Bioactivity of Essential Oils of Laggera pterodonta and Laggera aurita against Larvae of Anopheles gambiae, Malaria Vector †
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Essential Oil Extraction
2.3. Gas Chromatography-Mass Spectrometry (GC-MS)
2.4. Collection and Rearing of Mosquitoes
2.5. Bioassay of Essential Oils of L. pterodonta and L. aurita
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of the Essential Oils by GC-MS
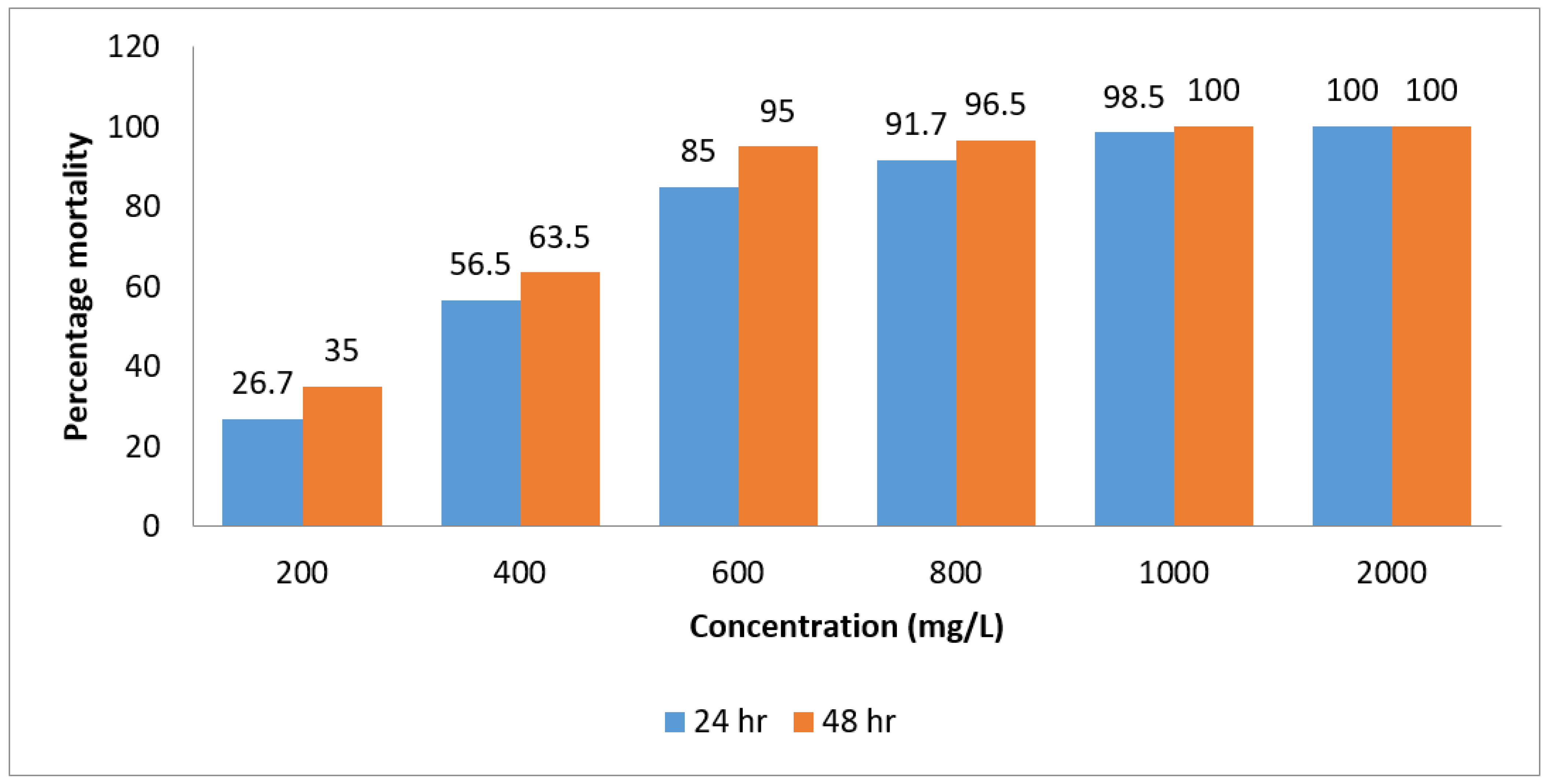
3.2. Larvicidal Activity of Essential Oil of Laggera pterodonta
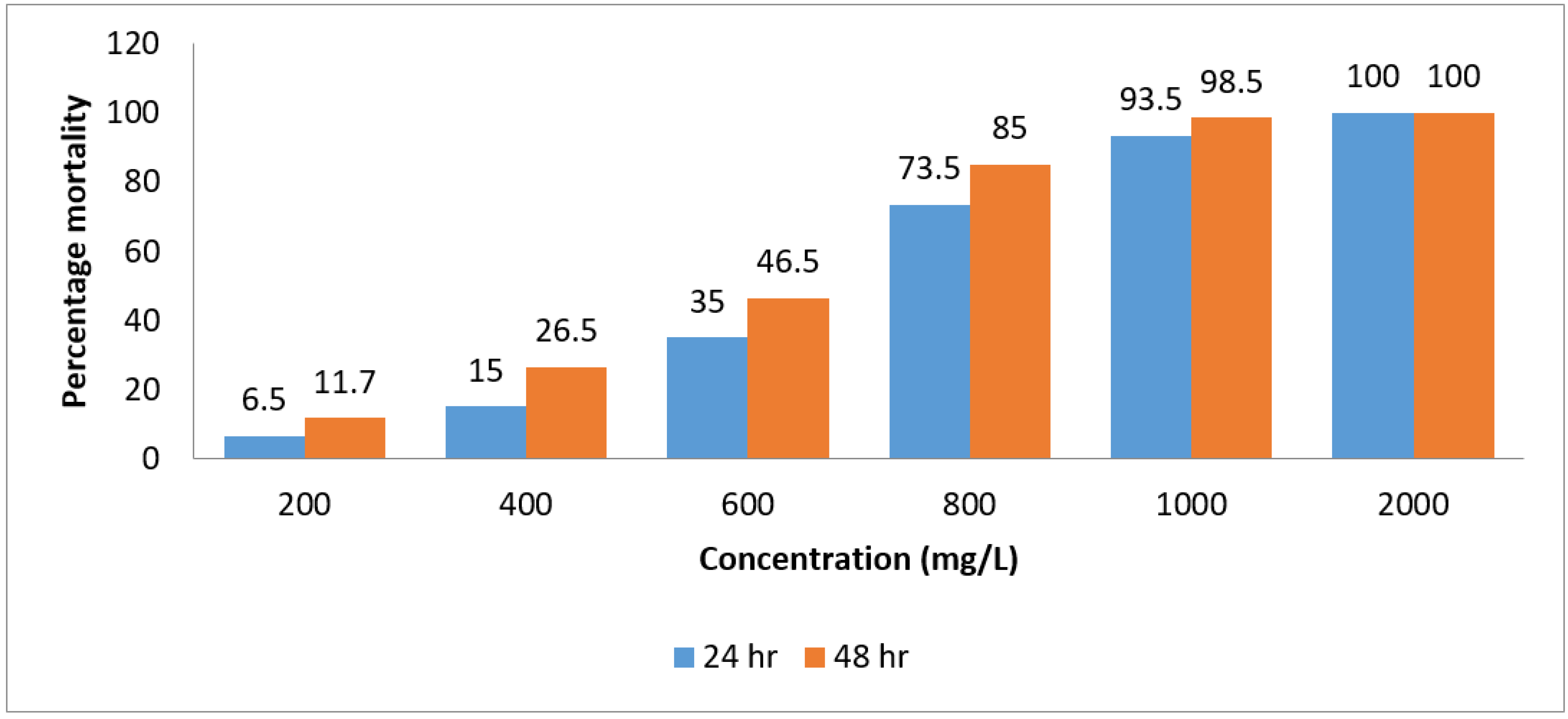
3.3. Larvicidal Activity of Essential Oil of Laggera aurita
3.4. Lethal Concentration of Essential Oils against Larvae of An. gambiae
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Maharaj, R.; Maharaj, V.; Crouch, N.R.; Bhagwandin, N.; Folb, P.I.; Pillay, P.; Gayaram, R. Screening for adulticidal bioactivity of South African plants against Anopheles arabiensis. Malar. J. 2011, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mudalungu, C.M.; Matasyoh, J.C.; Vulule, J.M.; Chepkorir, R. Larvicidal compounds from the plant Fagaropsis angolensis leaves against malaria vector (Anopheles gambiae). Int. J. Malar. Res. Rev. 2013, 1, 1–7. [Google Scholar]
