Abstract
Rubia cordifolia L. is an important plant used in Ayurvedic and Siddha medicinal systems of India for treatment of blood disorders. Of all the plant parts, roots of R. cordifolia are the most suitable source of effective secondary metabolites. The present work investigated phytochemical content and antioxidant potential of R. cordifolia root powder extracted in different solvents. Total polyphenols and flavonoids content were estimated. High antioxidant activity was corroborated with DPPH, hydrogen peroxide, nitric oxide, reducing power and total antioxidant assays. Obtained results showed that ethanol extracts were most potent over methanol, aqueous, and PBS extracts for DPPH, hydrogen peroxide, and reducing power assays. In contrast, methanol and aqueous extracts had higher potency in nitric oxide and total antioxidant assays. Encouraging results were obtained for antioxidant activity even upon PVPP treatment that removed the polyphenols from the extracts. The results suggest a potential of ethanol and methanol extracts for cancer cytotoxicity.
1. Introduction
Rubia cordifolia L. belongs to the family Rubiaceae and is largely distributed from Africa to tropical Asia, China, Japan, and Australia. The active compounds of R. cordifolia are 1-hydroxy,2-methoxy anthraquinone,3-dimethoxy 2 carboxy anthraquinone, rubiadin, mangistin, alizarin, garancin, rubiprasin A,B,C, ruiearbonls, mollugin and furomollugin [1]. The roots of R. cordifolia are used for the preparation of aqueous, ethanol, methanol, chloroform, and dichloromethane extracts. Some of their therapeutic activities are listed in Table 1.

Table 1.
Known potential/activities of R. cordifolia.
Different extracts have shown varied potential and a comparative account among the extracts is missing. We hereby determined antioxidant activities of these extracts. We identified ethanol and methanol extracts of the root as the most suitable solvent for antioxidant activities.
2. Experiments
2.1. Plant Collection
Powder of R. cordifolia (root) was collected from Maharashtra Arogya Mandal, Hadapsar Pune, Maharashtra, India. They were stored in airtight containers for future use.
2.2. Preparation of Extracts
Extracts of powders were prepared in ethanol (HiMedia, Mumbai, India), methanol (HiMedia, India) or distilled water as described. In brief, powder of R. cordifolia were extracted with solvent (ethanol, methanol or aqueous) by conventional Soxhlet apparatus (4951, Goel Scientific, Vadodara, India) extraction at the temperature of 60 °C. After the exhaustive extraction, each extract was evaporated to dryness by rotary evaporator (Aditya Scientific, Hyderabad, India) and if it was not dried then the extract was further concentrated using concentrator (5305000304, Eppendorf India Pvt. Ltd, Chennai, India) and stored at room temperature for future use. Phosphate Buffer Saline (PBS) extracts of R. cordifolia were made by mixing the powder of the root in PBS or media (50 mg/mL).
2.3. Phytochemical Screening
The presence of secondary metabolites viz. alkaloids, saponins, tannins, phenols, glycosides, terpenes, carotenoids and quinones was detected using the standard tests [10].
2.4. Removal of Polyphenols from Plant Extracts
The plant extracts were treated with 10% Polyvinylpolypyrrolidone (PVPP) (HiMedia, India) made in respective solvents for the removal of polyphenols. The extracts (5 mL) were treated with PVPP (5 mL) in respective solvents and kept on a shaking incubator at 37 °C overnight. The supernatant was used for further experiments [11].
2.5. Quantification of Phenols
The phenolic content was determined according to the method given earlier [12]. A total of 1 mL of 1 mg/mL extract or gallic acid (HiMedia, India) with the concentration of 20, 40, 60, 80 and 100 µg/mL was mixed with 0.5 mL of 1N Folin–Ciocalteu reagent. Mixture was kept for 5 min, followed by addition of 1 mL of 20% sodium carbonate (HiMedia, India). After 10 min incubation at room temperature, absorbance was measured at 730 nm using UV-Vis spectrophotometer. Gallic acid was used as the standard.
