Abstract
An ever-increasing amount of research is being performed on the stability and recovery of soil methane-oxidizing bacteria since this is one of the fundamental processes controlling the amount of methane in the atmosphere. Mineral fertilizers may alter the methane oxidation processes in agricultural soils when they are introduced. Although ammonium (NH4+) is believed to have a significant impact on aerobic methane oxidation activity in soils, there is still little data on how it reacts with lanthanum (La). The recent identification of a novel class of lanthanum-containing enzymes in methanotrophic bacteria may be the foundation for controlling the function of the soil “methane filter” and related microbiota. In the current study, microcosms with agricultural sod-podzolic soils were created and incubated in air or 20% CH4 in the gas phase with the addition of NH4+ (100 µg/g) and La (5 µg/g) to the soil. Using GC analysis and high-performance 16S rRNA sequencing, the methane oxidation potential and composition of soil bacterial communities were studied over the month of incubation. A negative impact of NH4+ on the oxidation of methane was observed, whereas La had a somewhat beneficial effect. Ammonium had an impact on the composition of methanotrophs, and a significant shift was observed upon La addition. Proteobacteria made up a larger share of the soil microbial community, and Gammaproteobacteria dominated the methanotrophic populations. Methylobacter, a methanotroph, and Methylotenera, an obligatory methylotroph, were the two absolute dominants in the La-amended variants. These findings could help evaluate how lanthanum regulates methanotrophic communities in agricultural soils and lead to the creation of new strategies for controlling the “methane filter” in soil.
1. Introduction
Although methane only makes up less than 0.02% of the atmosphere, it is a significant greenhouse gas that is thought to be responsible for 15% of present global warming [1]. Only microbial communities in aerobic soils are capable of naturally removing methane from the atmosphere; thus, any changes to the rate at which this process takes place could have a huge global impact.
Various substances supplied by atmospheric precipitation, fertilizers, and pollutants from enterprises and vehicles are constantly influencing agricultural soils [2]. There is very little data on how the application of fertilizers affects methanotrophic communities, and nitrogen compounds are typically taken into account. It is unclear how other elements, such as lanthanides, affect the microbial communities in soil. The lanthanides that humans introduce to the soil through mineral and organic fertilizers and microfertilizers are also in a bioavailable state [3].
Recently, it was shown that rare earth elements have a significant role in the metabolism of methylotrophs [4]. Lanthanides regulate the genes responsible for alternate methanol dehydrogenase production and also alter the variety of methanotrophs and satellite microorganisms [5]. However, the authors are not aware of any studies on the impact of La on soil methanotrophs.
In this work, we investigated how varied fertilization conditions selected for particular methanotrophic populations and affected methane oxidation activity using the soil microcosms approach. We measured the abundance of methanotrophic and related bacteria in response to ammonium and lanthanum treatment using 16S rRNA gene amplicon analysis.
2. Materials and Methods
2.1. Field Site Description, Soil Collection, and Characteristics
We used sod-podzolic medium loamy soil from a fallow grass meadow in the Yaroslavl region, Russia, for our microcosm studies. For more than 20 years, this soil has been subjected to crop rotation while receiving heavy dosages of organic fertilizer. A mixed sample was collected in June 2019 from the surface 0–20 cm horizon. Before the studies, the soil was held at a temperature of 4 °C.
The soil has a pH of 5.4, a significant amount of organic matter (19.3–21.7 g/kg according to the Tyurin method), a lot of nitrate (261 mg/kg), and a small amount of ammonium nitrogen (5 mg/kg).
2.2. Experimental Design
In November 2019, a microcosm experiment commenced as described in our previous study [6]. The experimental options were La (lanthanum chloride solution; 5 µg La/g) and NH4+ (ammonium sulfate solution; 100 µg N/g). A salt-free option served as a control.
Air was used to incubate half of the samples in desiccators. Vials were regularly taken, and the potential for methane oxidation was calculated as described previously [7]. Other samples were used to determine the variety of soil bacterial communities and were incubated with a methane–air mixture (20:90) to promote the growth of methanotrophs.
To measure the diversity of the bacterial community, DNA was directly extracted from the soil at the beginning and end of the experiment, and 16S rRNA genes were analyzed via next-generation sequencing (LLC BIOSPARK, Troitsk, Moscow, Russia).
The next-generation microbiome bioinformatics platform QIIME 2 was used to merge nucleotide sequences in OTE (97% similarity level) and assess the diversity indices. The Pielou index measured OTE alignment in the community whereas the Shannon index measured alpha diversity. As a measure of phylogenetic diversity, the Faith PD index was used.
Three samples’ value of data is displayed as averages. The observed treatment effects in each study were considered statistically significant at p ≤ 0.05. Excel (Microsoft Office Excel 2011) was the tool used to perform the statistical analysis.
