Abstract
Plants are constantly faced with both abiotic and biotic stresses, which seriously reduce their productivity. We evaluated the potential of endophytic bacteria Bacillus subtilis (strain 10-4) alone, and when mixed with salicylic acid (SA), to meliorate drought and Fusarium root rot (FRR) stresses in Triticum aestivum L. All microbiological, molecular, and physio-biochemical parameters were assessed using classical and modern methods. The findings demonstrated B. subtilis 10-4 alone, and especially mixed with SA, significantly improved wheat growth and ameliorated the damaging influence of drought, FRR, and drought + FRR. An important contribution to observed growth-stimulating and protective effects of B. subtilis 10-4 and SA on plants revealed the ability of bacteria to produce auxins, siderophores, lipopeptides surfactin, and to colonize internal plant tissues. Additionally, bio-priming of seeds with B. subtilis 10-4 alone, and especially mixed with SA, decreased stress-induced (drought, FRR, drought + FRR) lipid peroxidation and amino acid proline accumulation in plants, thereby indicating a protective effect on plant cells against reactive oxygen species and osmotic damages. Current research provides novel insights into the potential and mechanism of B. subtilis 10-4 and SA in the mitigation of combined drought + FRR stresses in wheat.
1. Introduction
Triticum aestivum L. (wheat) is a valuable food crop of great importance for ensuring food security around the world [1]. One of the most pressing problems related to agricultural and food industries is wheat yield losses (up to 50–82%) from drought, Fusarium-caused diseases, and the combination of these stresses [1,2,3]. Traditionally, chemical pesticides have played a central role in plant protection against diseases and abiotic stresses. However, the negative impact of pesticides on the environment and human health due to their high toxicity and their ability to accumulate in products in quantities exceeding sanitary standards leads to the need to reduce their use in food production, and encourages scientists around the world to show interest in the development of environmentally friendly and safe biologicals that are based on beneficial bacterial endophytes, such as Bacillus subtilis [4]. B. subtilis is capable of improving host plant growth and inducing systemic resistance/tolerance to a wide range of pathogens and abiotic stresses [1,4]. One of the main reasons hindering the development of Bacillus-based biologicals is the lack of knowledge about underlying interactions between host plants and endophytic B. subtilis under biotic/abiotic stresses. Moreover, it is difficult to select an individually effective microbial strain with a broad spectrum of simultaneous activity against a range of pathogens and abiotic stresses. That is why there is great interest in the co-application of B. subtilis with other biological methods in order to unravel its mechanisms of action [1]. Of particular interest is the use of endophytic B. subtilis in combination with natural and safe signaling molecules with pronounced anti-stress activity, such as salicylic acid (SA)—a recognized inducer of plant systemic resistance/tolerance to diseases and abiotic stresses [4,5]. To date, a large body of information has accumulated that indicates the participation of SA in the regulation of defense reactions in different plant species to phytopathogens and abiotic stresses (salinity, drought, hypo/hyperthermia, heavy metals, etc.) [5,6]. However, information on the joint use of B. subtilis and SA is limited, especially in relation to combined biotic/abiotic stresses.
This study aimed to investigate the effect of endophytic bacteria Bacillus subtilis 10-4 and its combination with salicylic (SA) on some physio-biochemical traits of Triticum aestivum L. (wheat) plants under the influence of combined biotic (Fusarium infection) and abiotic (drought) stresses.
2. Experiments
The experiments were carried out on hydroponically growth wheat (Triticum aestivum L., variety Omskaya 35) seedlings in the early stages of ontogenesis (the first 1–3 weeks). Before sowing, seeds were treated with B. subtilis (105 CFU mL−1) [7,8], SA (0.05 mM) [5], B. subtilis (105 CFU mL−1) + SA (0.05 mM), or distilled water (control) by immersion in solution for 1 h. Then, treated and non-treated seeds were grown for 3 days on filter paper moistened with water (t = 20–22 °C, 16 h light period, 12–16,000 lux). Then, the seedlings were transplanted into glasses with solutions of 12% PEG-6000 (drought condition) or water (normal condition). Infection of wheat with the phytopathogenic fungus Fusarium spp. was carried out by introducing a suspension of fungus conidia in water (106 spores mL−1) into the base of the stems of 5-day-old seedlings. The intensity of disease development was assessed by visual symptoms (0 points—no symptoms; 1 point—damage from 1 to 25%; 2 points—from 26 to 50%; 3 points—from 51 to 75%; 4 points—more than 75%; 5 points—dead plant). In each variant, 50 plants were used in 2–3 repetitions. The tested strain 10-4 was isolated and identified in our previous work [7]. Phytopathogenic fungus Fusarium culmorum was obtained from the collection of BRIA UFRC RAS (Ufa, Russia).
