Abstract
Using degenerated primers (LC1–LC2c) and two novel primer pairs, namely (KSLB–LC6) for Aspergillus niger and (AFl1F–LC2) for Aspergillus tubingensis, created for the acyl transferase (AT) and the KS sequences of fungal PKSs genes, a 700 pb PCR-derived DNA fragment was isolated from Aspergillus carbonarius, Aspergillus niger, and Aspergillus tubingensis. Testing was performed on DNA from most of the black Aspergillus species currently known to exist. This article describes the identification and characterisation of a portion of a novel putative OTA-polyketide synthase gene in A. tubingensis “AT Pks,” A. niger “AN Pks,” and A. carbonarius “AC Pks”. Phylogenetic methods were used to align and evaluate the sequences. The study’s primers demonstrated broad application, and several Aspergillus species from the section Nigri, particularly A. niger and A. tubingensis, were amplified satisfactorily. Predicted amino acid sequences known as “AC Pks” showed 66–81% similarity to several polyketide synthase genes, whereas “AN Pks” and “AT Pks” showed 68–71% and 81–97% similarity, respectively. The AT and KS sequences were linked to PKSs engaged in various mycotoxin production routes, including ochratoxin A, and they seemed to be specific for a specific kind of fungal PKSs. The sequences that have been reported in this paper are particularly useful in finding new fungal PKS gene clusters.
1. Introduction
Numerous mycotoxins and other secondary metabolites are produced by fungal specific polyketide synthases, or PKSs. Ochratoxin A (OTA) is a fungal secondary metabolite [1,2]. For humans, it is a strong teratogen and carcinogen [3,4]. To create OTA, a polyketide synthase (PKS) successively condenses several acetate units. This mycotoxin originates from the partially understood fungal polyketide biosynthesis pathway [5]. The contribution of the species included in the Aspergillus section Nigri group, mainly Aspergillus carbonarius and the members of the A. niger aggregate (A. niger and A. tubingensis) in the contamination of grapes by ochratoxin A (OTA) has been recently reported worldwide [6,7]. Genes involved in the production of secondary metabolites in fungi often cluster together, and these groups can include one or more regulatory genes [8,9].
The most clusters associated with the polyketide biosynthesis contain a single PKS gene and several genes encoding enzymes [10,11].
Two fatty acid synthases are involved in the production of ochratoxin; they generate a completely reduced hexanoyl chain, which serves as a primer for the PKS to finish the backbone [12]. Despite a striking range of final products, each polyketide biosynthesis route appears to adhere to a similar fundamental chemical design. The key chain-building step of this reaction scheme is a decarboxylative condensation analogous to the chain elongation step of classical fatty acid biosynthesis [13,14]. A class of multifunctional enzyme systems known as polyketide synthases (PKSs) catalyses the consecutive condensation step of tiny carbon precursor acid in the biosynthesis of the majority of polyketide metabolites [15]. Ketosynthetase (KS), acyltransferase (AT), and acyl carrier protein (ACP) are the principal domains that make up a normal fungal PKS. Optional domains include dehydratase (DH), enoyl reductase (ER), ketoreductase (KR), and thioesterase (TE) [16]. Using degenerate primers (LC1–LC2c) and two designed primer pairs (KSLB–LC6) and (AFL1F–LC2c) to target, respectively, PKS genes in A. niger and A. tubingenis as OTA-producing fungi in grapes, we report the characterisation of PKS genes (AC Pks) required for the biosynthesis of OTA in Aspergillus species.
2. Materiel and Methods
2.1. Fungal Strains and Culture Condition
For seven days leading up to sporulation, the isolated fungal cultures of A. carbonarius, A. niger, and A. tubingensis were cultured on MEA (Malt Extract Agar–DIFCO) supplemented with 100 µg/mL chloramphenicol (Sigma). Before extracting DNA, cultures were cultured at 25 °C. Unless otherwise noted in the text, chemicals were acquired from Difco Laboratories (Detroit, MI, USA).
2.2. Fungal DNA Extraction
The right moment was chosen before the sporulation stage, basically during seven days of fungal culture to harvest the mycelia, weigh them, and freeze them right away at −80 °C. In accordance with the manufacturer’s instructions, DNA was isolated using the Fungal DNA miniprep kit (QIAGEN-France). Using a NanoDrop® ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA), DNA concentrations were ascertained.
2.3. Amplification of Fungal DNA
All samples’ genomic isolated DNA was subjected to PCR tests utilising degenerate primers (LC1–LC2c) [17]. The PCR was run in the following manner: 36 consecutive cycles were run following the initial denaturising cycle, which lasted 8 min at 95 °C. Every cycle had three steps: 95 °C for one minute, 55 °C for one minute, and 72 °C for three minutes. A final extension step of 72 °C for ten minutes came last.
Using sequence alignments of the PKS gene from over fifty Aspergillus and Penicillium strains with varying origins, specific primer sets were created and obtained from databases. DNA STAR Lasergene MegAlign tool was used to modify and align a large number of sequences using the Clustal W method (Lasergene, Madison, WI, USA).
Both new pair primers, (KSLB/Lc6) and (Afl1F/LC2c), were designed in the laboratory (Table 1).

