Abstract
(1) Background: Hand hygiene with chemical disinfectants is an important measure to reduce the spread of infections, but frequent use can cause skin irritation. In recent years, it has become widely accepted that visible light can also have an antimicrobial effect, and visible light has even been applied to the disinfection of wounds. The present study aims to evaluate whether hand disinfection with visible light is a realistic alternative to chemical disinfectants. (2) Methods: Human hands were irradiated with a dose of 10 or 33 J/cm2 of visible violet light (405 nm) for 3 or 10 min, respectively. The reducing effect of the visible violet light was determined by comparing the contact agar plate results of irradiated and non-irradiated hands. Comparative experiments with a conventional hand disinfecting gel were also performed. Applicable standards were consulted to evaluate skin exposure to the irradiation. (3) Results: Irradiation of the hands with 10 and 33 J/cm2 resulted in an average reduction of microorganisms on the skin of 0.43 and 0.76 log-levels, respectively. These disinfection results with visible violet light are far behind those of the disinfectant gel, which achieved a reduction of 2.17 log-levels. Additionally, due to legal limits, a 3-min irradiation can only be performed five times per day and a 10-min procedure only once. (4) Conclusion: Since the irradiation doses applied up to now have not provided a substantial antimicrobial effect, and since an increase in the dose in a short time period is not arbitrarily possible without heating the hand unpleasantly, visible light of 405 nm seems rather unsuitable for repeated hand disinfection.
1. Introduction
Hand hygiene is one of the most important measures in hospitals taken to prevent nosocomial infections [1,2,3]. Doctors and nursing staff have to disinfect their hands up to 100 times a day for this purpose [4]. This is usually accomplished with alcohol-based liquid disinfectants, which can, however, lead to unpleasant skin irritations that reduce user compliance [1,2,3,5,6].
In a previous study, it was investigated as to whether Far-UVC irradiation (200–230 nm) could be an alternative to conventional hand disinfection [7], since Far-UVC has a strong antimicrobial effect and, due to favorable spectral properties, is already absorbed in the outermost skin layer and does not harm vital human cells. In principle, the approach works, especially when gloves are worn, but on the one hand, multiple daily application in Europe is limited by applicable standards, and on the other hand, no studies exist for the effects of longer-lasting frequent Far-UVC irradiation of the skin.
In contrast to UV radiation, visible light is considered to be much less harmful to humans. Nevertheless, visible blue and violet light in particular also exhibit antimicrobial properties and are capable of inactivating bacteria and fungi, among other things [8,9,10,11,12,13]. This effect is based on the fact that microorganisms contain endogenous photosensitizers such as flavins and porphyrins, which absorb blue and violet light and subsequently generate so-called reactive oxygen species. These attack membranes, proteins, and DNA in the cell, and if the resulting damage is great enough, the microorganism dies [14,15,16,17]. These properties of visible light have already been investigated for potential therapeutical applications like wound disinfection [18,19,20].
In the context of the present study, it should therefore be investigated as to whether hand disinfection is also possible with visible violet light of 405 nm wavelength. At this wavelength, strong LEDs exist, and the most important photosensitizers (porphyrins) feature strong absorptions, so this wavelength seems to be the most suitable for potential application in hand disinfection.
For the irradiation of skin with visible light with wavelengths above 400 nm, there are, in principle, no legal irradiation limits for daily use. However, 405 nm LEDs are not monochromatic and also exhibit weak emissions in the UV range (below 400 nm) for which irradiation limits exist [21]. The extent to which these limits restrict the application of violet light for skin disinfection is also to be determined.
2. Materials and Methods
2.1. Hand Disinfection with 405 nm Irradiation
The applied, home-built light source was already described in detail in [22]. An area of about 9.5 × 9.5 cm2 was irradiated from below and above by 128 LEDs (NVSU119CT type of Nichia, Tokushima, Japan) on printed circuit boards, as illustrated in Figure 1.

Figure 1.
Photograph of the 405 nm illumination device with 64 LEDs at the top and 64 LEDs at the bottom.