Comparative analyses of the influence of B. subtilis 10-4, SA, and B. subtilis 10-4 + SA on the length of seedlings (roots, shoots) of wheat (variety Omskaya 35) and fresh (FW) and dry (DW) biomass accumulation under normal growing conditions, when infected with phytopathogenic fungi Fusarium spp. and exposed to drought (12% PEG-6000), were carried out using classical physiological methods [9]. In each variant, 50 plants were used in 2–3 repetitions.
The concentration of amino acid proline (pro) accumulation and the degree of lipid peroxidation were determined according to the methods described by Bates et al. [10] and Health and Packer [11], respectively. The ability of B. subtilis 10-4 to colonize the internal parts of seedlings was assessed using surface-sterilized plant segments and RAPD-PCR analysis [7], indole-3-acetic acid (IAA)-production using LB medium with L-tryptophan [12], phosphate solubilization using Pikovskaya medium [13], nitrogen fixation using gas chromatography [14], and siderophore production using Chrome Azurol S dye [15]. Other physio-biochemical properties of strain 10-4 were tested using test-system Set N° 2 (MicroGen, Nizhny Novgorod, Russia). The ability of B. subtilis 10-4 to produce metabolites was tested on the MOLP medium, extracted with n-butanol, and analyzed by the UHPLC-MS method [16]. Shortly after this, cells of B. subtilis 10-4 were cultured in MOLP medium (130 rpm, 36 °C, 6 days) and cells were pelleted by centrifugation (5000 rpm, 60 min). LP was extracted with n-butanol and the organic phase was evaporated on a rotary evaporator, the residue was lyophilized, dissolved in 10% methanol, and analyzed. B. subtilis-produced metabolites and LP-extracted antifungal activity were tested according to [8,16].
All experiments were carried out in three biological and three analytical replicates. The data were presented as the mean of standard error (±SEM). Statistically significant differences between the mean values were evaluated using analysis of variance (ANOVA), followed by the Tukey test (p < 0.05).
3. Results and Discussion
3.1. Characteristics and Properties of the Tested Bacillus subtilis 10-4 Strain
3.1.1. Some Plant Growth-Promoting (PGP) and Physio-Biochemical Properties
Bacterial strain 10-4 was previously isolated from the arable soils (Republic of Bashkortostan, Russia) and identified as Bacillus subtilis using 16S rRNA gene sequence analysis (similarity 99.8%) [7]. Further studies allowed found that B. subtilis 10-4 had plant growth-promoting (PGP) characteristics, such as the production of indole-3–acetic acid (IAA), siderophores, and fixed atmospheric nitrogen (Figure 1). Moreover, strain 10-4 showed oxidase-negative and catalase-positive reactions, did not utilized galactose, did not dispose of inositol, and did not form indoles or hydrogen sulfide. Additionally, strain 10-4 gave a positive Voges–Proskauer reaction and was negative for PAD activity (Figure 1a). Among PGP traits, IAA plays a vital role in plant growth and development [17]. Our finding demonstrated that, in the presence of L-TRP, which is considered to be the main precursor for IAA formation in microorganisms [18], B. subtilis 10-4 produced IAA (Figure 1a). Another important PGP trait detected for strain 10-4 is siderophore production, which significantly influences plant growth due to its contribution to the transport of Fe3+ inside plant cells [17]. The results showed that B. subtilis 10-4 at different concentrations improved the growth of 6-day-old wheat seedlings (Figure 1b); however, the most optimal concentration for stimulating the lengths and FW and DW accumulation in both roots and shoots was 105 CFU mL−1 (Figure 1c). Obviously, the detected ability of B. subtilis 10-4 to produce bioactive compounds (Figure 1a) makes a significant contribution to plant growth. Additionally, plant growth stimulation may occur due to the direct penetration of bacteria into the internal tissues and organs of plants [19].
