Role of Class III Peroxidases in Stem Lignification of Zinnia elegans Jacq. †
Abstract
:1. Introduction
2. Experiments
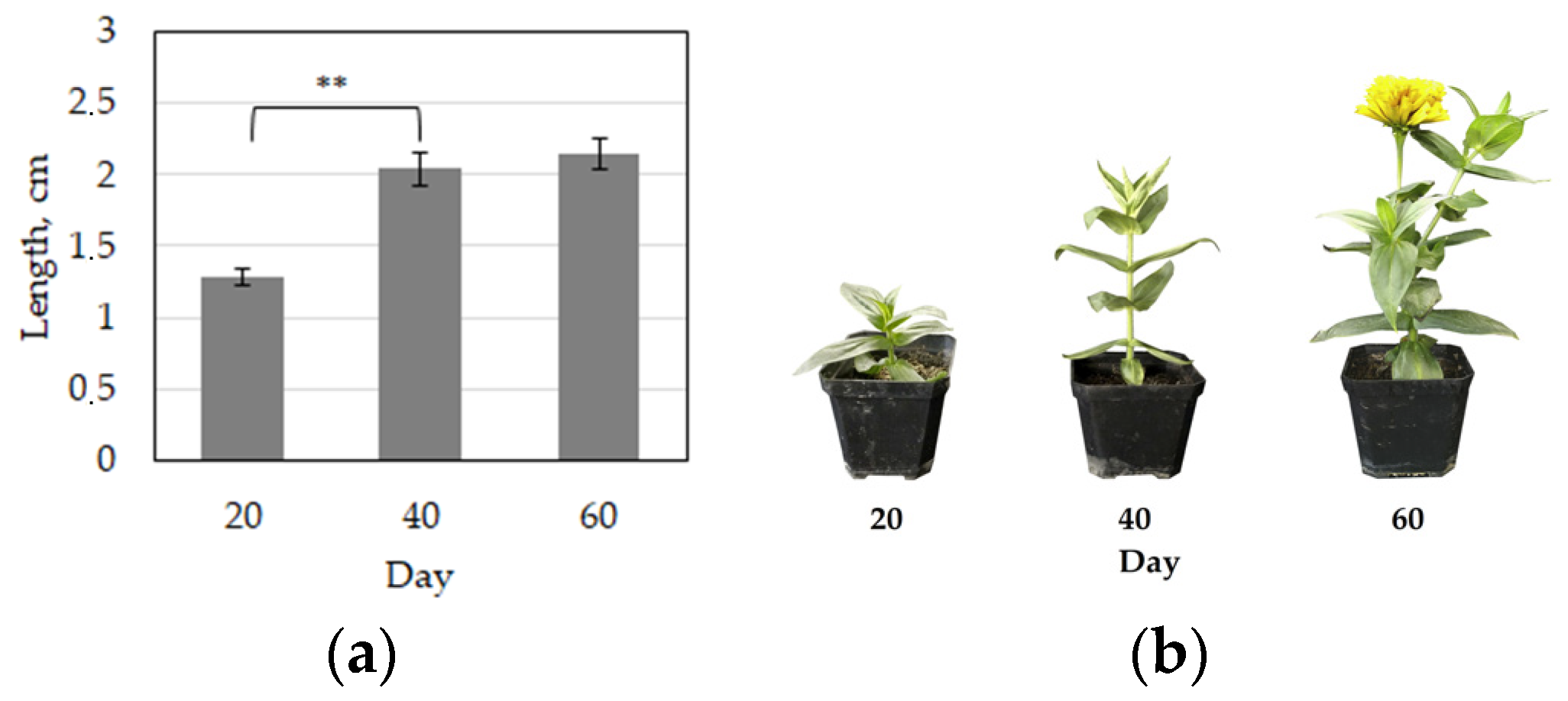
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPOX | Benzidine peroxidase |
| GPOX | Guaiacol peroxidase |
| PX | Protoxylem |
| MX | Metaxylem |
| SC | Sclerenchyma |
References
- Passardi, F.; Longet, D.; Penel, C.; Dunand, C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004, 65, 1879–1893. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 2, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Kukavica, B.; Vidovic, M.; Morina, F.; Menckhoff, L. Class III peroxidases: Functions, localization and redox regulation of isoenzymes. Antioxid. Antioxid. Enzym. High. Plants 2018, 269–300. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001, 5, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcelo, A.R.; Gomez Ros, L.V.; Carrasco, A.E. Looking for syringyl peroxidases. Trends Plant Sci. 2007, 12, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Gabaldon, C.; Lopez-Serrano, M.; Pedreño, M.A.; Barcelo, A.R. Cloning and molecular characterization of the basic peroxidase isoenzyme from Zinnia elegans, an enzyme involved in lignin biosynthesis. Plant Physiol. 2005, 3, 1138–1154. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Demura, T.; Yamawaki, K.; Inoue, Y.; Sato, S.; Sugiyama, M.; Fukuda, H. Isolation and characterization of a novel peroxidase gene ZPO-C whose expression and function are closely associated with lignification during tracheary element differentiation. Plant Cell Physiol. 2006, 4, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Maksimovic, J.D.; Maksimović, V.; Živanović, B.; Šukalović, V.H.-T.; Vuletić, M. Peroxidase activity and phenolic compounds content in maize root and leaf apoplast and their association with growth. Plant Sci. 2008, 175, 656–662. [Google Scholar] [CrossRef]
- Liljegren, S. Phloroglucinol stain for lignin. Cold Spring Harb. Protoc. 2010, 2010, 4954. [Google Scholar] [CrossRef]
- Goldfischer, S.; Essner, R. Further observation of the peroxidase activities of microbodies (peroxisomes). J. Histochem. Cytochem. 1969, 17, 681–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrvoje Lepeduš, H.; Cesar, V.; Krsnik-Rasol, M. Guaiacol peroxidases in carrot (Daucus carota L.) root. Food Technol. Biotechnol. 2004, 42, 33–36. [Google Scholar]
