRD4, HSP47, LOLX2, and IKKB proteins individually. Further, in vitro and animal model-based in vivo experimental analysis needs to be explored to validate the potential of quercitrin and hinokinin for anti-liver fibrosis in the future.
1. Introduction
Chronic liver injury, liver inflammation, and hepatic stellate cell (HSC) activation are some of the key features of liver fibrosis. HSCs are precursor fibroblastic, liver-resident cells that transdifferentiate to become actively proliferating fibroblastic cells to secrete extracellular matrix (ECM) components like collagen and proteoglycans [1]. ‘Perpetuation phase’ is a series of HSCs phenotypic activation events, which includes uncontrolled proliferation, retinoid reduction, fibrogenesis, cholesterol metabolism, changes in matrix degradation, contractility, endoplasmic reticulum (ER) and oxidative stresses, epigenetics, chemotaxis, inflammatory responses and receptor-mediated signaling [2,3]. While elevated proliferation and activation of HSCs majorly contribute to liver fibrosis [4], enhanced stabilization of ECM components, especially collagen, along with the promotion of collagen synthesis [5,6], also contributes to matrix stiffness and recurrence of fibrotic injury [7]. Likewise, chronic inflammatory cues precede hepatic fibrosis, and its persistence drives various signaling cascades of liver fibrosis [8].
Phyllanthus niruri, an Indian herb belonging to the Euphorbiaceae family, is lauded for its anti-cancer [9] and hepato-protective effects [10]. Numerous studies have vouched for the potential of multiple natural plant-derived phytochemicals to alleviate or improve hepatic fibrosis via an array of molecular mechanisms. Key transcription cum epigenetic regulator protein, Bromodomain protein 4 (BRD4), is found to be overexpressed in HSCs in promoting its trans-activation via inhibition of its apoptosis through the P300/H3K27ac/PLK1 axis [11,12]. The heat shock protein 47 (HSP47) is a collagen-specific ER-chaperon, which aids collagen triple helix formation and efficient folding. Additionally, the interaction between HSP47 and the NLRP3 inflammasome further promotes HSC activation and fibrosis progression [13,14]. The ECM crosslinking cum stabilizing protein lysyl oxidase-like 2 (LOXL2) has been identified to have prime mediation in collagen remodeling and stabilization [15,16]. Similarly, the IKKβ alias IKK2 kinase has been accorded to activate the NF-κB via phosphorylation for aggravating hepatic inflammation and HSCs activation cum apoptosis inhibition via TGF-β1 and IL-6 stimulation [17,18]. This study aimed to explore novel P. niruri phytochemicals-based inhibitors of LOXL2, HSP47, BRD4, and IKKβ which have been speculated to impact some of the key features of liver fibrosis like activation of HSCs, stabilization of collagen fibrils and induction of hepatic inflammation using in silico analysis. By conducting in silico ADME analysis and molecular docking studies, a comprehensive assessment of the binding interactions and druggability of many P. niruri compounds against liver fibrosis has been explored in this research. The schematic workflow adopted for this research is shown in Figure 1.
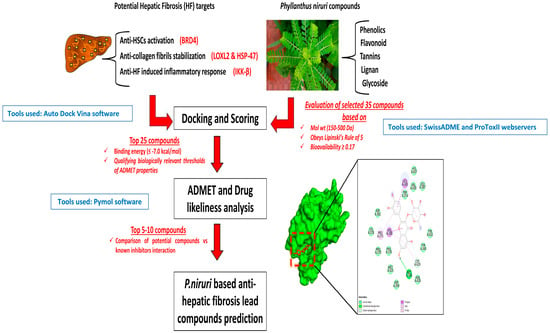
Figure 1.
Schematic workflow.
