Volatile Organic Compounds Emitted by C3 or CAM-Induced Mesembryanthemum crystallinum Plants †
Abstract
:1. Introduction
2. Experiments
2.1. Plant Material
2.2. Gas-Exchange and VOC Emission
2.3. Statistical Analysis
3. Results and Discussion
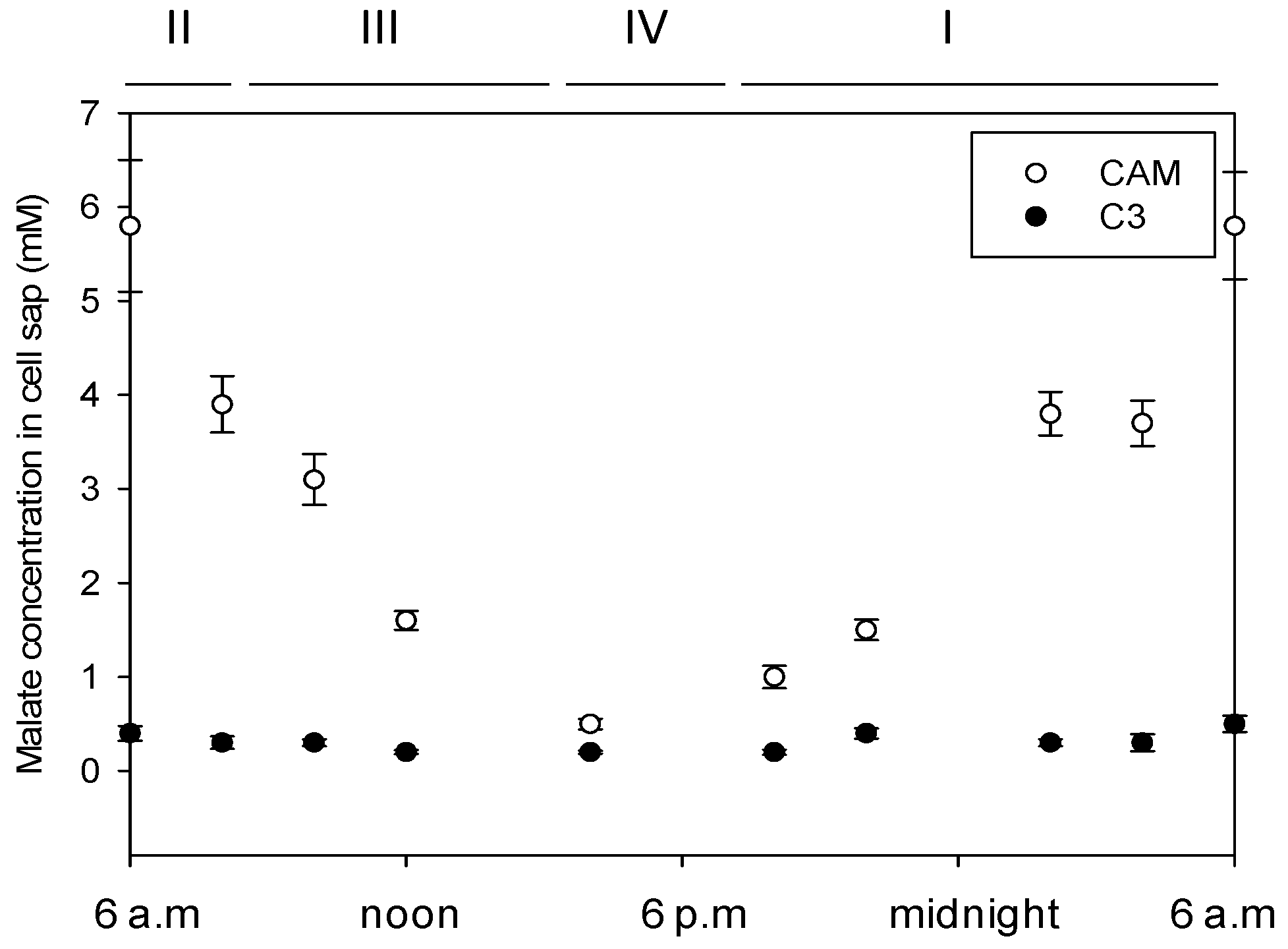
3.1. Malate Concentration
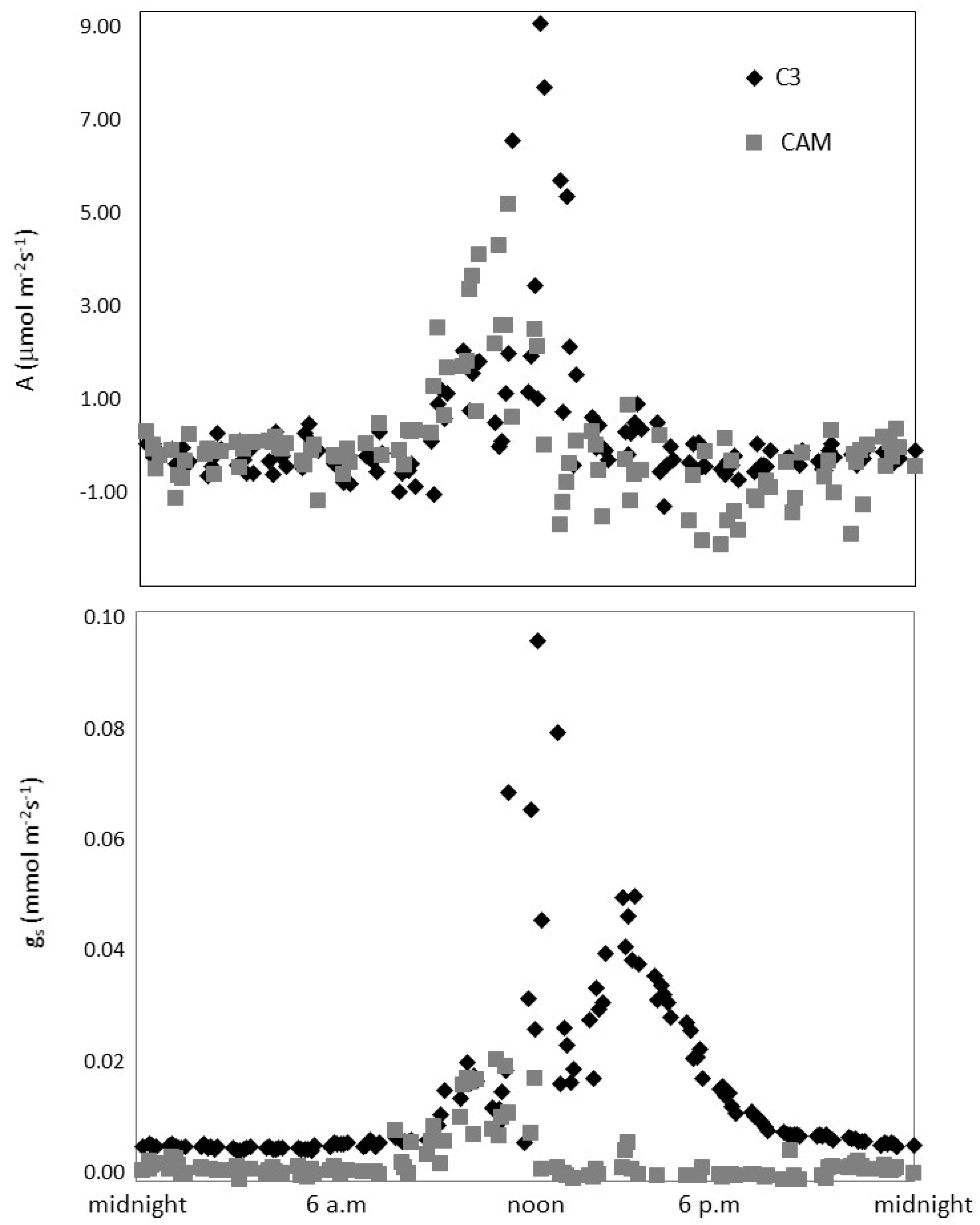
3.2. Gas Exchange
3.3. VOC Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CAM | Crassulacean acid metabolism |
| VOC | Volatile organic compound |
References
- Osmond, C.B. Crassulacean acid metabolism: A curiosity in context. Ann. Rev. Plant Physiol. 1978, 29, 379–414. [Google Scholar] [CrossRef]
- Winter, K.; Garcia, M.; Holtum, J.A.M. On the nature of facultative and constitutive CAM: Environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. J. Exp. Bot. 2008, 59, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Lüttge, U. The role of crassulacean acid metabolism (CAM) in adaptation of plants to salinity. New Phytol. 1993, 125, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Lüttge, U. CO2-concentrating: Consequences in crassulacean acid metabolism. J. Exp. Bot. 2002, 53, 2131–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushman, J.C.; Michalowski, C.B.; Bohnert, H.J. Developmental control of crassulacean acid metabolism inducibility by salt stress in the common ice plant. Plant. Physiol. 1990, 94, 1137–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.; Dai, Z.; Ku, M.S.B.; Edwards, G. Induction of Crassulacean acid metabolism in the facultative halophyte Mesembryanthemum crystallinum by abscisic acid. Plant Physiol. 1990, 93, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Broetto, F.; Lüttge, U.; Ratajczak, R. Influence of light intensity and salt-treatment on the mode of photosynthesis and enzymes of the antioxidant response system of Mesembryanthemum crystallinum. Funct. Plant Biol. 2002, 29, 13–23. [Google Scholar] [CrossRef]