At a total LED current of 2 A, each LED board generated an irradiance of 55 mW/cm2, with the spectral distribution given in Figure 2. This was neither the maximum possible current nor the highest possible irradiance, but one that could be well sustained during the irradiation tests without the skin becoming too hot. Irradiation durations of 3 and 10 min were applied, corresponding to irradiation doses of 10 and 33 J/cm2. In laboratory tests on Staphylococcus aureus and Staphylococcus epidermis, these violet light doses were sufficient for bacterial reduction in the order of 1–2 log-levels [8].
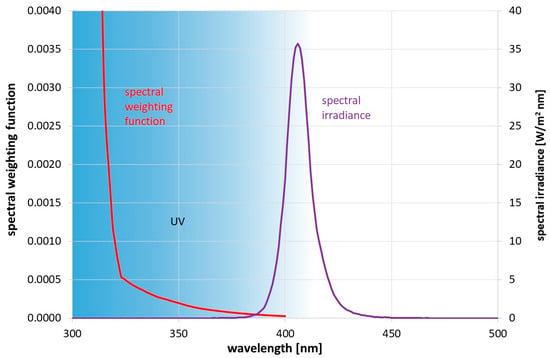
Figure 2.
Applied spectral irradiance Eλ(λ) on the skin and spectral weighting function S(λ) according to DIN EN 1500 [23] for calculating the effective irradiance Heff.
Since the 405 nm device and the 55 mm agar plates employed are not large enough to irradiate and sample the whole hand, only the microbial contamination of the 3 middle fingers was examined. For this purpose, three fingers—each of a non-disinfected and a disinfected hand—were pressed onto a Caso contact agar plate of VWR/Avantor (Darmstadt, Germany) and rolled slightly forward. Afterwards, both plates were incubated for about 24 h at 37 °C, and the number of visible colonies were counted and compared to each other. Both samples were always from the same person, and prior to the disinfection procedure and sampling, both hands were rubbed against each other to reach more or less the same contamination on both hands.
As mentioned above, there are limits for the daily skin exposure by UV radiation [21]. The effective irradiance Heff defined as
with the spectral irradiance Eλ(λ,t) and the spectral weighting function S(λ) [21], both illustrated in Figure 2, is limited to 30 J/m2 per day. This allowed us to calculate the possible number of disinfection procedures per day. A 1 min exposure, based on the data in Figure 2, leads to an effective irradiance of 1.74 J/m2.
2.2. Hand Disinfection with Commercial Disinfection Gel
For comparison, conventional hand disinfection with a commercial disinfection gel was also investigated. Both hands were rubbed against each other to even the microbial concentrations on both hands. Then, the three middle fingers of one hand were pressed onto the Caso contact agar plates. Afterwards, 3 mL of the disinfection gel OSA VITA HANDHYGIENE GEL of OSA Brands (Schorndorf, Germany), approved according to the standard DIN EN 1500, was applied as described in DIN EN 1500 [23]. Subsequently, the three middle fingers of the other hand were pressed on an agar plate, and after 24 h at 37 °C, the colony number were evaluated.
3. Results
For each disinfection approach (10 J/cm2 @405 nm; 33 J/cm2 @405 nm disinfection gel), at least eight runs were performed and analyzed. Figure 3 gives an example of the contact agar plates for a hand that was irradiated for 10 min with 405 nm and an unirradiated hand.
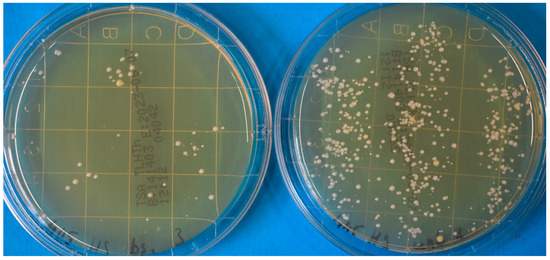
Figure 3.
Photograph of contact agar plates for a hand that was irradiated for 10 min with 405 nm (left) and an unirradiated hand (right).