- Ohimain, E.I.; Angaye, T.C.N.; Bassey, S.E. Comparative larvicidal activities of the leaves, bark, stem and root of Jatropha curcas (Euphorbiaceae) against malaria vector Anopheles gambiae. Sky J. Biochem. Res. 2014, 3, 29–32. [Google Scholar]
- Ombito, O.J.; Matasyoh, C.J.; Vulule, M.J. Chemical composition and larvicidal activity of Zanthoxylum gilletii essential oil against Anopheles gambiae. Afr. J. Biotechnol. 2014, 13, 2175–2180. [Google Scholar] [CrossRef]
- Langsi, D.J.; Nukenine, E.N.; Fokunang, C.N.; Goudoungon, J.W.; Kosini, D.; Fotso, T.G.; Tchinda, G.; Agbor, G. Evaluation of the insecticidal properties of fractionated extracts of Ocimum canum and Laggera pterodonta on stored maize against the infestation of Sitophilus zeamis (Coleoptera: Curculidae). Int. J. Agron. Agric. Res. 2017, 10, 41–50. [Google Scholar]
- Ollengo, M.A.; Vulule, J.M.; Matasyoh, J.C. Isolation, structure elucidation and larvicidal activity of Laggera alata extracts. Res. J. Pharmacogn. Phytochem. 2016, 8, 153–164. [Google Scholar] [CrossRef]
