Abstract
Chicha de güiñapo (ChG) is an ancestral beverage from the culture and gastronomy of Arequipa, Peru. This traditional drink is made from purple corn (Zea mays L.), cultivated across various Peruvian regions. Purple corn is renowned for its nutritional content and high bioactive compound value, such as antioxidants (20.5 ± 2.0 μmol TE/g), total phenolic compounds (2.5 ± 0.3 mg GAE/g), and anthocyanins (1.8 ± 0.2 mg/g). This research aimed to explore the technological development of an instant powder product derived from chicha de güiñapo (ChG) utilizing spray-drying technology. The purple corn (Zea mays L.) used in this study was from Peru; it was first processed by boiling the güiñapo at 100 °C 1 h, followed by cooling and fermenting under controlled conditions for 5–7 days until achieving the desired characteristics referenced from previous studies, such as pH, alcohol content (v/v), and degrees Brix. Upon attaining the desired fermentation characteristics, the ChG was centrifuged, filtered, and dehydrated by spray-drying technology with the following parameters: air inlet temperature (165 °C), airflow (0.89 mL/min), feed flow (1.67 mL/min), and outlet temperature (93 °C). These optimal parameters were determined using the response surface methodology after 15 runs. Then, a fine purple powder was produced with 6.61% moisture, pH 4.83, and 1.5 °Brix. The results of proximal analysis before and after spray-drying were for carbohydrates (1.77% to 82.67%), ash (0.02% to 4.91%), protein (0.10% to 5.81%), and alcohol (3.17% to 0.64%). This study highlights the biodiversity, sustainability, and food security of ancestral crops to contribute to cultural heritage valorization.
1. Introduction
Chicha de güiñapo (ChG) is an ancestral drink deeply rooted in the culture and gastronomy of Arequipa, Peru. Made from purple corn (Zea mays L.) grown in several Peruvian regions, this traditional drink is recognized for its high nutritional content and bioactive properties [1].
The main raw material to make ChG is purple corn (Zea mays L.), which is partially germinated and then ground to produce güiñapo, a key ingredient in the traditional preparation of ChG. Purple corn is grown in several regions of Peru, with approximately 5000–6000 hectares dedicated to its production. This crop, originally from the Andean region, grows in environments up to 3000 m.a.s.l. and is known for its high content of anthocyanins, such as cyanidin-3-glucoside, characteristic of its purple color [2]. The use of güiñapo in ChG, particularly in the Arequipa region, is attributed to tradition, making it unique compared to chicha from other regions of Peru [1]. Arequipa, located at an average altitude of 2335 m, is known for its unique blend of Andean and Spanish culinary traditions, with ChG being singled out for its use of partially germinated purple corn, a practice preserved since the pre-Incan period [1].
Spray-drying, a technique that turns liquid food products into powder, helps preserve nutritional and bioactive properties, ensuring product stability and quality [3,4].
This research aimed to convert ChG into powder using spray-drying technology.
2. Materials and Methods
2.1. Raw Materials and Preparation of Chicha de Güiñapo
The raw material used was purple corn (Zea mays L.), which is traditionally cultivated in various regions of Peru, particularly in the Andean highlands, and it was purchased at the San Camilo market located in the city of Arequipa. This corn is partially germinated and then milled to produce germinated güiñapo. The purple corn (Zea mays L.), originating from the rich agricultural traditions of several Peruvian regions, used in this study was processed by boiling the güiñapo at 100 °C for one hour, followed by cooling and fermentation under controlled conditions for 5–7 days until reaching the desired characteristics referenced in previous studies, such as pH, alcohol content (v/v), and degrees Brix [1]. The fermentation process is critical in developing the distinctive taste and bioactive properties of the ChG. After reaching the desired fermentation characteristics, the ChG is centrifuged and filtered to remove solid residues, preparing it for the subsequent spray-drying process. Fermentation occurs naturally, based on the native microorganisms present in the environment and in the raw materials. No external inoculum is added, which allows the process to conserve the traditional methods and flavors of the region of Arequipa [4].
2.2. Spray-Drying Process
The spray-drying process was employed to convert ChG into a fine powder, optimizing key parameters to maintain the beverage’s nutritional and functional qualities. The ChG was first prepared as described previously, then dried via spray-drying (Büchi B-290, Büchi Labortechnik AG, Flawil, Switzerland). The optimal conditions were determined through the response surface methodology with 15 runs in Minitab 19 software (Stat-Ease, Inc., Minneapolis, MN, USA) [3]. Three independent variables were used: X1—temperature (135–165 °C); X2—air flow (0.85–0.95 mL/min); and X3—feed flow (0.1–0.2 mL/min). Water activity was measured using a water-in-food activity meter, (WA-60A, Guangzhou Landtek Instruments Co., Guangzhou, China), and the result was expressed for each run. The yield was calculated with the weight of the solution before entering the spray-drying process divided by the weight of the resulting solution after the drying process and expressed as a percentage. Alcohol was measured in terms of variables of response (Table 1).