3. Results and Discussion
3.1. Effect of Ammonium and Lanthanum on Methane-Oxidizing Activity
Ammonium added to the soil reduced the methane-oxidizing activity whereas lanthanum increased it (Figure 1). The differences were not significant (p > 0.05) after 10 days of the experiment.
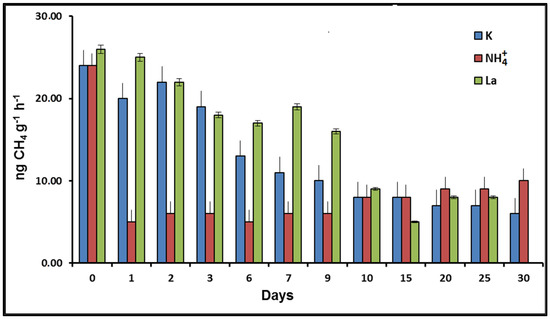
Figure 1.
Dynamics of potential methane oxidation rates of soil samples as affected by ammonium and lanthanum addition.
3.2. Effects of Ammonium and Lanthanum on Soil Microbial Community Composition
Figure 2 illustrates the relative abundance of the bacterial phyla before (Soil) and after (K, NH4+, and La) incubation.
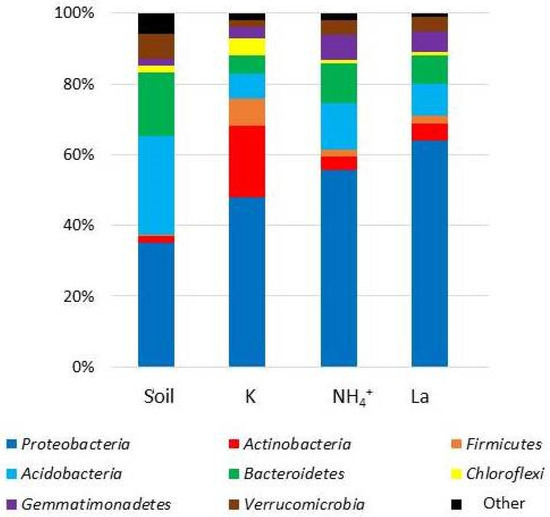
Figure 2.
Relative abundance of the prokaryotic communities in soil samples at the phylum level.
The predominant bacterial phyla were discovered to be the same in both the initial soil and the incubation experiment samples; however, their representation varied considerably. With the addition of salts, the representation of certain phyla increased. For instance, in microcosm soil samples without salt treatment, the percentage of Proteobacteria was 48%; in samples with ammonium, it was 55%; and in samples with lanthanum, it was 66%. Furthermore, the salt-amended samples exhibited an increase in the representation of Acidobacteria and Bacteroidetes in comparison to the control.
It is fascinating to see that adding salts significantly reduces the number of Gram-positive bacteria. As a result, the proportion of Actinobacteria dropped from 20% in the control sample to 5% and 4% in the La and NH4+ samples. In a similar vein, the percentage of Firmicutes dropped from 8% to 2% and 4%, respectively.
3.3. Effect of Ammonium and Lanthanum on the Diversity of Soil Microbial Communities
Table 1 displays the attributes of alpha diversity, such as the number of phyla and OTE and diversity indices, including Pielou alignment, Shannon diversity, and phylogenetic diversity (Faith PD index).

Table 1.
Features of the diverse microbial communities of the soils used in the lab microcosm experiment and native sod-podzolic soil.
It was found that the addition of lanthanum and ammonium caused distinct reactions in the bacterial communities. The microbial communities’ diversity somewhat declined when they were cultured with ammonium. In terms of the Shannon index, the number of bacterial phyla and genera was similar to that of the control variation, and the alignment Pielou indicator’s value even increased. The Faith PD index value decreased during the same period, indicating that more taxa started to cluster together quite closely on the phylogenetic tree.
Only 203 genera from the 16 phyla were identified, demonstrating a dramatic reduction in the variety of soil microbes caused by the addition of lanthanum. The fact that the Shannon diversity indices and PD were significantly (p < 0.05) lower than the corresponding values in the control soil provides evidence that lanthanum salt had a deleterious effect on diversity. Furthermore, the community’s taxon alignment value decreased (Table 1).
The findings are consistent with other research that demonstrated that applying mineral fertilizers [8] resulted in a reduction in the diversity of the bacterial community. Because some groups of microorganisms have not adjusted to the new conditions, the established combination of ecological niches appears to be disrupted at the same time that their relative abundance rises.
3.4. Ammonium and Lanthanum’s Effects on the Diversity of Soil Methanotrophs
Methane incubation altered the composition of the methanotrophic community and increased the proportion of methanotrophs (Table 2). The Methylosinus concentration rose to 0.38% after 30 days of methane incubation. There were also Methylobacter and Methylocella in the community. Therefore, by an order of magnitude, there were more methanotrophs, which made up around 1% of all of the sequences.