- Singh, S.P.; Mittal, P.K. Mosquito repellent and oviposition deterrent activities of Laggera aurita plant extract against malaria vector Anopheles stephensi. Entomol. Appl. Sci. Lett. 2015, 2, 18–22. [Google Scholar]
- Papachristos, D.P.; Stamopoutous, D.C. Fumigant toxicity of three essential oils on eggs of Acanthoscelides obtectus Say (Coleooptera: Bruchidae). J. Stored Prod. Res. 2004, 40, 517–525. [Google Scholar] [CrossRef]
- Omoregie, E.H.; Oluyemisi, K.F.; Koma, O.S.; Ibumeh, O.J. Chemical Constituents of the Essential Oil of Laggera pterodonta (DC.) Sch. Bip. from North-Central. Niger. J. Appl. Pharm. Sci. 2012, 2, 198–202. [Google Scholar] [CrossRef][Green Version]
- Okhale, S.E.; Ugbabe, G.E.; Oladosu, P.O.; Ibrahim, J.A.; Egharevba, H.O.; Kunle, O.F.; Elisha, E.P.; Chibuike, A.J.; Ettah, U.O. Chemical constituents and antimicrobial activity of the leaf essential oil of Ixora coccinea L (Rubiaceae) collected from North Central Nigeria. Int. J. Bioassays 2018, 7, 5630–5637. [Google Scholar]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). S. Afr. Inst. Med Res. Johannesbg. 1968, 2, 1–343. [Google Scholar]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; 2005; Volume 13, 39p, Available online: https://apps.who.int/iris/handle/10665/69101 (accessed on 11 September 2020).