2.6. Quantification of Flavonoids
Flavonoid content in the extract was determined according to the method given earlier [13]. A total of 1 mL of extract or quercetin (HiMedia, India) with the concentration of 100, 200, 300, 400 and 500 µg/mL was mixed with 1.25 mL of distilled water and 75 µL of 5% of sodium nitrite (HiMedia, India). This solution was incubated for 5 min, and then 150 µL of 10% aluminum chloride (Sigma-Aldrich, St. Louis, MO, USA) solution was added. After incubation of 6 min, 500 µL of 1 M sodium hydroxide (HiMedia, India) and 275 µL of distilled water were added to prepare the final mixture. The absorbance was read at 510 nm using UV-Vis spectrophotometer. Quercetin was used as the standard.
2.7. Antioxidant Assays
2.7.1. DPPH Assay
DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging activity was measured with spectrophotometer method described previously [14]. To the 0.5 mL extract solution with or without PVPP, made in respective solvents of concentration ranging from 20–100 µg, 1 mL of 0.2 mM DPPH (HiMedia, India) made in ethanol was added and volume was made up to 2 mL with methanol, and incubated for 30 min at room temperature. The absorbance was measured at 517 nm against blank. Ascorbic acid (HiMedia, India) was used as the standard. The percentage of inhibition of DPPH was calculated as follows:
2.7.2. Hydrogen Peroxide Assay
The scavenging effect of hydrogen peroxide was determined as described previously [15]. A total of 1 mL of extract solution treated with or without PVPP, of concentration ranging from 20–100 µg was treated with 0.6 mL, 40 mM of hydrogen peroxide (Thermo Fisher, Concord, NH, USA) prepared in phosphate buffer (pH 7.4) for 10 min. The absorbance was read at 230 nm against blank. Ascorbic acid was used as standard.
2.7.3. Scavenging Activity of Nitric Oxide
Nitric oxide was generated from sodium nitroprusside and its scavenging effect was determined as per [16]. Different concentration from 20–100 µg of 1 mL of extract solution with or without PVPP, phosphate buffer 1 mL (pH 7.4) was used to prepare sodium nitroprusside (HiMedia, India) 0.5 mL, 10 mM, and then incubated for 5 h at 25 °C. After 5 h of incubation, 0.5 mL of supernatant liquid was removed and 0.5 mL of Griess reagent (Thermo Fisher, USA) (1 mM) prepared in distilled water was added. The absorbance was read at 546 nm. Ascorbic acid was used as standard.
2.7.4. Total Antioxidant Capacity
The total antioxidant capacity was determined by phosphomolybdate assay [17]. A total of 1 mL of extract with or without PVPP, of concentration prepared in respective solvents, ranging from 20–100 μg was taken in centrifuge tube and 1 mL of reagent containing 0.6 M sulfuric acid (HiMedia, India), 28 mM sodium phosphate (Thermo Fisher, USA) and 4 mM ammonium molybdate (Thermo Fisher, USA) was added. The tubes were incubated at 95 °C for 90 min, and were cooled to room temperature, and absorbance was read at 695 nm. Ascorbic acid was used as standard.
2.7.5. Assay of Reducing Power
The reducing power assay was determined by Spectrophotometric method of Oyaizu (1986) [18]. 2.5 mL of extract solution made in respective solvents, of various concentrations ranging from 20–100 µg was treated with 2.5 mL of 0.2 M phosphate buffer (pH 6.6), 2.5 mL of 1% potassium ferricyanide (Thermo Fisher, USA), incubated at 50 °C for 20 min, cooled, 2.5 mL of 10% trichloro acetic acid (HiMedia, India) was added and centrifuged at 3000 rpm for 10 min. The upper layer (2.5 mL) of the solution was removed and 2.5 mL of methanol and 0.5 mL of 0.1% ferric chloride (HiMedia, India) solutions were added, the absorbance of the resulting solution was read at 700 nm. Ascorbic acid was used as standard.