Table 1.
PCR primers used to identify PKSs genes in Aspergillus strains producing OTA.
2.4. DNA Cloning and Sequencing
Following the manufacturer’s instructions, the purified PCR products were ligated into the pGEMT vector (Promega). Using the primers PU–PR as insert-specific primers in the vector pGEMT, this vector was used to transform into competent Escherichia coli (JM 109) cells. On Luria–Bertani (LB) plates supplemented with ampicillin, isopropythio-β-D-galactoside (IPTG), and 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal), transformed colonies of E. Coli JM 109 were chosen [18]. Five millilitres of LB broth was inoculated using individual clones, which were then cultured at 37 °C for the whole night. To identify the recombinant colonies, plasmid DNA was extracted from E. coli using a small-scale preparation method called miniprep.
The ABI Prism “CEQ™ 8000 Genetic Analysis System” was used for PCR sequence analysis. Aspergillus PKS sequences obtained were compared against other sequences available online with the Basic Local Alignment Search Tool algorithm (Blast, National Center for Biotechnology Information, National Institutes of Health).
3. Results
We looked at Pks sequencing data from the recognised fungal strains that produce OTA, including A. carbonarius, A. niger, and, most recently, A. tubingensis, which has been identified as an OTA-producing species. A. carbonarius is the principal producer of OTA in grapes and its derivatives.
3.1. Amplification and Identification of Pks Gene Sequence in A. carbonarius
Targeting KS domains from Pks genes in the powerful OTA-producing strains of A. carbonarius, A. niger, and A. tubingenis discovered from Tunisian grapes was the goal of one pair of degenerate primers, LC1/LC2c. The outcome was a 700 bp fragment. After further sequencing and comparison studies of this sequence, specifically AC Pks, it was discovered to be identical to the KS domain sequences previously discovered in A. carbonarius by Atoui et al. [19]. Specifically, AC Pks (Figure 1) exhibited the highest similarity (81%) to the coding sequence of a predicted PKS protein in A. oryzae, resulting from the sequencing and analysis of the AP007162 strain. Moreover, a similarity, around 76%, to KS domains, was obtained with A. ochraceus (AAS98198) and A. terreus (CAB44699). While a similarity of 66% was obtained with A. flavus (AAS90022) (see Table 2).

Figure 1.
Alignment of the deduced amino acid sequences “AC Pks” of A. carbonarius (Tun) isolated from Tunisian grapes with the corresponding PKSs regions of A. oryzae (Accession No. AP007162), A. ochraceus (Accession No. AY272043), A. terreus (Accession No. CAB44699), and A. flavus (Accession No. AAS90022).

Table 2.
Similarities between the sequences encoding proteins putatively involved in the synthesis of ochratoxin A precursors isolated from A. carbonarius species and PKSs reported in several toxigenic fungi.
The deduced PKS sequence “AC PKS” was aligned with PKSs submitted online (Figure 1).
The isolated Pks sequence “AC Pks” obtained is described as following (Figure 2).

Figure 2.
Pks gene sequence from A. carbonarius.
3.2. Amplification and Identification of PKS Gene Sequences in A. niger and A. tubingensis
In order to examine the existence of the AN PKS gene in A. niger and the AT PKS gene in A. tubingensis, genomic DNA was extracted and subsequently amplified using the identical specific pair primer (LC1/LC2c) and amplification conditions employed for A. carbonarius.
The two species (A. niger and A. tubingensis) showed a positive response with these degenerate pair primers and full similarity was obtained with the amino acid sequence “AC PKS”.
In order to isolate new PKSs genes from A. niger and A. tubingensis, a new molecular approach was optimised using new primers designed in the laboratory (Table 1).
3.2.1. Cloning of AN Pks Gene in A. niger
Using the pair primer (KSLB–LC6), a 700 bp fragment was amplified following the PCR process. The PCR results were sequenced after being cloned into the pGEMT plasmid. The sequenced fragments were aligned in an effort to find consensus.
From the sequencing analysis, the amplicons exhibited a similarity of about 71% to the corresponding PKS of P. chrysogenum (CAP95405) and A. ochraceus (AAS98204). A. niger PKS gene sequence revealed a similarity of 68% with A. carbonarius (ACH47947) and A. oryzae (BAE56814) (see Table 3).