- Asgar, E.; Roya, K.; Jalal, J.S.; Parisa, H.; Rahim, M.A. Toxicity and Physiological effects of essential oil from Agantachefoeniculum (Pursh)Kuntze against Tribolium casteneum Herbst(Coleoptera:Tenebrionidae) larvae. Annual Rev. Res. Bio. 2013, 3, 649–658. [Google Scholar]
- Gangwar, P.; Tiwari, S.N. Insecticidal Activity of Curcuma longa Essential Oil and its Fractions against Sitophilus oryzae L. and Rhyzopertha dominica F. (Coleoptera). Int. J. Pure Appl. Biosci. 2017, 5, 912–921. [Google Scholar]
- Ali, A.; Tabanca, N.; Kurkcuoglu, M.; Duran, A.; Bylthe, E.K.; Khan, I.A.; Baser, K.H. Chemical composition, larvicidal and biting deterrent activity of essential oils of two subspecies of Tanacetum argenteum (Asterales: Asteraceae) and individual constituents against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 824–830. [Google Scholar] [CrossRef]
- Doria, G.A.A.; Silva, W.J.; Carvalho, G.A.; Alves, P.B.; Cavalcanti, S.C.H. A study of larvicidal activity of two Croton species from northeastern Brazil against Aedes aegypti. Pharm. Biol. 2010, 48, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tian, Y.J. Chemical composition and larvicidal activity of essential oil of Artemesia gilvescens against Anopheles anthropophagus. Parasitol. Res. 2013, 112, 1137–1142. [Google Scholar] [CrossRef]
- Chintem, W.D.; Nzelibe, C.H. Larvicidal activity of leaf extracts and purified fractions of Ocimum gratissimum against Culex quinquefasciatus mosquito larvae. Int. J. Sci. Res. 2015, 4, 12. [Google Scholar]
- Zhu, S.; Liu, X.C.; Liu, Z.L.; Xu, X. Chemical composition of Salvia plebeian R. Br. essential oil and its larvicidal activity against Aedes aegypti L. Trop. J. Pharm. Res. 2015, 14, 831–836. [Google Scholar] [CrossRef]
- Guo, S.S.; Zhang, W.J.; You, C.X.; Liang, J.Y.; Yang, K.; Geng, Z.F.; Du, S.S.; Wang, C.F. Chemical Composition of Essential Oil Extracted from Laggera pterodonta and its Bioactivities against Two Stored Product Insects. J. Food Process. Preserv. 2017, 41, 21–24. [Google Scholar] [CrossRef]
| ID | Compound | Percentage (%) | |
|---|---|---|---|
| L. pterondata | L. aurita | ||
| 1 | Bicylo[3.1.0]hex-2-ene, 2-methyl-5 | 0.33 | - |
| 2 | Tricyclo[4.1.1.0(2,5)] octane | - | 0.15 |
| 3 | trans-beta-Ocimene | 1.48 | - |
| 4 | Nortricyclyl bromide | - | 0.16 |
| 5 | Benzenepropanoyl bromide | 0.93 | - |
| 6 | Sabinene | 0.47 | - |
| 7 | s-Triazaborane | - | 0.04 |
| 8 | α –Phellandrene | 2.36 | 4.36 |
| 9 | (+)-4-Carene$$ | 10.40 | 5.58 |
| 10 | o-Cymene | 1.85 | 0.64 |
| 11 | β –Methylmercaptoeethylamine | - | 0.10 |
| 12 | α –Limonene | 0.93 | - |
| 13 | Hyacinthin | 0.68 | - |
| 14 | ɤ-Terpinene | 12.72 | 8.92 |
| 15 | β –Linalool | 2.25 | 0.43 |
| 16 | 2-Carene | 3.46 | 1.38 |
| 17 | Linalool | 2.51 | 1.16 |
| 18 | 1-(2-methylphenyl)-Ethanone | 0.26 | - |
| 19 | 4-Isopropyl-1-methyl-2-cyclohexane | 0.80 | - |
| 20 | 3(10)-Caren-4-ol, aceacetic acid es | 0.53 | - |
| 21 | 6-Methylenebicyclo[3.1.0]hexane | 0.18 | - |
| 22 | 1,4-Dimethyl-delta-3-tetrahydroace | 0.39 | - |
| 23 | Pyrrole-2-aldehyde | 0.14 | - |
| 24 | 4-Carvomenthenol | 9.22 | 3.43 |
| 25 | p-Menthan-8-ol | 0.31 | 0.07 |
| 26 | ɤ-Acetopropanol | 0.06 | - |
| 27 | 2,3,4,5-Tetrahydropyridazine | 0.06 | - |
| 28 | Dimethylethylbenzene | 0.34 | - |