Table 2.
The methanotrophic community structure in the lab microcosm experiment’s sod-podzolic soils.
The addition of lanthanum significantly changed the number and variety of methanotrophic communities. Following a month of incubation, Table 2 displays a figure of 4% for Methylobacter and 0.1% for Methylosinus. The Methylosinus OTUs had 99.24% similarity with M. sporium, while all 16S rRNA sequences of Methylobacter OTE displayed high similarity with those of M. tundripaludum (98.12–99.06%).
The high percentage of obligate methylotroph Methylotenera 16S rRNA gene sequences exceeded 10% (six OTUs). These sequences demonstrated 98.12–98.46% similarity with M. versatilis, which is noteworthy. Previously, methylotrophs of the genus Methylotenera joined forces with methanotrophic bacteria in shallow methane seeps [9]. Additionally, two OTUs of methylotrophic Hyphomicrobium facile (0.4–0.2%) were found.
Therefore, it has been established that the coordinated dominance of the methanotroph Methylobacter and the obligatory methylotroph Methylotenera may occur from the addition of lanthanum to soils with an elevated level of methane, which is characteristic of agricultural soils with a high content of organic matter. To fully understand their cooperative role in controlling the exchange of methane between the soil and the atmosphere, more research is required.
4. Conclusions
Our findings indicate that the soil microbial population is significantly impacted by the introduction of ammonium and lanthanum compounds. These components regularly enter the soils of agroecosystems via mineral and organic nitrogen fertilizers, phosphorites, and lanthanum-containing microfertilizers.
According to the results of our experiments, the entry of ammonium and lanthanum compounds into the soil with nitrogen (mineral and organic), phosphorous (phosphorites), and lanthanum-containing fertilizers has a significant impact on the soil microbial community.
More investigations are needed into the potential mechanisms underlying lanthanum’s influence on soil methane oxidation. The study’s results may help to develop modern methods for figuring out the appropriate amounts of ammonium fertilizers and micronutrients, including lanthanum, for use in central Russian agriculture. We make the assumption that information on methanotrophic community activity and composition may be utilized to evaluate the effects of anthropogenic activities on soils.
Funding
This research was funded by the Russian Science Foundation, grant number 22–24–00418.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed during the current study are available upon request.
Acknowledgments
The author is greatly thankful to Lomonosov MSU students Lev Sizov and Liana Gogmachadze for their laboratory assistance.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Saunois, M.; Bousquet, P.; Poulter, B.; Peregon, A.; Ciais, P.; Canadell, J.G.; Dlugokencky, E.J.; Etiope, G.; Bastviken, D.; Houweling, S.; et al. The global methane budget 2000–2012. Earth Syst. Sci. Data 2016, 8, 697–751. [Google Scholar]
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; et al. Global change pressures on soils from land use and management. Glob. Change Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R. Rare earth elements in the soil environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Chistoserdova, L. Lanthanides: New life metals? World J. Microbiol. Biotechnol. 2016, 32, 138. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.M.B.; Johnson, T.; Karunaratne, Y.S.; Fu, Y.; Beck, D.A.C.; Chistoserdova, L.; Lidstrom, M.E. Lanthanide-dependent cross-feeding of methane-derived carbon is linked by microbial community interactions. Proc. Natl. Acad. Sci. USA 2017, 114, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Sizov, L.R.; Khusnetdinova, K.A.; Kravchenko, I.K. Effect of lanthanum on nitrifying activity and composition of microbial communities of sod-podzolic agrosoil. J. Agricult. Environ. 2022, 5, 1–6. [Google Scholar]
- Kravchenko, I.K.; Semenov, V.M.; Kuznetsova, T.V.; Bykova, S.A.; Dulov, L.E.; Pardin, G.; Gispert, M.; Boeckx, P.; Van Cleemput, O.; Galchenko, V.F. Physicochemical and biological factors affecting atmospheric methane oxidation in gray forest soils. Microbiology 2005, 74, 216–220. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.-Z.; Gao, J.; Peng, F.; Gao, P. Long-term combined application of manure and chemical fertilizer sustained higher nutrient status and rhizospheric bacterial diversity in reddish paddy soil of Central South China. J. Sci. Rep. 2018, 8, 16554. [Google Scholar] [CrossRef] [PubMed]
- Danilova, O.V.; Ivanova, A.A.; Terent’eva, I.E.; Glagolev, M.V.; Sabrekov, A.F. Microbial community composition of floodplains shallow-water seeps in the Bolshaya Rechka floodplain, Western Siberia. Microbiology 2021, 90, 632–642. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).