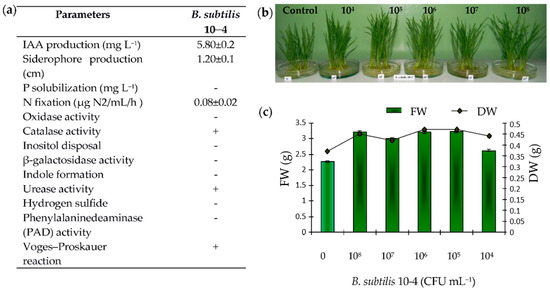
Figure 1.
Some PGP traits and physio-biochemical properties of bacterial strain B. subtilis 10-4 (a), and the effect of pre-inoculation with B. subtilis 10-4 in different concentrations (0 (control), 104, 105, 106, 107, 108 CFU mL−1) on the growth of 6-day-old wheat seedlings (b) and their fresh (FW) and dry (DW) biomass accumulations (c).
3.1.2. The Ability of B. subtilis 10-4 to Colonize Internal Wheat Tissues
Among PGP bacteria, there is a special interest in endophytic bacteria due to their ability to colonize internal host plant tissues and create more effective relationships without having a negative influence on plants [18,19]. Using surface-sterilized wheat seedlings (grown in sterile conditions) and RAPD–PCR analysis, we observed that B. subtilis 10-4 effectively colonized internal wheat tissues after pre-sowing inoculation (Figure 2). Around control (non–inoculated) plant segments, bacterial growth was absent (Figure 1a); however, around B. subtilis (10-4)-inoculated segments, endophytic bacteria growth was observed (Figure 2b). RAPD-PCR analysis confirmed that bacteria grown around plants are identical to the native bacterial strain of 10-4, which is used for pre-sowing inoculation of seeds (Figure 2c).
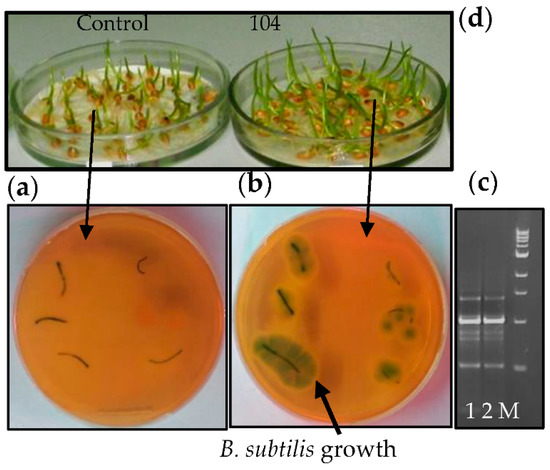
Figure 2.
Testing the ability of B. subtilis 10-4 to colonize internal wheat seedling (3-days-old) upon pre-sowing inoculation of surface-sterilized seeds and after growing in sterile conditions for 3 days: (a) the absence of bacterial growth around non-bacterial inoculated lant segments; (b) bacterial growth (light green colonies) around the surface-sterilized leaf and root segments of wheat seedlings pre-inoculated with B. subtilis 10-4; (c) electropherogram of PAAG: 1—DNA of origin B. subtilis 10-4; 2—DNA of bacteria isolated from the bacteria growing around wheat segments; M—DNA marker; (d)—appearance of 3-day-old wheat seedlings non-inoculated (Control) and pre-inoculated with B. subtilis 10-4 (104) and taking for analysis.