Table 1.
Response surface methodology with 15 runs.
2.3. Proximal Analysis
The proximal composition analysis was carried out according to official methods [4]. The moisture content was determined at 110 °C until constant weight using a halogen moisture analyzer (Sartorius MA-30, Sartorius AG, Göttingen, Germany). The total protein content was determined as % nitrogen × 6.25 using a Kjeldahl analyzer (UDK 139, VELP, Usmate Velate, Italy). The ash content was determined by incineration at 550 °C for 72 h in a muffle furnace. The fat content was determined by extraction with hexane for 4 h, as described in [4].
2.4. Total Phenolic Content
The total phenolic content (TPC) was determined by the Folin–Ciocalteau method [4] at 760 nm using a spectrophotometer (UV 1280 Vis Spectrophotometer Shimadzu, Kyoto, Japan). The results were expressed as µg of gallic acid equivalent (GAE)/g powder. Analyses were performed in triplicate and are presented as mean values.
2.5. Antioxidant Activity
The antioxidant activity was determined by the DPPH method [4] with some modifications at 517 nm by spectrometry (UV 1280 Vis Spectrophotometer Shimadzu, Kyoto, Japan). The results were expressed as µg Trolox/g sample. Analyzes were performed in triplicate and presented as mean values.
2.6. Analysis of pH, °Brix, and Alcohol Content
The pH of the samples was measured using a digital pH meter (Metrohm 827 pH Lab, Metrohm AG, Herisau, Switzerland). The soluble solid content (°Brix) was measured with a portable digital refractometer (Atago PAL-1, Atago Co., Tokyo, Japan). The alcohol content was initially measured using a portable digital refractometer (Atago PAL-34S, Atago Co., Tokyo, Japan) with a measurement range of 0 to 45 g/100 g, and the results were then converted to percentage volume/volume (v/v) based on the density of the solution. These measurements were conducted to monitor the fermentation parameters of ChG.
2.7. Statistical Analysis
Results were expressed as mean ± standard deviation. All measurements were determined in duplicate or triplicate. Analysis of variance (ANOVA) followed by a post hoc test was used to determine the statistical significance of differences between sample means. It was used to analyze the data acquired at a 95% significance level with Minitab 19.0 Software (Minitab Inc., State College, Palo Alto, CA, USA).
3. Results and Discussion
3.1. Spray-Drying
The optimum conditions for the ChG powder drying process were predicted using the response surface methodology with a Box–Behnken design (Table 1). The p-value of the coefficients related to the variables were as follows: Temperature (°C), Air flow (mL/min), and Feed flow mL/min. All terms resulted in a p-value less than or equal to α = 0.05, so there was an association between the response variable and the terms presented. Regression statistics, such as coefficient of determination R2 (94.01%), adjusted coefficient of determination R2adj (83.24%), and predicted coefficient of determination R2pred (78.42%), would indicate an acceptable degree of evaluation between the model and the response variable.
The optimization of the ChG drying process through the second-order quadratic model is presented in Figure 1. The optimal conditions of the Box–Behnken design were Temperature (165 °C), Air flow (0.89 mL/min), and Feed flow (1.67 mL/min) with 6.61% moisture, 0.52 water activity, 0.50% alcohol, and 0.30% yield.
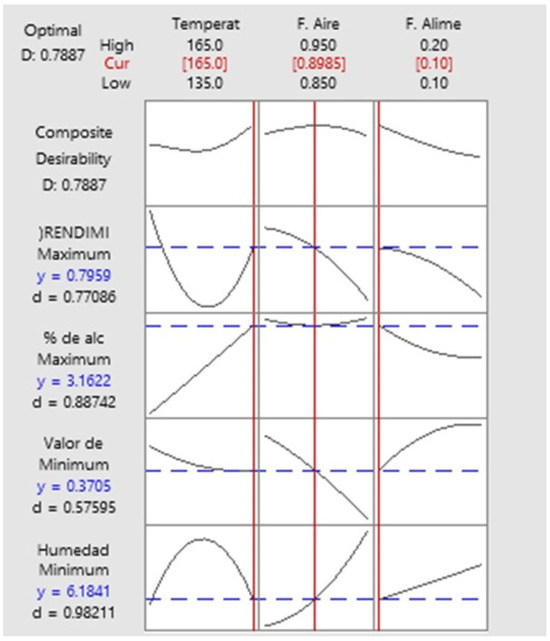
Figure 1.
Optimization of the Box–Behnken design obtained using Minitab 19 software.
The shapes of the contour curves can be seen in Figure 2. The shapes of the curves indicate the importance of the interactions between the variables studied (temperature, air flow, and feed flow) with the response variables.