However, the 405 nm irradiation results were not always this obvious but exhibited large variations. The mean log-reduction after 3 min of 405 nm irradiation was 0.429, which is a reduction of about 63%. A 10-min irradiation led to an average log-reduction of 0.757 or a drop of 82.5% in the detected number of microorganisms. In comparison, the hand disinfection gel inactivated 99.3% of the microorganisms, which corresponds to a reduction of 2.168 log-steps. The results of the different approaches, with their average and median reductions and the scattering of the results, are illustrated in the box plot in Figure 4.
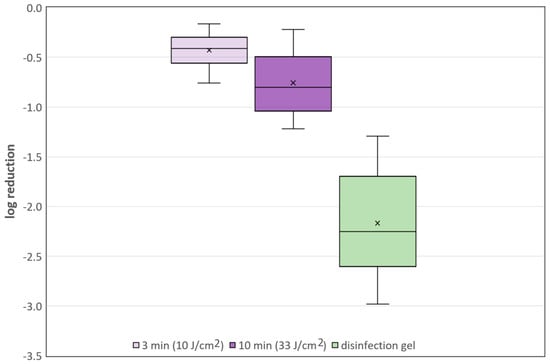
Figure 4.
Box plots with average and median log-reduction and scattering of the single results for all three disinfection approaches.
Concerning the daily irradiation limit according to the directive 2006/25/EC [21], this irradiation intensity may be applied for 1033 s or 17.2 min. Accordingly, only five 3-min procedures or one 10-min disinfection procedure would be allowed per day.
4. Discussion
Hand disinfection with the commercial gel provides good reduction results with more than 2 log-levels. In contrast, the antimicrobial effect of the visible violet light is rather sobering. The reduction effect is clearly visible, but at least with the two 405 nm irradiation doses applied, the effect of the commercial gel cannot be achieved.
Also, this dose cannot realistically be increased arbitrarily. On the one hand, the duration of the application speaks against it; already, the shorter of the two irradiation times was 3 min. On the other hand, if, for example, the irradiance is doubled to 110 mW/cm2, in order to halve the application time, a significant warming of the skin is to be expected, since 110 mW/cm2 corresponds to the maximum irradiation at midday in summer. There is also a legal limit to skin irradiation intensity to prevent skin burns by infrared and visible light. Slightly simplified, this limit is not exceeded as long as the irradiation intensity stays below 355 mW/cm2, which is about 6.5 times the current intensity.
Also, the dose cannot be increased arbitrarily via elongation of the irradiation, as this is also limited by the European directive due to the UV components of the LED emission; a maximum daily irradiation of 57 J/cm2 would be possible for these LEDs. It has not been experimentally verified here, but it can be assumed that even this dose would not have achieved the reduction effect of the disinfection gel since it does not even correspond to a doubling of the examined maximum dose of 33 J/cm2.
It would still be conceivable to apply 450 nm LEDs which do not exhibit UV emissions. Based on the known log-reduction doses for 405 and 450 nm [8,9] it is to be expected that even higher 450 nm irradiation doses, and thus longer irradiation durations, would be necessary. However, the application of very high doses of 450 nm or an even higher wavelength may be complicated by another effect. Human cells also contain endogenous photosensitizers; it is therefore not surprising that high irradiation doses of visible light can also have a skin-damaging effect [24,25].
Funding
The development of the 405 nm irradiation device was funded by the Else-Kröner-Fresenius-Stiftung (2020_EKKP.140).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Technische Hochschule Ulm (No. 2023-01 of 4th of July 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI). Händehygiene in Einrichtungen des Gesundheitswesens: Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2016, 59, 1189–1220. [Google Scholar] [CrossRef]
- Stadler, R.N.; Tschudin-Sutter, S. What is new with hand hygiene? Curr. Opin. Infect. Dis. 2020, 33, 327–332. [Google Scholar] [CrossRef]
- Lotfinejad, N.; Peters, A.; Tartari, E.; Fankhauser-Rodriguez, C.; Pires, D.; Pittet, D. Hand hygiene in health care: 20 years of ongoing advances and perspectives. Lancet Infect. Dis. 2021, 21, e209–e221. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Clean Hands Count for Safe Healthcare. Available online: https://www.cdc.gov/patientsafety/features/clean-hands-count.html (accessed on 15 August 2023).
