Study of the Release Kinetics of Capsaicin Extracted from Charapita Chili (Capsicum frutescens) from an O/W Emulsion Made with Sacha Inchi Oil (Plukenetia volubilis) and Encapsulated in Calcium Alginate Beads †
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing
2.2. Capsaicin Extraction Process
2.3. Emulsion Preparation Process
2.4. Emulsion Encapsulation Process
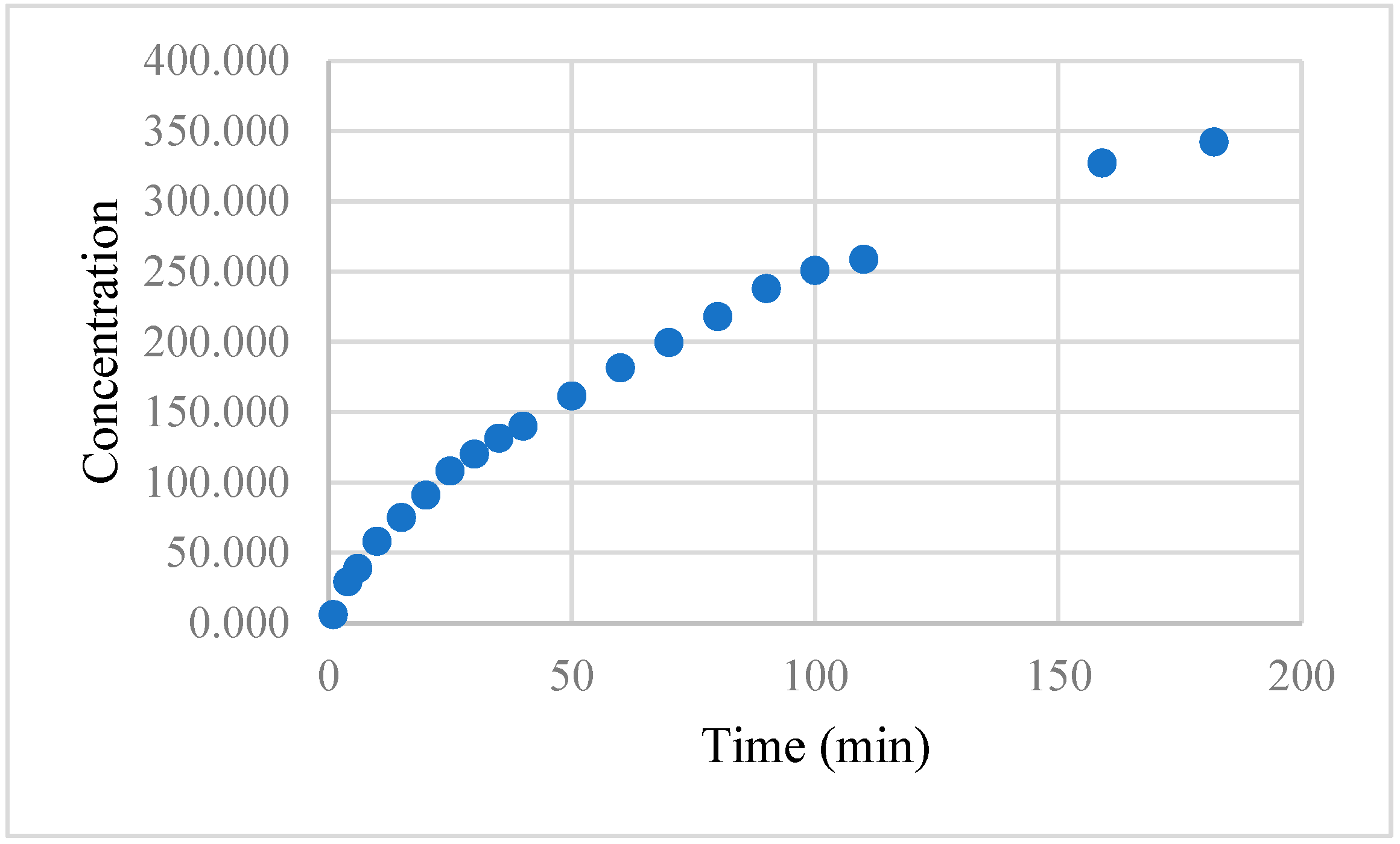