2.8. Statistical Analysis
All experiments were performed in triplicate. All the values were expressed as mean ± standard error of mean. The data were analyzed by Student–Newman–Keuls test using Sigma Plot version 14 (Systat software Inc., San Jose, CA, USA) and IC50 values calculated using Origin software version 8.1 (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. Extracts of Plant Powders Show Presence of Several Phytochemicals
3.1.1. Qualitative Analysis of Secondary Metabolites
The ethanol extract of the R. cordifolia powder showed the presence of alkaloids, flavonoids, glycoside, phenols and terpenes while the methanol extract showed the presence of alkaloids, tannins, phenols, flavonoids, and terpenes. The aqueous extract of plant powder showed the presence of alkaloids, saponins, tannins, phenols, flavonoids, and terpenes. The PBS extract of powder showed the presence of alkaloids, flavonoids, terpenes and phenols (Table 2).

Table 2.
Phytochemical screening of R. cordifolia extracts in different solvents—Qualitative Assay.
3.1.2. Quantification of Phenols and Flavonoids in Plant Extracts
Standard curves were generated for phenols (with gallic acid) and flavonoids (with quercetin) to quantify phenols and flavonoids in the extracts. These assays were repeated for presence and absence of PVPP (Figure 1). Ethanol and methanol extracts of powders had 75% more phenol and flavonoid content compared to aqueous and PBS extracts for 1 mg/mL concentration of extracts (Table 3 and Table 4).
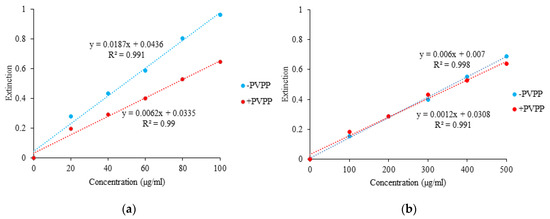
Figure 1.
(a) Standard curve of gallic acid without (blue) and with (red) PVPP for Phenols; (b) Standard curve of quercetin without (blue) and with (red) PVPP for Flavonoids.

Table 3.
Quantification of phenol contents of root powder extracts of R. cordifolia.

Table 4.
Quantification of flavonoid contents of powder extracts of R. cordifolia.
3.2. Plant Extracts Have Antioxidant Activity
Ascorbic acid showed significant radical scavenging activity (p ≤ 0.05) (Figure 2 and Figure 3, Table 5). Prior to PVPP treatment, in antioxidant assays viz. DPPH, hydrogen peroxide, and reducing power assay, the ethanol extract had 0.55, 0.54 and 0.95 times scavenging activity as that of ascorbic acid, respectively. In nitric oxide and total antioxidant assay, methanol extract was 93% and 82% more potent as compared to standard.
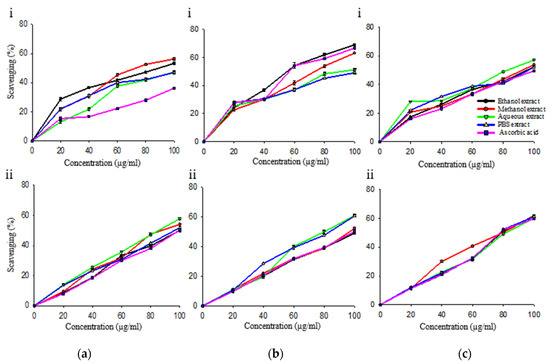
Figure 2.
In vitro antioxidant assays of R. cordifolia without (i) and with (ii) PVPP for (a) DPPH assay; (b) hydrogen peroxide assay; (c) nitric oxide assay.
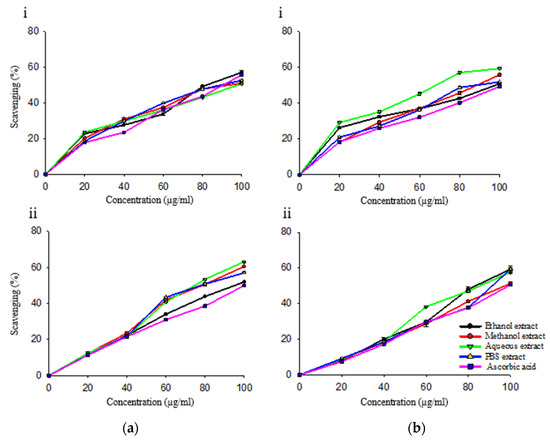
Figure 3.