2. Materials and Methods
2.1. Initial Compounds Selection and ADME Studies
The phytoconstituents of P. niruri were obtained through the manual literature search and from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/ (accessed on 6 September 2024)). The active phyto-compounds were examined for molecular weight (Mol wt), oral bioavailability (OB), drug-likeness (DL), compliance to Lipinski’s rule, and the additional literature evidence for hepato-protective potential using online webservers Swiss ADME and Molsoft [19]. The selection criteria for Mol wt (150–500 Da), OB (>0.17), DL (>0.18), and non-violation of Lipinski’s rule were considered as parameters for selecting potentially pharmacological ingredients, and additionally, phytocompounds with the literature evidence for hepato-protective potential but having violations to the examined ADME parameters were also included in the further analysis.
2.2. Preparation of Ligands, Protein Structures
The MGLTools and AutoDock 1.5.7 software were utilized to prepare the ligand and protein structures. The reference inhibitor compounds and the P. niruri phytocompounds that were selected based on initial ADME studies were individually downloaded.SDF files were further prepared and saved.PDBQT files. The protein structures were retrieved as.PDB files from the RCSB database (https://www.rcsb.org (accessed on 8 September 2024)) and saved as.PDBQT files post-preparation include the removal of HOH molecules and the addition of Gasteiger charges. The prediction of grid box parameters to facilitate docking was also performed [20]. The list of details about the PDB files and the corresponding inhibitor compounds used as reference for docking analysis is listed in Table 1.

Table 1.
A list of details about protein PDBs and inhibitor references was used in this study.
2.3. In Silico Molecular Docking Analysis
The AutoDock Vina virtual screening algorithm was utilized to predict the binding. Top 15 compounds exhibiting docking scores ≤ −7.0 kcal/mol were chosen for further analysis. The binding interactions of the docked ligand and protein results were visualized using Pymol version 3.1.1 and Discovery Studio visualizer tools.
2.4. In Silico Prediction of Toxicity Parameters
The Absorption, distribution, metabolism, excretion, and toxicity (ADMET) toxicity parameters like predicted LD50, predicted toxicity class, hepato-toxicity, immunogenicity, carcinogenicity, and activity status of Tox21 nuclear receptor parameters like the PPAR-γ, MMP, and p53 were explored using the Protox II web server [24].
3. Results and Discussion
3.1. ADME/T Profiles of the Best
Around 35 compounds have been listed in Table 2 as potential P. niruri phytochemicals based on ideal molecular weight, oral bioavailability (OB), drug likeliness score (DL), and non-violation of Lipinski’s rule. Additionally, phytocompounds with the literature evidence for hepato-protective potential but having violations of the examined ADME parameters are also included for further analysis.

Table 2.
List of selected P. niruri compounds based on initial ADMET profiles.
3.2. Molecular Docking Analysis
The docked interactions of selected 8 P. niruri compounds like astragalin, quercitrin, niruriflavone, amariin, corilagin, hinokinin, pectolinaroside, and isocorilagin were analyzed for each of the four target proteins taken for the current study.
When comparing the interaction of BRD4 inhibitor against the P. niruri compounds, it was identified that astragalin, quercitrin, niruriflavone, corilagin, and hinokinin had been found to interact with many of the crucial sites that the BRD4 inhibitor has been identified to bind within the BRD4 protein via our docking analysis and also to sites closer to the desirable residues (Figure 2A). With respect to binding energy scores, hinokinin with −8.1 kcal/mol and quercitrin with −8.8 kcal/mol binding energy are nearer to the BRD4 inhibitor score of −9.7 kcal/mol. Similarly, the eight P. niruri compounds were also reported to interact with HSP47 (Figure 2B), LOLX2 (Figure 2C), and IKKB (Figure 2D) target proteins. Among the compounds, quercitrin and hinokinin have been identified to show interactions with many of the desirable residues of all four target proteins.