3.1.3. Biocontrol Activity of B. subtilis 10-4 Cells, Metabolites, and Lipopeptides against Fusarium spp.
The obtained results of in vitro assays showed that B. subtilis 10-4 exerts antagonistic activity against the growth of phytopathogenic fungus F. culmorum (Figure 3a). Microscopic observation showed that the mycelia structure was well organized in the absence of bacterial cells (Figure 3b), while numerous gaps in mycelia appeared in the presence of B. subtilis 10-4 (Figure 3c). The observed fungal suppression (Figure 3a) is most likely related to the ability of B. subtilis 10-4 to produce various metabolites with strong antibiotic activities (Figure 3d). Our results showed that B. subtilis 10-4-produces the LPs surfactin C14, C15, and C15 (Figure 3e), which are characterized by strong antifungal activity (Figure 3f).
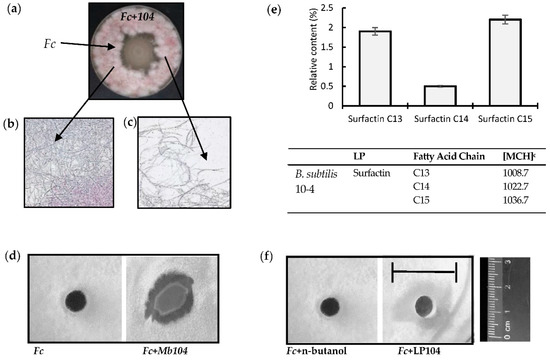
Figure 3.
In vitro antagonistic activity of B. subtilis 10-4 (Bs104) against Fusarium culmorum (Fc) (a) and microscopic visualizations of the Fc fungal growth and morphology in the absence (b) and on the border with B. subtilis 10-4 (104) (c). (d) Inhibition of Fc by B. subtilis 10-4-produced metabolites (Mb104); (e) relative content of lipopeptides (LP) produced by B. subtilis 10-4; (f) inhibition of Fc by B. subtilis 10-4-produced LP (LP104).
3.2. Effect of B. subtilis 10-4 and SA on Wheat Growth under Drought, Fusarium Infection, and a Combination of These Stresses
In nature, plants are constantly exposed to various abiotic and biotic stresses, as well as a combination of these stresses, that can significantly inhibit the growth and productivity of major agricultural crops, including wheat [1,2,3,4,5]. Among biotic and abiotic stresses, Fusarium spp.-caused diseases and drought are the most common and important combinations of stresses that significantly reduce wheat yields [1,2,3,4,5].
It was found that pre-inoculation with endophyte B. subtilis 10-4 alone, and together with SA under normal growing conditions, promoted the activation of seedling growth (Figure 4a), with the best growth stimulation occurring after the joint application of endophyte and SA (Figure 4a). Drought, FRR, and combined drought + FRR resulted in decreased seedling growth (Figure 4b,e,f). Pre-treatment with B. subtilis 10-4, SA, and B. subtilis 10-4 + SA softened (to varying degrees) the level of damage caused by these stresses on plant growth, a fact that was reflected in higher indicators of root length and shoots of plants in comparison with untreated controls. Moreover, the greatest protective effect from the damaging effect of these stresses on growth was observed when endophyte was used in conjunction with SA (Figure 4b,e,f). Similarities between B. subtilis 10-4, SA, and B. subtilis 10-4 + SA were also revealed during the assessment of plants’ fresh (FW) and dry (DW) weight accumulation, both under normal conditions (Figure 4c) and in the case of drought (Figure 4d), FRR (Figure 4j), and drought + FRR (Figure 4h). The findings indicate that the most effective method for stimulating growth processes under normal conditions while protecting wheat plants from the negative impact of drought, FRR, and drought + FRR, is the use of B. subtilis 10-4 in conjunction with SA, since it is with this combination the maximum positive effect on growth indicators, including growth in the lower (roots) and aboveground (shoots) tissues and their accumulation (FW, DW), is observed (Figure 4a–d).