In vitro antioxidant assays of R. cordifolia without (i) and with (ii) PVPP for (a) Assay of reducing power; (b) Total antioxidant assay.

Table 5.
IC50 values of DPPH, Hydrogen peroxide, Nitric oxide, Reducing power and Total antioxidant assay of R. cordifolia.
4. Discussion
R. cordifolia L. produces a variety of secondary metabolites. These metabolites are responsible for spatiotemporal and sustainable growth of the plant. They also act as important defense compounds. Utility of secondary metabolites for human health has achieved high recognition owing to the usage in traditional medication. R. cordifolia L. has been a less explored system and identification of suitable extract with maximal components is urgently required. In the present study, we have selected four solvent system for extracting secondary metabolites from roots of R. cordifolia L. Quantitative analysis indicated a good number of phenols and flavonoids in the root extracts. Our method of Soxhlet extraction, led to more release of phenols (in ethanol, and methanol extracts) from R. cordifolia root powder than that obtained from field [19].
Presence of antioxidant in the extract is crucial. Results of DPPH assay for ethanol extract reported by Zhang et al. [20] had EC50 in the range of 23.88 to 65.23 µg/mL. They used ultrasonic-assisted extraction process. These values are much lower than our range of 78.25–88.63 µg/mL. We believe suitability of extraction method is driver of differential results. Basu and Hazra [21] reported a range of 153.7–310.3 µg/mL for methanol and aqueous extracts as evaluated by nitric oxide assay. Here the authors used filtrate of direct solubilization of extracts in respective solvents. Our results have a better range (94.46–108.21 µg/mL), possibly due to our choice of method of Soxhlet exhaustive extraction process. We are also reporting for the first time, results of R. cordifolia extracts (ethanol, methanol, aqueous and PBS) treated with PVPP for antioxidant assays. The IC50 of R. cordifolia, extracts (ethanol, methanol, aqueous, PBS extracts), for DPPH assay (98.26, 89.47, 85.53 and 97.55 µg/mL), hydrogen peroxide free radical scavenging assay (101.34, 97.71, 80.85 and 81.05 µg/mL), nitric oxide assay (82.17, 78.46, 84.23 and 81.95 µg/mL), reducing power assay (93.72, 79.79, 77.62 and 81.81 µg/mL), total antioxidant assay (87.92, 97.52, 85.14 and 91.92 µg/mL). Hence, even after removal of phenols and flavonoids, antioxidant activity is not hampered. This suggests antioxidant potential for different classes of secondary metabolites. High presence of antioxidant may be used for anti-proliferative properties in certain cancers [1].
5. Conclusions
Our study has revealed presence of high antioxidants in root extracts of R. cordifolia. Methods of extraction is important as observed when compared reports. This work provides initial steps required in suitability of solvents for R. cordifolia extract preparations. Further work regarding anticancer potential needs to be evaluated to verify the extent of utility of antioxidant nature.
Author Contributions
S.C.K., A.A.K., and R.B.H. conceived and designed the experiments; R.B.H. performed the experiments; S.C.K., R.B.H., J.S. and A.A.K. analyzed the data; S.C.K. and A.A.K. contributed reagents/materials/analysis tools; S.C.K. and J.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All the relevant data is presented in this study itself.