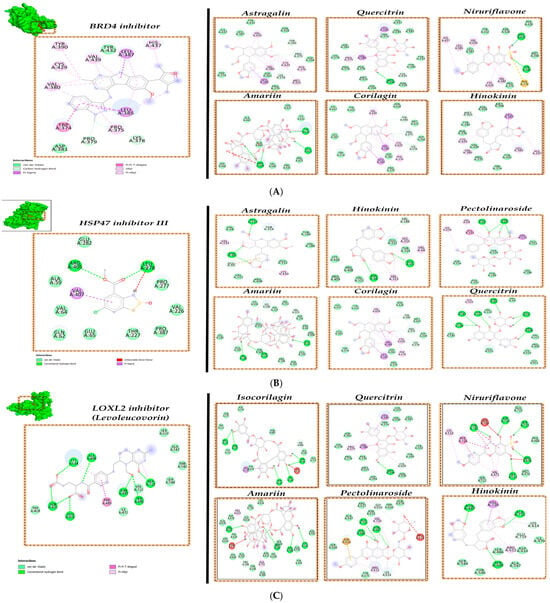
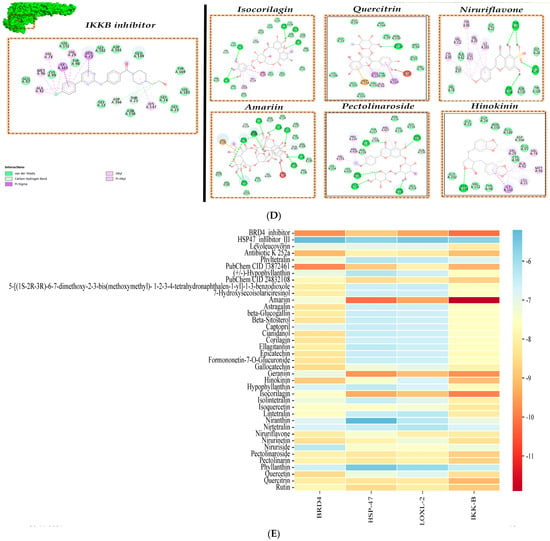
Figure 2.
Molecular interactions of phytochemicals of P. niruri. (A) BRD4 protein, (B) HSP47 protein, (C) LOXL2 protein, (D) IKKB protein, and (E) Heat Map of Binding energy (scores kcal/mol).
The cross-binding interactions studies of each of the inhibitor compounds against the other target proteins for example, docking of BRD4 inhibitor against other target proteins, namely HSP47, LOXL2, and IKKB, were also carried out, and we identified that certain inhibitors like BRD4 inhibitor and antibiotic K255 an (IKKB inhibitor) have been identified to even show high interaction to other proteins like IKKB and BRD4, respectively (Figure 2E).
With respect to the molecular docking binding energy (BE) scores, compounds interacting with BE scores lower than −7 kcal/mol are accorded as hit compounds, as shown in the heat map profile (Figure 2E). The potentially suitable P. niruri hit compounds, which also show interactions against target proteins at key residues and satisfy the BE scores requirement, are identified as desirable anti-liver fibrosis inhibitor compounds. In this study, P. niruri compounds like astragalin, quercitrin, corilagin, and hinokinin have been identified to interact with most of the key residues of BRD4 target protein, and amongst them, hinokinin exhibits higher BE of −8.8 kcal/mol as shown in Figure 2A,E. Similarly, pectolinaroside, hinokinin, and quercitrin show binding to some of the key residue sites where HSP47 inhibitor III binds along with lower BE scores, as in Figure 2B,E. Compounds isocorilagin and amariin have been identified to interact with sufficient desirable residues of LOXL2 protein shown in Figure 2C,E. Compounds like quercitrin, niruriflavone, and hinokinin also promisingly interact with the IKKB target, as shown in Figure 2D,E.
Overall, the molecular docking results of these eight P. niruri compounds demonstrate promising interactions with BRD4, HSP47, LOXL2, and IKKB, suggesting that they may exhibit therapeutic efficacy against the prime features of liver fibrosis. Compounds quercitrin and hinokinin show preferential interaction cum desirably lower BE scores with all four target proteins.
3.3. In Silico Toxicity Prediction
The in silico toxicity activeness for hepato-toxicity, carcinogenicity, and immunogenicity of P. niruri compounds and the reference inhibitor compounds explored in this current research was examined using the ProTox-III webserver. Amongst the inhibitor compounds, only levoleucovorin (LOXL2 inhibitor reference) showed inactivity for the toxicity potential Table 3.

Table 3.
In silico toxicity results for P. niruri compounds.