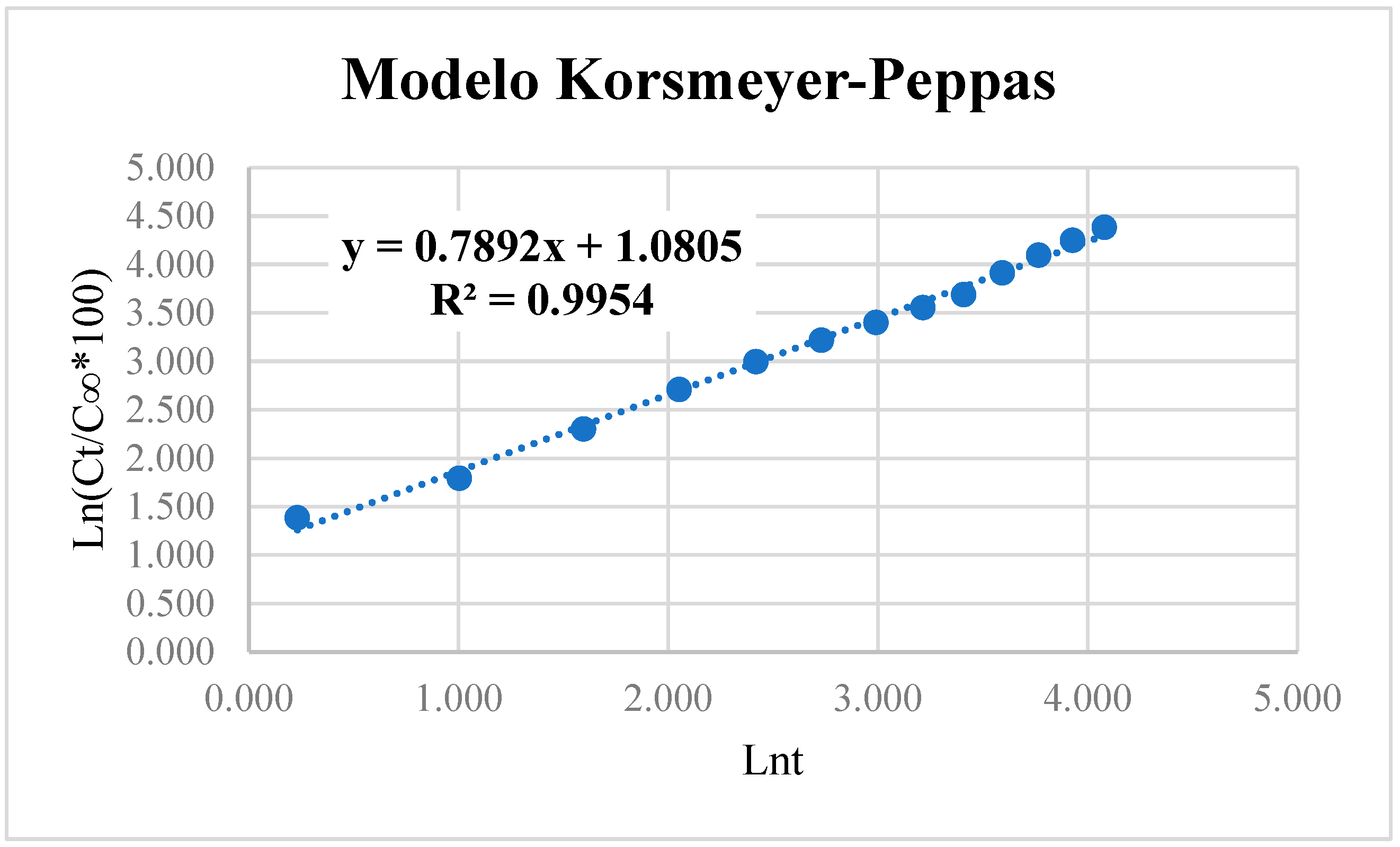
2.5. Capsaicin Release Kinetics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Universidad Nacional Agraria La Molina. Ajíes Peruanos, Sazón para el Mundo, 1st ed.; Sociedad Peruana de Gastronomía: Lima, Perú, 2009; Available online: https://www.librosperuanos.com/libros/detalle/16842/Ajies-peruanos-Sazon-para-el-mundo (accessed on 1 June 2024).