- Kramer, A.; Hübner, N.-O.; Assadian, O. Anforderungen an die chirurgische Handedesinfektion und veraendertes Prozedere: Requirements on surgical hand disinfection and modified procedures. GMS Krankenhaushyg Interdiszip 2007, 2, Doc55. [Google Scholar]
- Labadie, J.C.; Kampf, G.; Lejeune, B.; Exner, M.; Cottron, O.; Girard, R.; Orlick, M.; Goetz, M.L.; Darbord, J.-C.; Kramer, A. Recommendations for surgical hand disinfection—Requirements, implementation and need for research. A proposal by representatives of the SFHH, DGHM and DGKH for a European discussion. J. Hosp. Infect. 2002, 51, 312–315. [Google Scholar] [CrossRef]
- Hessling, M.; Sicks, B.; Lau, B. Far-UVC Radiation for Disinfecting Hands or Gloves? Pathogens 2023, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths—A review on existing data. FEMS Microbiol. Lett. 2016, 364, fnw270. [Google Scholar] [CrossRef] [PubMed]
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; Maclean, M. Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380–480 nm Violet-Blue Light. Photochem. Photobiol. 2018, 94, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Guffey, J.S.; Wilborn, J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed. Laser Surg. 2006, 24, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 285, 227–232. [Google Scholar] [CrossRef]
- Dai, T.; Hamblin, M.R. Visible Blue Light is Capable of Inactivating Candida albicans and Other Fungal Species. Photomed. Laser Surg. 2017, 35, 345–346. [Google Scholar] [CrossRef]
- Feuerstein, O.; Ginsburg, I.; Dayan, E.; Veler, D.; Weiss, E.I. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem. Photobiol. 2005, 81, 1186–1189. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef]
- Plavskii, V.Y.; Mikulich, A.V.; Tretyakova, A.I.; Leusenka, I.A.; Plavskaya, L.G.; Kazyuchits, O.A.; Dobysh, I.I.; Krasnenkova, T.P. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J. Photochem. Photobiol. B 2018, 183, 172–183. [Google Scholar] [CrossRef]
- Cieplik, F.; Spath, A.; Leibl, C.; Gollmer, A.; Regensburger, J.; Tabenski, L.; Hiller, K.-A.; Maisch, T.; Schmalz, G. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin. Oral Investig. 2014, 18, 1763–1769. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.-Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: Efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Plattfaut, I.; Demir, E.; Fuchs, P.C.; Schiefer, J.L.; Stürmer, E.K.; Brüning, A.K.E.; Opländer, C. Characterization of Blue Light Treatment for Infected Wounds: Antibacterial Efficacy of 420, 455, and 480 nm Light-Emitting Diode Arrays Against Common Skin Pathogens Versus Blue Light-Induced Skin Cell Toxicity. Photobiomodul. Photomed. Laser Surg. 2021, 39, 339–348. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Directive 2006/25/EC of the European Parliament and of the Council on the minimum health and safety requirements regarding the exposure of workers to risks arising from physical agents (artificial optical radiation). Off. J. Eur. Union 2006, L114, 38–59. [Google Scholar]
- Lau, B.; Becher, D.; Hessling, M. High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces. Photonics 2021, 8, 414. [Google Scholar] [CrossRef]
- DIN EN 1500; Chemische Desinfektionsmittel und Antiseptika_-Hygienische Händedesinfektion_-Prüfverfahren und Anforderungen (Phase 2/Stufe_2); Deutsche Fassung EN_1500:2013. Beuth Verlag GmbH: Berlin, Germany, 2017.
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Effects of visible light on mechanisms of skin photoaging. Photodermatol. Photoimmunol. Photomed. 2022, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.; Forbes, P.D. Examining the role of visible light in photocarcinogenesis—Lessons from the past. J. Photochem. Photobiol. 2023, 17, 100201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).