The predicted toxicity class and LD50 results show that P. niruri compounds administration could potentially be harmful only beyond high dosages as per the predicted LD50 values, and also, these compounds have been predicted to belong to mostly class IV and V, unlike the reference inhibitor compounds. The Tox21 nuclear receptor signaling activeness results shown in Table 3 imply that almost all the analyzed inhibitor compounds and P. niruri compounds, except the IKKB inhibitor and flavonoid astragalin, show inactivity towards triggering the PPAR-γ, MMP, and p53 cascades to induce cytotoxic effects at concentration beyond the predicted LD50 threshold.
4. Discussion
The selected eight P. niruri compounds have been identified to be non-hepatotoxic, though most compounds show active immune-toxic potential, which could possibly emphasize the anti-oxidant, radical scavenging potency of phytochemicals, and their applicability as anti-cancer agents [25]. Hinokinin has earlier been reported to mediate beneficial health effects like neuroprotective and anti-cancer abilities [26,27]. The anti-myocardial fibrotic prowess of hinokinin [28] substantially advocates the predictive inference of this current research for a similar anti-liver fibrosis effect. Quercitrin has been identified to prevent mitochondrial damage in liver cells [29], reduce hepatic steatosis [30], and suppress HCC metastasis [31], and its potential therapeutic effect against liver fibrosis is attempted for exploration in this current research. Previous studies on P. niruri extracts report reduced hepatic and nephrotic injury cum lowered fibrotic scores in animal model studies [32,33], which also supports the computational modeling outcomes of the current research work.
P. niruri has many substantial previous studies emphasizing its therapeutic potential, like anti-cancer and hepato-protective efficacy [34,35]. For instance, one of the major P. niruri phytochemical phyllanthin has been identified to protect from hepatic injuries cum toxicity [36] and impart anti-fibrosis effect via downregulation of TGF-β1 cascade [37] and TNF-α/NF-κB levels in vitro [38]. Quercetin, a prominent flavonoid, has also been proficient against pulmonary liver fibrosis and NAFLD [39,40,41,42]. Likewise, methanolic extracts of P. niruri have been identified to mitigate liver fibrosis progression and NAFLD complexities concerned with atherosclerosis [43].
5. Conclusions
This computational analysis provides comprehensive insights into the anti-liver fibrosis potential of Phyllanthus niruri compounds against some of the prominent therapeutic targets influencing key features of liver fibrosis. The in silico molecular docking and in silico ADMET studies provide valuable insights into hit compounds of P. niruri, like quercitrin and hinokinin, to have good docking scores below −7 kcal/mol threshold and molecular interactions with many key residues of all the four liver fibrosis targets explored in this research namely the BRD4, HSP47, LOLX2, and IKKB proteins. Both quercitrin and hinokinin compounds have been previously accorded for their health benefits. This present study holds promising computational predictive findings of both quercitrin and hinokinin as anti-liver fibrosis therapeutic druggable compounds. Further in vitro and animal model based in vivo experimental analysis needs to be explored for future validations.
Author Contributions
C.R.: Writing, software, computational analysis, methodology, investigation. K.S.: Conceptualization, review cum editing, supervision, methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Computational study, so not applicable.
Informed Consent Statement
It is not applicable; it is a computational modeling study.
Data Availability Statement
All the relevant data are disclosed in this manuscript.