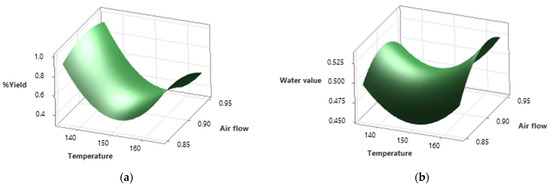
Figure 2.
Surface plot of the optimized ChG drying process. (a) Surface graph of the efficiency (%) of the optimized ChG drying process as a function of temperature (°C) and air flow (mL/min). (b) Surface graph of the water value of the optimized ChG drying process as a function of temperature (°C) and air flow (mL/min).
3.2. Characterization
Table 2 shows the results of pH, °Brix, and % of alcohol of ChG development. Under optimal conditions, the pH (5.08 ± 0.13) of the ChG development was higher than that of the commercial ChG (3.22 ± 0.11). The °Brix (1.97 ± 0.50) and % of alcohol (2.5 ± 1.00%) of ChG development were lower than the those of the commercial ChG (7.27 ± 0.50 °Brix and 13 ± 3.60% alcohol). This is because in the commercial ChG, sugars such as sucrose are added to increase the °Brix and % of alcohol [4].

Table 2.
Parameters physicochemicals obtained after the fermentation of chicha de güiñapo (ChG).
The proximal analysis of purple Corn, güiñapo flour, commercial ChG, and powdered ChG is shown in Table 3.

Table 3.
Proximal analysis of purple corn, güiñapo flour, commercial ChG, and powdered ChG.
The total phenolic and antioxidant content (DPPH) of güiñapo flour, commercial ChG, and development ChG are shown in Table 4. The spray-dried powdered ChG showed a higher antioxidant capacity (7743.454 ± 48.767 mg Trolox/100 g d.m) and a high phenolic content (0.700 ± 0.194 mg GAE/100 g d.m). The güiñapo flour presented lower antioxidant (9502.90 ± 14.66 mg Trolox/100 g d.m) and phenolic content (0.085 ± 0.070 mg GAE/100 g d.m).

Table 4.
Total phenolic content and antioxidant activity (DPPH) in güiñapo flour, commercial ChG, and development ChG.
The commercial ChG showed a higher antioxidant capacity (0.185 ± 0.001 mg Trolox/100 g d.m) and a high phenolic content (0.00 ± 0.00 mgGAE/100 g d.m) compared to the development ChG antioxidant capacity (0.198 ± 0.004 mg Trolox/100 g d.m) and phelonic content (0.00 ± 0.00 mg GAE/100 g d.m).
These results suggest that the spray-drying process, under optimized conditions, effectively preserves the bioactive compounds in the final powder product.
4. Conclusions
This research demonstrates the potential of using spray-drying technology to develop a stable, nutritionally rich, and culturally significant instant powder product from güiñapo ChG. The optimized spray-drying parameters ensured high retention of essential nutrients and bioactive compounds; the process not only preserved the traditional qualities of güiñapo ChG but also enhanced its potential for broader consumption, contributing to the valorization of cultural heritage and the promotion of ancestral crops.
Author Contributions
Conceptualization, J.M., O.M. and N.A.C.; methodology, J.M., O.M. and N.A.C.; software, N.A.C.; formal analysis, J.M. and O.M.; investigation, J.M., O.M. and N.A.C.; resources, N.A.C.; data curation, J.M. and O.M.; writing—original draft preparation, J.M. and O.M.; supervision, N.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the manuscript.
Acknowledgments
This work was supported and developed at Laboratorio de Alimentos Funcionales de la Carrera de Ingeniería Industrial, Universidad de Lima, Peru.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vargas, D.; Aguilar, B.; Pezo, N. Ancestral Peruvian ethnic fermented beverage “Chicha” based on purple corn (Zea mays L.): Unraveling the health-relevant functional benefits. J. Ethn. Foods 2020, 7, 35. [Google Scholar] [CrossRef]
- Medina, A.; Narro, L.; Chávez, A. Purple corn (Zea mays L.) crop in the Peruvian Highlands: Adaptation and identification of high-yield and high anthocyanin content cultivars. Agric. Res. 2017, 56, 291–299. [Google Scholar] [CrossRef]
- Gül, O.; Atalar, İ.; Törnük, F.; Akgün, A. Process optimization of a cereal-based fermented beverage (Boza) powder and investigating upscaling conditions. Food Bioprod. Process. 2018, 96, e14248. [Google Scholar] [CrossRef]
- Chasquibol, N.; Sotelo, A.; Alarcón, R. Development of Powdered Beverage with Cushuro (Nostoc commune) Concentrated Protein and Quinoa (Chenopodium quinoa). Biol. Life Sci. Forum 2023, 25, 2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).