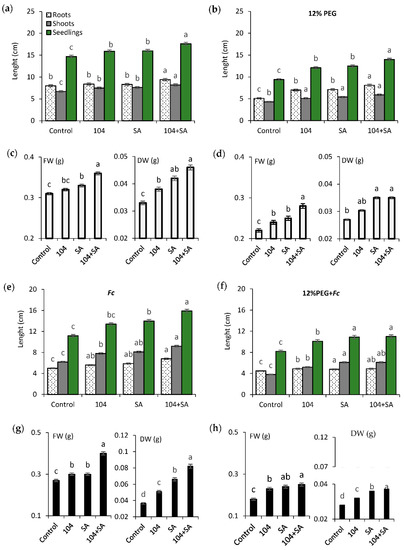
Figure 4.
Effect of pre-sowing treatment with endophyte B. subtilis 10-4 (104), SA, and B. subtilis 10-4 + SA on growth of wheat seedlings (6-day-old) (length of roots, shoots) and their biomass accumulation under normal (a,c), drought (12%PEG) (b,d), Fusarium culmorum infection (Fc) (e,g), and combined drought + F. culmorum infection (12%PEG + Fc) (f,h) conditions. Different letters indicate a significant difference between the means at the probability level of p < 0.05.
3.3. Changes in the Level of Lipid Peroxidation (MDA) and Proline (Pro) Content in Wheat Plants Pre-Treated with B. subtilis 10-4, SA, and B. subtilis 10-4 + SA under Combined Drought and Fusarium Infection
Abiotic/biotic stresses and their combination result in strong oxidative and osmotic damages at the cellular level due to the abnormal formation of reactive oxygen species (superoxide radicals, hydrogen peroxide, etc.), and water regime disturbances [1,4,20]. As biomarkers of the development of plants’ defence reactions against oxidative and osmotic stresses are the final product of lipid peroxidation, malondialdehyde (MDA) and osmoprotectant proline (Pro), which also play the role of an antioxidant and low molecular weight chaperone, and are involved in maintaining the native structure of enzymes, reduce the degree of damage to cellular structures caused by dehydration [1,10,20]. Our results showed that seeds bio-primed with endophytic B. subtilis 10-4 alone, and especially when mixed with SA, resulted in decreased stress-induced (drought, FRR, drought + FRR) lipid peroxidation (Figure 5a) and Pro accumulation in plants (Figure 5b), thereby indicating a protective effect of plant cells against reactive oxygen species and osmotic damages [20].
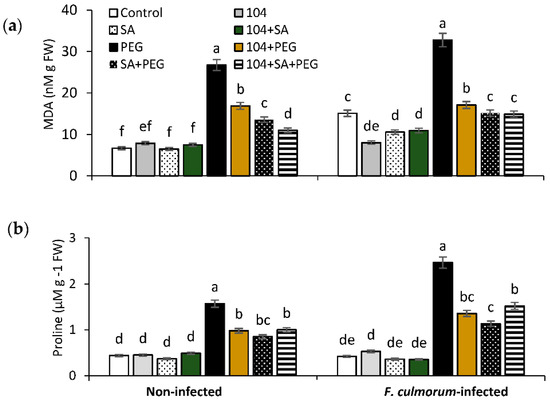
Figure 5.
Changes in the content of malondialdehyde (MDA) (a) and osmoprotectant proline (Pro) (b) in healthy (non-infected) and Fusarium culmorum (F. culmorum)-infected seedlings pre-treated before sowing with B. subtilis 10-4 (104), SA, and B. subtilis 10-4 + SA, and grown under normal and combined drought stress conditions. Different letters indicate a significant difference between the means at the probability level of p < 0.05.
4. Conclusions
Thus, endophytic bacteria B. subtilis 10-4 alone, and especially when mixed with SA, significantly enhances wheat growth and softens the damaging influence of drought, FRR, and drought + FRR. An important contribution to the observed growth-stimulating and protective effects of B. subtilis 10-4 and SA on the plants revealed the ability of bacteria to produce bioactive compounds such as auxins, siderophores, LP surfactin (C13–C15), and to colonize internal plant tissues, thereby forming a close interaction with host plants. Data on the formation of an effective interaction between B. subtilis 10-4 and wheat indicate decreased stress-induced oxidative (MDA) and osmotic (Pro) damages to plant cells thanks to the influence of B. subtilis 10-4. Moreover, the results clearly demonstrated that SA significantly increased the positive actions of B. subtilis 10-4 on plants under normal and stress conditions. The findings indicate that B. subtilis 10-4 alone, and especially when mixed with SA, has potential in relation to its use as an eco-friendly agent for improving wheat growth and tolerance under drought, FRR, and drought + FRR stresses.