Acknowledgments
C.R. acknowledges the Anna Research Fellowship (ARF) scholarship, awarded by the Centre for Research (CFR), Anna University, Chennai, Tamil Nadu, India.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsukada, S.; Parsons, C.J.; Rippe, R.A. Mechanisms of liver fibrosis. Clin. Chim. Acta 2006, 364, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-T.; Liao, Z.-X.; Ping, J.; Xu, D.; Wang, H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J. Gastroenterol. 2008, 43, 419–428. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Khomich, O.; Ivanov, A.V.; Bartosch, B. Metabolic Hallmarks of Hepatic Stellate Cells in Liver Fibrosis. Cells 2019, 9, 24. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Liu, Q.; Tang, Q.; Jia, Q.; Xiong, Y.; He, J.; Li, Y. CREKA-modified liposomes target activated hepatic stellate cells to alleviate liver fibrosis by inhibiting collagen synthesis and angiogenesis. Acta Biomater. 2023, 168, 484–496. [Google Scholar] [CrossRef]
- Liu, S.B.; Ikenaga, N.; Peng, Z.; Sverdlov, D.Y.; Greenstein, A.; Smith, V.; Schuppan, D.; Popov, Y. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016, 30, 1599–1609. [Google Scholar] [CrossRef]
- Saneyasu, T.; Akhtar, R.; Sakai, T. Molecular Cues Guiding Matrix Stiffness in Liver Fibrosis. BioMed Res. Int. 2016, 2016, 2646212. [Google Scholar] [CrossRef]
- Czaja, A.J.; Carpenter, H.A. Progressive fibrosis during corticosteroid therapy of autoimmune hepatitis. Hepatology 2004, 39, 1631–1638. [Google Scholar] [CrossRef]
- Abdel-Sattar, O.E.; Allam, R.M.; Al-Abd, A.M.; Avula, B.; Katragunta, K.; Khan, I.A.; El-Desoky, A.M.; Mohamed, S.O.; El-Halawany, A.; Abdel-Sattar, E.; et al. Cytotoxic and chemomodulatory effects of Phyllanthus niruri in MCF-7 and MCF-7ADR breast cancer cells. Sci. Rep. 2023, 13, 2683. [Google Scholar] [CrossRef]
- Sowjanya, K.; Girish, C.; Bammigatti, C.; Lakshmi, N.C.P. Efficacy of Phyllanthus niruri on improving liver functions in patients with alcoholic hepatitis. Indian J. Pharmacol. 2021, 53, 448–456. [Google Scholar] [CrossRef]
- Cheng, M.; Li, J.J.; Niu, X.N.; Zhu, L.; Liu, J.Y.; Jia, P.C.; Zhu, S.; Meng, H.W.; Lv, X.W.; Huang, C.; et al. BRD4 promotes hepatic stellate cells activation and hepatic fibrosis via mediating P300/H3K27ac/PLK1 axis. Biochem. Pharmacol. 2023, 210, 115497. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Lu, S.; Pan, N.; Zhao, F.; Jia, X.; Wang, S.; Zhang, Y.; Zhou, Y. Bromodomain protein 4 is a key molecular driver of TGFβ1-induced hepatic stellate cell activation. Biochim. Et Biophys. Acta BBA—Mol. Cell Res. 2023, 1870, 119569. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Jin, L.; Wang, J. HSP47 and Its Involvement in Fibrotic Disorders; Springer: Berlin/Heidelberg, Germany, 2019; pp. 299–312. [Google Scholar]
- Yuan, W.; Qiu, T.; Yao, X.; Wu, C.; Shi, Y.; Wang, N.; Zhang, J.; Jiang, L.; Liu, X.; Yang, G.; et al. Hsp47 acts as a bridge between NLRP3 inflammasome and hepatic stellate cells activation in arsenic-induced liver fibrosis. Toxicol. Lett. 2022, 370, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Klepfish, M.; Gross, T.; Vugman, M.; Afratis, N.A.; Havusha-Laufer, S.; Brazowski, E.; Solomonov, I.; Varol, C.; Sagi, I. LOXL2 Inhibition Paves the Way for Macrophage-Mediated Collagen Degradation in Liver Fibrosis. Front. Immunol. 2020, 11, 480. [Google Scholar] [CrossRef]
- Ikenaga, N.; Peng, Z.W.; Vaid, K.A.; Liu, S.B.; Yoshida, S.; Sverdlov, D.Y.; Mikels-Vigdal, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut 2017, 66, 1697–1708. [Google Scholar] [CrossRef]