- Ślesak, I.; Libik, M.; Miszalski, Z. Superoxide dismutase activity in callus from the C3-CAM intermediate plant Mesembryanthemum crystallinum L. Plant Cell Tissue Organ Cult. 2003, 75, 49–55. [Google Scholar] [CrossRef]
- Niewiadomska, E.; Miszalski, Z.; Ślesak, I.; Ratajczak, R. CAT activity during C3-CAM transition in Mesembryanthemum crystallinum L. leaves. Free Radic. Res. 1999, 31, S251–S256. [Google Scholar] [CrossRef] [PubMed]
- Ślesak, I.; Libik, M.; Miszalski, Z. The foliar concentration of hydrogen peroxide during salt-induced C3-CAM transition in Mesembryanthemum crystallinum L. Plant Sci. 2008, 174, 221–226. [Google Scholar] [CrossRef]
- Niewiadomska, E.; Bilger, W.; Gruca, M.; Mulisch, M.; Miszalski, Z.; Krupinska, K. CAM-related changes in chloroplastic metabolism of Mesembryanthemum crystallinum L. Planta 2011, 233, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fall, R. Abundant oxygenates in the atmosphere: A biochemical perspective. Chem. Rev. 2003, 103, 4941–4951. [Google Scholar] [CrossRef] [PubMed]
- Possell, M.; Loreto, L. The Role of Volatile Organic Compounds in Plant Resistance to Abiotic Stresses: Responses and Mechanisms. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 209–235. [Google Scholar] [CrossRef]
- Dodd, A.N.; Griffith, S.H.; Taybi, T.; Cushman, J.C.; Borland, A.M. Integrating diel starch metabolism with the circadian and environmental regulation of crassulacean acid metabolism in Mesembryanthemum crystallinum. Planta 2003, 216, 789–797. [Google Scholar] [CrossRef] [PubMed]
| Emission Rates from M. crystallinum (nmol m−2 sec−1) | |||
|---|---|---|---|
| Compound | C3 | CAM (Phase I) | CAM (Phase II) |
| Aldehydes | |||
| Hexanal | 0.022 ± 0.008 a | 0.018 ± 0.002 a | 0.004 ± 0.002 b |
| Octanal | 0.033 ± 0.001 a | 0.036 ± 0.004 a | 0.002 ± 0.0002 b |
| Nonanal | 0.014 ± 0.001 a | - | 0.008 ± 0.001 b |