Acknowledgments
S.C.K. would like to acknowledge Department of Technology, Savitribai Phule Pune University, Pune, India for departmental funds. A.A.K. would like to acknowledge Department of Botany, Savitribai Phule Pune University, Pune, India for departmental funds. R.B.H. and S.C.K. acknowledge the authenticated plant material gifted by Sushil Deshmukh, Maharashtra Arogya Mandal, Pune, India.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| PBS | Phosphate Buffer Saline |
| PVPP | Polyvinylpolypyrrolidone |
References
- Verma, A.; Kumar, B.; Alam, P.; Singh, V.; Kumar Gupta, S. Rubia cordifolia—A Review on Pharmaconosy and Phytochemistry. Int. J. Pharm. Sci. Res. 2016, 7, 2720. [Google Scholar]
- Chen, Y.; Chen, P.; Bao, B.; Shan, M.; Zhang, K.; Cheng, F.; Cao, Y.D.; Zhang, L. Anti-thrombotic and pro-angiogenic effects of Rubia cordifolia extract in zebrafish. J. Ethnopharmacol. 2018, 219, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Joharapurkar, A.A.; Zambad, S.P.; Wanjari, M.M.; Umathe, S.N. In vivo evaluation of antioxidant activity of alcoholic extract of Rubia cordifolia Linn. and its influence on ethanol-induced immunosuppression. Indian J. Pharmacol. 2003, 35, 232–236. [Google Scholar]
- Singh, P.; Agrawal, M.; Hishikar, R.; Joshi, U.; Maheshwari, B. Adverse drug reactions at adverse drug reaction monitoring center in Raipur: Analysis of spontaneous reports during 1 year. Indian J. Pharmacol. 2017, 49, 438–444. [Google Scholar]
- Shilpa, P.N.; Sivaramakrishnan, V.; Devaraj, S.N. Induction of Apoptosis by Methanolic Extract of Rubia cordifolia Linn in Hep-G2 Cell Line is Mediated by Reactive Oxygen Species. Asian Pac. J. Cancer Prev. 2012, 13, 2753–2758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Sarma, M.D.; Amarendra, P.; Hazra, B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. J. Pharm. Pharmacol. 2010, 62, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Sun, Y.; Chen, W.; Guo, X.; Guan, J.; Li, D. Anti-diarrheal and anti-inflammatory activities of aqueous extract of the aerial part of Rubia cordifolia. BMC Complement. Altern. Med. 2017, 17, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuanyuan, S.; Xuepeng, G.; Tan, J.Y.; Kang, L.; Dongyan, L.; Vikash; Yang, J.; Guang, D. In vitro antiviral activity of Rubia cordifolia aerial part extract against Rotavirus. Front. Pharmacol. 2016, 7, 1–15. [Google Scholar]
- Adwankar, M.K.; Chitnis, M.P. In vivo anti-Cancer activity of RC-18. Chemotherapy 1982, 28, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.C.; Evans, D.; Trease, G.E. Trease and Evans Pharmacognosy, 16th ed.; Saunders/Elsevier: Edinburgh, UK; New York, NY, USA, 2009. [Google Scholar]
- Ranatunge, I.; Adikary, S.; Dasanayake, P.; Fernando, C.D.; Soysa, P. Development of a Rapid and Simple Method to Remove Polyphenols from Plant Extracts. Int. J. Anal. Chem. 2017, 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaiman, C.T.; Balachandran, I. Total phenolics and total flavonoids in selected indian medicinal plants. Indian J. Pharm. Sci. 2012, 74, 258–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhishen J, Mengcheng T, Jianming W. Determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Lobo, R.; Shirwaikar, A. Free Radical Scavenging Actvity of Aqueous Extract of Sphaetanthus indicus (Linn). Pharmacologyonline 2009, 476, 468–476. [Google Scholar]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese Green Tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Pandithurai, M.; Murugesan, S. Free radical scavenging activity of methanolic extract of brown alga Spatoglossum asperum. J. Chem. Pharm. Res. 2014, 6, 128–132. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reactions: Antioxidative Activities of Product of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Kudale, S.; Ghatge, S.; Desai, N. Quantification of Phytochemicals in hairy root cultures of Rubia cordifolia Linn. Int. J. 2015, 3, 903–913. [Google Scholar]
- Zhang, X.; Liu, L.-J.; Song, T.-T.; Wang, Y.-Q.; Yang, X. An approach based on antioxidant fingerprint—Efficacy relationship and TLC bioautography assay to quality evaluation of Rubia cordifolia from various sources. J. Nat. Med. 2014, 68, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Hazra, B. Evaluation of Nitric Oxide Scavenging Activity, In Vitro and Ex Vivo, of Selected Medicinal Plants Traditionally Used in Inflammatory Diseases. Phyther. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 900, 896–900. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).