- Beraza, N.; Malato, Y.; Borght, S.V.; Liedtke, C.; Wasmuth, H.E.; Dreano, M.; de Vos, R.; Roskams, T.; Trautwein, C. Pharmacological IKK2 inhibition blocks liver steatosis and initiation of non-alcoholic steatohepatitis. Gut 2008, 57, 655–663. [Google Scholar] [CrossRef]
- Wei, J. IκB kinase-beta inhibitor attenuates hepatic fibrosis in mice. World J. Gastroenterol. 2011, 17, 5203. [Google Scholar] [CrossRef]
- Şahin, S. 3,4-difluoro-2-(((4-phenoxyphenyl)imino)methyl)phenol with in silico predictions: Synthesis, spectral analyses, ADME studies, targets and biological activity, toxicity and adverse effects, site of metabolism, taste activity. J. Mol. Struct. 2024, 1317, 139136. [Google Scholar] [CrossRef]
- Shah-Abadi, M.E.; Ariaei, A.; Moradi, F.; Rustamzadeh, A.; Tanha, R.R.; Sadigh, N.; Marzban, M.; Heydari, M.; Ferdousie, V.T. In Silico Interactions of Natural and Synthetic Compounds with Key Proteins Involved in Alzheimer’s Disease: Prospects for Designing New Therapeutics Compound. Neurotox. Res. 2023, 41, 408–430. [Google Scholar] [CrossRef]
- Wu, S.; Liang, C.; Xie, X.; Huang, H.; Fu, J.; Wang, C.; Su, Z.; Wang, Y.; Qu, X.; Li, J.; et al. Hsp47 Inhibitor Col003 Attenuates Collagen-Induced Platelet Activation and Cerebral Ischemic–Reperfusion Injury in Rats. Front. Pharmacol. 2022, 12, 792263. [Google Scholar] [CrossRef]
- Deshpande, H. Levoleucovorin inhibits LOXL2 (lysyl oxidase like-2) to control breast cancer proliferation: A repurposing approach. J. Biomol. Struct. Dyn. 2024, 42, 5104–5113. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Sorokina, I.V.; Zhukova, N.A.; Frolova, T.S.; Tolstikova, T.G.; Shults, E.E.; Turks, M. Lupane-type conjugates with aminoacids, 1,3,4- oxadiazole and 1,2,5-oxadiazole-2-oxide derivatives: Synthesis, anti-inflammatory activity and in silico evaluation of target affinity. Steroids 2019, 150, 108443. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Polito, L.; Djemil, A.; Bortolotti, M. Plant Toxin-Based Immunotoxins for Cancer Therapy: A Short Overview. Biomedicines 2016, 4, 12. [Google Scholar] [CrossRef]
- Tarbiat, S.; Unver, D.; Tuncay, S.; Isik, S.; Yeman, K.B.; Mohseni, A.R. Neuroprotective effects of Cubebin and Hinokinin lignan fractions of Piper cubeba fruit in Alzheimer’s disease in vitro model. Turk. J. Biochem. 2023, 48, 303–310. [Google Scholar] [CrossRef]
- Cunha, N.; Teixeira, G.; Martins, T.; Souza, A.; Oliveira, P.; Símaro, G.; Rezende, K.C.; dos Santos Gonçalves, N.; Souza, D.G.; Tavares, D.C.; et al. (−)-Hinokinin Induces G2/M Arrest and Contributes to the Antiproliferative Effects of Doxorubicin in Breast Cancer Cells. Planta Med. 2016, 82, 530–538. [Google Scholar] [CrossRef]
- Lu, Q.; Zheng, R.; Zhu, P.; Bian, J.; Liu, Z.; Du, J. Hinokinin alleviates high fat diet/streptozotocin-induced cardiac injury in mice through modulation in oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2021, 137, 111361. [Google Scholar] [CrossRef]
- Xiong, W.; Yuan, Z.; Wang, T.; Wu, S.; Xiong, Y.; Yao, Y.; Yang, Y.; Wu, H. Quercitrin Attenuates Acetaminophen-Induced Acute Liver Injury by Maintaining Mitochondrial Complex I Activity. Front. Pharmacol. 2021, 12, 586010. [Google Scholar] [CrossRef]
- Hur, H.J.; Jeong, Y.-H.; Lee, S.H.; Sung, M.J. Quercitrin Ameliorates Hyperlipidemia and Hepatic Steatosis in Ovariectomized Mice. Life 2020, 10, 243. [Google Scholar] [CrossRef]
- Guang Li, H.; Jun Gao, H.; Feng Liu, F.; Liu, J. Quercitrin Suppresses Hepatocellular Carcinoma Metastasis and Angiogenesis by Targeting the Nrf2 Signaling Pathway. 2017. Available online: https://www.oncotarget.com/article/23777/ (accessed on 13 February 2025).