| 29 | Thymol methyl ether | 0.62 | 2.19 |
| 30 | 4-Ethylformanilide | - | 0.06 |
| 31 | Trifluoromethyl peroxynitrate | - | 0.03 |
| 32 | 2,2′-azobis[2-methyl propionitrile | - | 0.27 |
| 33 | 1-methylpyrrole | - | 0.04 |
| 34 | Benzene, 2-tert-butyl-1,4-dimethoxy | 12.66 | 25.24 |
| 35 | Caryophyllene | 5.83 | 12.25 |
| 36 | α –Caryophyllene | 3.29 | 2.69 |
| 37 | Isolongifolan-8-ol | 2.34 | - |
| 38 | 1,2-Benzenediol, O-(4-butylbenzoyl) | 0.05 | 0.20 |
| 39 | (2S,4R)-p-Mentha-[1(7),8]-diene 2-1 | - | 0.60 |
| 40 | Cadina-1(10),4-diene | 0.84 | - |
| 41 | α –Bourbonene | 0.78 | - |
| 42 | Caryophyllene oxide | 1.16 | - |
| 43 | Ethane, 1-(2-bromoethoxy)-2-metho | 0.18 | - |
| 44 | Cyclohexane, butylidene | 0.20 | - |
| 45 | ɤ -Eudesmol | 8.67 | - |
| 46 | 2-Isopropenyl-1,3-dimethylcyclopen | - | 1.34 |
| 47 | δ-Cadinol,(-)- | 2.03 | - |
| 48 | α –Cadinol | - | 5.29 |
| 49 | ɤ-Eudesmol | 1.42 | - |
| 50 | α –Muurolene | - | 0.30 |
| 51 | tau-Muurolol | 1.94 | 5.91 |
| 52 | Cedrol | - | 1.51 |
| 53 | Cadina-1(10),4-diene | 0.84 | 4.20 |
| 54 | (+/−)-Camphor | - | 1.29 |
| 55 | Champaca camphor | 1.95 | - |
| 56 | Juniper camphor | 2.40 | - |
| 57 | Benzenepropanoyl bromide | - | 0.03 |
| 58 | 2,4-Diisopropenyl-1-methylcylohexane | - | 1.30 |
| 59 | Borazine | - | 0.04 |
| 60 | α –Bourbonene | 0.78 | 6.30 |
| 61 | 1,11-Dodecadiyne | - | 0.34 |
| 62 | 3,5-Dithiono-6-methyl-1,2,4-triazine | - | 0.08 |
| 63 | Isopiperitenone | - | 0.06 |
| 64 | Patchoulane | - | 0.83 |
| 65 | s-Triazine | - | 0.03 |
| 66 | (−)-Spathulenol | - | 0.92 |
| 67 | 3-Buten-1-one, 2,2-dimethyl-1-phen | - | 0.08 |
| 68 | 4-Quinolinol,2-methyl-$$ 2-Methy | - | 0.03 |
| 69 | 2-(methylthio)-Ethanamine | - | 0.03 |
| 70 | (Z,Z)-α-Famesene | 0.17 | - |
| 71 | Ethyl(isopropyl)propylborane | 0.14 | - |
| 72 | Phthalic acid, cyclobutyl tridecly est | 0.57 | 0.12 |
| 73 | 4-Hydroxytranylcypromine | 0.11 | - |
| Total | 100 | 100 | |
| Extracts | LC50 (LB-UB)mg/L | LC90 (LB-UB)mg/L | ||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| Laggera pterodonta | 418 (284–503) | 404 (283–475) | 753 (655–892) | 628 (543–762) |
| Laggera aurita | 688 (603–740) | 642 (485–704) | 973 (896–1139) | 870 (804–1059) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantanko, F.; Malann, Y.D. Bioactivity of Essential Oils of Laggera pterodonta and Laggera aurita against Larvae of Anopheles gambiae, Malaria Vector. Biol. Life Sci. Forum 2021, 4, 93. https://doi.org/10.3390/IECPS2020-08651
Dantanko F, Malann YD. Bioactivity of Essential Oils of Laggera pterodonta and Laggera aurita against Larvae of Anopheles gambiae, Malaria Vector. Biology and Life Sciences Forum. 2021; 4(1):93. https://doi.org/10.3390/IECPS2020-08651
Chicago/Turabian StyleDantanko, Fatima, and Yoila D. Malann. 2021. "Bioactivity of Essential Oils of Laggera pterodonta and Laggera aurita against Larvae of Anopheles gambiae, Malaria Vector" Biology and Life Sciences Forum 4, no. 1: 93. https://doi.org/10.3390/IECPS2020-08651
APA StyleDantanko, F., & Malann, Y. D. (2021). Bioactivity of Essential Oils of Laggera pterodonta and Laggera aurita against Larvae of Anopheles gambiae, Malaria Vector. Biology and Life Sciences Forum, 4(1), 93. https://doi.org/10.3390/IECPS2020-08651