- Hoyos Olga, L.; Franco, J.M.; Solarte, C.E.; Orozco, M.I. Extracción y cuantificación de capsaicina a partir del fruto del ají (Capsicum spp.). Sci. Tech. 2007, 33, 33–35. Available online: https://www.researchgate.net/publication/230724632_Extraccion_y_cuantificacion_de_capsaicaps_a_partir_del_fruto_del_aji_Capsicum_spp (accessed on 1 June 2024).
- Liu, Z.; Cai, S.; Zhang, S.; Xiao, Y.; Devahastin, S.; Guo, C.; Wang, Y.; Wang, T.; Yi, J. A systematic review on fermented chili pepper products: Sensorial quality, health benefits, fermentation microbiomes, and metabolic pathways. Trends Food Sci. Technol. 2023, 141, 104189. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin-the Major Bioactive Ingredient of Chili Peppers: Bio-efficacy and Delivery Systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef] [PubMed]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Smutzer, G.; Jacob, J.C.; Tran, J.T.; Shah, D.I.; Gambhir, S.; Devassy, R.K.; Tran, E.B.; Hoang, B.T.; McCune, J.F. Detection and modulation of capsaicin perception in the human oral cavity. Physiol. Behav. 2018, 194, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Jiajia, R.; McClements, D.J. Food grade micro emulsions, nano emulsions and emulsions: Fabrication from sucrose Monopalmitato and lemon oil. Food Hydrocoll. 2011, 25, 1413–1423. [Google Scholar] [CrossRef]
- Chan, E.S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of salute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia, N.; Perez, W.; Navarro, L.; Maldonado, H.; Borja, A.; De Lama, G. Study of the Release Kinetics of Capsaicin Extracted from Charapita Chili (Capsicum frutescens) from an O/W Emulsion Made with Sacha Inchi Oil (Plukenetia volubilis) and Encapsulated in Calcium Alginate Beads. Biol. Life Sci. Forum 2024, 37, 5. https://doi.org/10.3390/blsf2024037005
Tapia N, Perez W, Navarro L, Maldonado H, Borja A, De Lama G. Study of the Release Kinetics of Capsaicin Extracted from Charapita Chili (Capsicum frutescens) from an O/W Emulsion Made with Sacha Inchi Oil (Plukenetia volubilis) and Encapsulated in Calcium Alginate Beads. Biology and Life Sciences Forum. 2024; 37(1):5. https://doi.org/10.3390/blsf2024037005
Chicago/Turabian StyleTapia, Nelson, Winnie Perez, Liset Navarro, Holger Maldonado, Ale Borja, and Gerardo De Lama. 2024. "Study of the Release Kinetics of Capsaicin Extracted from Charapita Chili (Capsicum frutescens) from an O/W Emulsion Made with Sacha Inchi Oil (Plukenetia volubilis) and Encapsulated in Calcium Alginate Beads" Biology and Life Sciences Forum 37, no. 1: 5. https://doi.org/10.3390/blsf2024037005
APA StyleTapia, N., Perez, W., Navarro, L., Maldonado, H., Borja, A., & De Lama, G. (2024). Study of the Release Kinetics of Capsaicin Extracted from Charapita Chili (Capsicum frutescens) from an O/W Emulsion Made with Sacha Inchi Oil (Plukenetia volubilis) and Encapsulated in Calcium Alginate Beads. Biology and Life Sciences Forum, 37(1), 5. https://doi.org/10.3390/blsf2024037005