| Decanal | 0.020 ± 0.0005 a | - | 0.019 ± 0.011 a |
| Benzenoids | |||
| Benzaldehyde | 0.006 ± 0.002 b | 0.015 ± 0.001 a | 0.002 ± 0.001 c |
| Xylene | 0.03 ± 0.01 a | 0.005 ± 0.001 c | 0.024 ± 0.004 b |
| Alkanes | |||
| Nonane | 0.029 ± 0.013 a | 0.031 ± 0.003 a | 0.0022 ± 0.0009 b |
| Undecane | 0.007 ± 0.001 b | 0.02 ± 0.002 a | 0.003 ± 0.001 c |
| Dodecane | 0.0035 ± 0.0007 a | 0.0037 ± 0.001 a | 0.0035 ± 0.001 a |
| Tetradecane | 0.018 ± −0.001 a | 0.0194 ± 0.0008 a | 0.0032 ± 0.001 b |
| Alcohols | |||
| Phenol | 0.010 ± 0.007 b | 0.018 ± 0.005 a | 0.019 ± 0.008 a |
| Benzylalcohol | 0.01 ± 0.0007 a | - | 0.006 ± 0.002 b |
| 2-Ethyl-1-Hexanol | 0.046 ± 0.011 a | 0.02 ± 0.004 b | 0.002 ± 0.001 c |
| Terpenes | |||
| a-Pinene | 0.019 ± 0.006 a | 0.021 ± 0.003 a | - |
| Carene | 0.016 ± 0.002 a | 0.009 ± 0.003 b | - |
| Limonene | 0.128 ± 0.024 a | 0.039 ± 0.01 b | - |
| Total | |||
| 0.410 ± 0.033 a | 0.257 ± 0.014 b | 0.088 ± 0.016 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogués, I.; Kocurek, M.; Miszalski, Z. Volatile Organic Compounds Emitted by C3 or CAM-Induced Mesembryanthemum crystallinum Plants. Biol. Life Sci. Forum 2021, 4, 86. https://doi.org/10.3390/IECPS2020-08723
Nogués I, Kocurek M, Miszalski Z. Volatile Organic Compounds Emitted by C3 or CAM-Induced Mesembryanthemum crystallinum Plants. Biology and Life Sciences Forum. 2021; 4(1):86. https://doi.org/10.3390/IECPS2020-08723
Chicago/Turabian StyleNogués, Isabel, Maciej Kocurek, and Zbigniew Miszalski. 2021. "Volatile Organic Compounds Emitted by C3 or CAM-Induced Mesembryanthemum crystallinum Plants" Biology and Life Sciences Forum 4, no. 1: 86. https://doi.org/10.3390/IECPS2020-08723
APA StyleNogués, I., Kocurek, M., & Miszalski, Z. (2021). Volatile Organic Compounds Emitted by C3 or CAM-Induced Mesembryanthemum crystallinum Plants. Biology and Life Sciences Forum, 4(1), 86. https://doi.org/10.3390/IECPS2020-08723