Author Contributions
O.L. conceived and designed the experiments; O.L., D.G. and A.I. performed the experiments; O.L. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Council for grants of the President of Russian Federation] grant number [MK643.2019.11] and partially by [Russian Science Foundation] grant number [18-76-00031].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The research was partially carried out within the state assignment of Russia [No. AAAA-A21-121011990120-7] using the instrument park of the RCCU “Agidel” of the UFRC RAS and “KODINK”. Additionally, we grateful to all colleagues and students who contributed to the research.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| SA | Salicylic acid |
| LP | Lipopeptide |
| FRR | Fusarium root rot |
| MDA | Malondialdehyde |
| Pro | Proline |
References
- Lastochkina, O.; Aliniaeifard, S.; Kalhor, M.S.; Yuldashev, R.; Pusenkova, L.; Garipova, S. Plant growth promoting bacteria—Biotic strategy to cope with abiotic stresses in wheat. In Wheat Production in Changing Environments: Management, Adaptation and Tolerance; Hasanuzzaman, M., Nahar, K., Hossain, A., Eds.; Springer: Singapore, 2019; Chapter 23; pp. 579–614. [Google Scholar]
- Juroszek, P.; von Tiedemann, A. Climate change and potential future risks through wheat diseases: A review. Eur. J. Plant Pathol. 2013, 136, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Afzal, F.; Chaudhari, K.S.; Gul, A.; Farooq, A.; Ali, H.; Nisar, S.; Sarfraz, B.; Shehzadi, K.J.; Mujeeb-Kaziet, A. Bread Wheat (Triticum aestivum L.) under biotic and abiotic stresses: An overview. In Crop Production and Global Environmental Issues; Hakeem, K., Ed.; Springer: Cham, Switzerland, 2015; pp. 293–317. [Google Scholar]
- Lastochkina, O. Bacillus subtilis-mediated abiotic stress tolerance in plants. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Islam, M.T., Rahman, M.M., Pandey, P., Boehme, M.H., Haesaert, G., Eds.; Springer Nature: Bremgarten, Switzerland, 2019; Volume 2, Chapter 6; pp. 97–133. [Google Scholar]
- Shakirova, F.M.; Avalbaev, A.M.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Maslennikova, D.R.; Yuldashev, R.A.; Allagulova, C.R.; Lastochkina, O.V. Hormonal intermediates in the protective action of exogenous phytohormones in wheat plants under salinity: A case study on wheat. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N., Nazar, R., Iqbal, N., Anjum, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 185–228. [Google Scholar]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [Green Version]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Netrusov, A.I.; Egorova, M.A.; Zakharchuk, L.M. Praktikum Po Mikrobiologii (A Practical Course in Microbiology); Tsentr “Akademiya”: Moscow, Russia, 2005. [Google Scholar]
- Mokronosov, A.T. Small Workshop on Plant Physiology; Moscow State University: Moscow, Russia, 1994; 184p. [Google Scholar]
- Bates, L.S.; Waldern, R.P.; Teare, D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Malik, D.K.; Sindhu, S.S. Production of indole acetic acid by Pseudomonas sp.: Effect of coinoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol. Mol. Biol. Plants 2011, 17, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Umarov, M.M. Associative Nitrogen Fixation; Moscow State University: Moscow, Russia, 1986; 136p. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018, 9, 1315. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Singh, M.C.; Singh, S.S.; Kumar, M.; Pathak, M.; Shakywar, R.C.; Pandey, A.K. Inside the plants: Endophytic bacteria and their functional attributes for plant growth promotion. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 11–21. [Google Scholar] [CrossRef]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; von Maltzahn, G.; et al. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolupaev, Y.E.; Yastreb, T.O. Physiological functions of nonenzymatic antioxidants of plants. Bull. Kharkiv Natl. Agrar. Univ. Ser. Biol. 2015, 2, 6–25. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).