- Giribabu, N.; Karim, K.; Kilari, E.K.; Salleh, N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J. Ethnopharmacol. 2017, 205, 123–137. [Google Scholar] [CrossRef]
- Abu Hassan, M.R.; Hj Md Said, R.; Zainuddin, Z.; Omar, H.; Md Ali, S.M.; Aris, S.A.; Chan, H.K. Effects of one-year supplementation with Phyllanthus niruri on fibrosis score and metabolic markers in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Heliyon 2023, 9, e16652. [Google Scholar] [CrossRef] [PubMed]
- Pahmi, K.; Sidratullah, M. Effect of Leafflower (Phyllanthus niruri Linn.) treatment on kidney and uterus in sodium chloride-induced fibrotic rats. Biosci. Med. J. Biomed. Transl. Res. 2021, 5, 263–267. [Google Scholar]
- Amin, Z.A.; Bilgen, M.; Alshawsh, M.A.; Ali, H.M.; Hadi, A.H.A.; Abdulla, M.A. Protective Role of Phyllanthus niruri Extract against Thioacetamide-Induced Liver Cirrhosis in Rat Model. Evid.-Based Complement. Altern. Med. 2012, 2012, 241583. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.L.; Loh, S.I.; Sattar, M.A.; Muhammad, T.S.T.; Sulaiman, S.F. Cytotoxic, caspase-3 induction and in vivo hepatoprotective effects of phyllanthin, a major constituent of Phyllanthus niruri. J. Funct. Foods 2015, 14, 236–243. [Google Scholar] [CrossRef]
- Krithika, R.; Jyothilakshmi, V.; Prashantha, K.; Verma, R.J. Mechanism of protective effect of phyllanthin against carbon tetrachloride-induced hepatotoxicity and experimental liver fibrosis in mice. Toxicol. Mech. Methods 2015, 25, 708–717. [Google Scholar] [CrossRef]
- Krithika, R.; Jyothilakshmi, V.; Verma, R.J. Phyllanthin inhibits CCl4-mediated oxidative stress and hepatic fibrosis by down-regulating TNF-α/NF-κB, and pro-fibrotic factor TGF-β1 mediating inflammatory signaling. Toxicol. Ind. Health 2016, 32, 953–960. [Google Scholar] [CrossRef]
- Hernández-Ortega, L.D.; Alcántar-Díaz, B.E.; Ruiz-Corro, L.A.; Sandoval-Rodriguez, A.; Bueno-Topete, M.; Armendariz-Borunda, J.; Salazar-Montes, A.M. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J. Gastroenterol. Hepatol. 2012, 27, 1865–1872. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Y.; Zhang, W.; Chen, X. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem. Cell Biol. 2018, 96, 742–751. [Google Scholar] [CrossRef]
- Marcolin, E.; San-Miguel, B.; Vallejo, D.; Tieppo, J.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Quercetin Treatment Ameliorates Inflammation and Fibrosis in Mice with Nonalcoholic Steatohepatitis3. J. Nutr. 2012, 142, 1821–1828. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Y.; Zhang, C.; Sullivan, M.A.; Chen, W.; Jing, X.; Yu, H.; Li, F.; Wang, Q.; Zhou, Z.; et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J. Nutr. Biochem. 2023, 120, 109414. [Google Scholar] [CrossRef]
- Al Zarzour, R.; Ahmad, M.; Asmawi Mohd Kaur, G.; Saeed, M.; Al-Mansoub, M.; Saghir, S.A.; Usman, N.S.; Al-Dulaimi, D.W.; Yam, M.F. Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague–Dawley Rats. Nutrients 2017, 9, 766. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).