- Zhivet’ev, M.A.; Rachenco, E.I.; Putilina, T.E.; Krasnobaev, V.A.; Graskova, I.A.; Voinikov, V.K. Activity and isoenzyme spectrum of peroxidases and dehydrins of some plant species, growing on the shores of lake Baikal, under abiotic stress. J. Stress Physiol. Biochem. 2010, 6, 42–50. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay catalase and peroxidase. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zaia, D.M.; Marques, F.R.; Zaia, C.V. Spectrophotometric determination of total proteins in blood plasma: A comparative study among dye-binding methods. Braz. Arch. Biol. Technol. 2005, 48, 385–388. [Google Scholar] [CrossRef]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonics is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrier, M.; Loppinet Serani, A.; Denux, D.; Lasnier, J.; Ham Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Begovic, L.; Lepeduš, H.; Lalić, A.; Štolfa, I.; Jurković, Z.; Kovačević, J.; Cesar, V. Involvement of peroxidases in structural changes of barley stem. Bragantia 2017, 76, 352–359. [Google Scholar] [CrossRef]
- Ros Barcelo, A.; Gomes Ros, L.V.; Gabaldon, C.; Lopez-Serrano, M.; Pomar, F.; Carrión, J.S.; Pedreño, M.A. Basic peroxidases: The gateway for lignin evolution? Phytochem. Rev. 2004, 3, 61–78. [Google Scholar] [CrossRef]
- Novo-Uzal, E.; Fernandez-Perez, F.; Herrero, J.; Gutierrez, J.; Gomez-Ros, L.V.; Bernal, M.A.; Diaz, J.; Cuello, J.; Pomar, F.; Pedreño, M.A. From Zinnia to Arabidopsis: Approaching the involvement of peroxidases in lignification. J. Exp. Bot. 2013, 64, 3499–3518. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Plant Age, Days | Klason Lignin, % | H2O2, µM g−1 Dry Weight | BPOX, Units mg−1 Protein * Min | GPOX, mM Guaiacol mg−1 Protein * Min |
|---|---|---|---|---|
| 20 (juvenile) | 14.30 ± 0.56 | 114.2 ± 6.8 | 1.03 ± 0.045 | 0.21 ± 0.03 |
| 40 (vegetative) | 17.71 ± 0.61 a | 171.2 ± 2.6 a | 2.99 ± 0.12 a | 0.42 ± 0.01 a |
| 60 (flowering) | 20.58 ± 1.62 b | 179.2 ± 5.3 | 0.27 ± 0.01 b | 0.61 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tugbaeva, A.; Ermoshin, A.; Plotnikov, D.; Wuriyanghan, H.; Kiseleva, I. Role of Class III Peroxidases in Stem Lignification of Zinnia elegans Jacq. Biol. Life Sci. Forum 2021, 4, 22. https://doi.org/10.3390/IECPS2020-08847
Tugbaeva A, Ermoshin A, Plotnikov D, Wuriyanghan H, Kiseleva I. Role of Class III Peroxidases in Stem Lignification of Zinnia elegans Jacq. Biology and Life Sciences Forum. 2021; 4(1):22. https://doi.org/10.3390/IECPS2020-08847
Chicago/Turabian StyleTugbaeva, Anastasia, Alexander Ermoshin, Dmitry Plotnikov, Hada Wuriyanghan, and Irina Kiseleva. 2021. "Role of Class III Peroxidases in Stem Lignification of Zinnia elegans Jacq." Biology and Life Sciences Forum 4, no. 1: 22. https://doi.org/10.3390/IECPS2020-08847
APA StyleTugbaeva, A., Ermoshin, A., Plotnikov, D., Wuriyanghan, H., & Kiseleva, I. (2021). Role of Class III Peroxidases in Stem Lignification of Zinnia elegans Jacq. Biology and Life Sciences Forum, 4(1), 22. https://doi.org/10.3390/IECPS2020-08847