Table 3.
Comparison of the deduced amino acid sequence for Aspergillus niger using (KSLB-LC6) and fungal PKSs sequences submitted in Genbank.
The new deduced protein sequence was aligned using genetic tools (Figure 3).
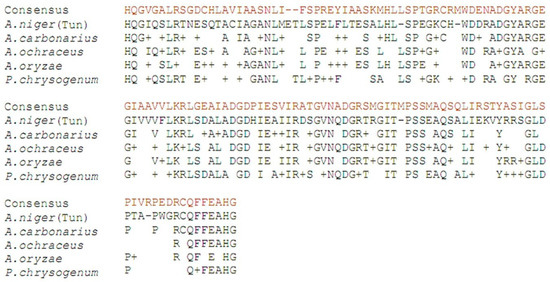
Figure 3.
Alignment of the deduced amino acid sequences “AN PKS” of A. niger (Tun) isolated from Tunisian grapes with the corresponding PKS regions of A. carbonarius (Accession No. ACH47947), A. ochraceus (Accession No. AAS98204), A. oryzae (Accession No. BAE56814), and P. chrysogenum (Accession No. CAP95405).
Here is a description of the A. niger PKS sequence that was determined (Figure 4).

Figure 4.
PKS gene sequence from Aspergillus niger.
The following table (Table 4) provides a comparison of the fungal PKSs sequences that have been submitted to Genbank with the Aspergillus niger deduced amino acid sequence using (Afl1F- LC2R).

Table 4.
Comparison of the deduced amino acid sequence for Aspergillus niger using (Afl1F-LC2R) and fungal PKSs sequences submitted in Genbank.
3.2.2. Cloning of ATPks Gene in A. tubingensis
PCR primers (AFL1F-LC2c) designed in the laboratory were used to target Pks genes in A. tubingensis species. Products obtained from the PCR reactions were sequenced and shown to be fragments from a undiscovered PKS gene. The sequence fragments displayed similarities with P. thomii (97%), A. niger (94%), A. ochraceus (88%), A. terreus (87%), A. flavus (82%), and 81% with A. ochraceoroseus (Table 5).

Table 5.
Similarities of the sequences encoding Pks putative proteins isolated from Aspergillus tubingensis and fungal PKSs from toxigenic strains.
The alignment of the predicted AT PKS was achieved (Figure 5).

Figure 5.
Alignment of the deduced amino acid sequences “AT PKS” of A. tubingensis (Tun) isolated from Tunisian grapes with the corresponding PKS regions of P. thomii (Accession No. AAS60178), A. niger (Accession No. CAK38306), A. ochraceus (Accession No. AAS98198), A. terreus (Accession No. BAB88689), A. flavus (Accession No. AAS89999), and A. ochraceoroseus (Accession No. ACH72912).
The derived Pks sequence from A. tubingensis is characterised as follows (Figure 6).

Figure 6.
The deduced Pks gene sequence from A. tubingensis.
4. Discussion
PKS proteins are widely distributed throughout various fungi and are principally engaged in the production of a diverse range of secondary metabolites. Since certain filamentous fungi genera are able to create a large variety of polyketide metabolites with a high degree of chemical diversity and require more than one type of polyketide backbone, numerous PKS genes can be found in various fungal genomes.
PKSs are multifunctional enzymes encoded by a single gene and typically possess up to eight types of functional domains [20,21].
Degenerate PCR primers (LC1–LC2c) were created in the current study to match particular homology regions. The primers were then utilised in PCR reactions with fungal genomic DNA from many species that are known to produce polyketides. These primer pairs showed full homology to A. carbonarius when further tested for A. niger and A. tubingensis. This approach has previously been successfully utilised to characterise five putative PKSs in A. carbonarius, which are KS domains [19]. Nevertheless, it is still unclear whether any of these PKS expressing DNA sequences are directly involved in OTA production in A. carbonarius. Furthermore, we report that primers designed based on the AC Pks sequence were utilised in a preliminary screening to monitor the presence of the gene in the genomes of Aspergillus isolates (A. niger and A. tubingensis). Degenerate PCR primers (LC1 and LC2c) were also used for the amplification of the ketosynthase domain fragments from fungal PKS genes to characterise endophyte fungi [22].
Amino acid sequences revealed by utilising these primers for Aspergillus section Nigri revealed similarities between the PKS genes for fungal toxigenic strains that ranged from 68% to 77%. The present investigation identified a novel PKS gene (AC PKS) using degenerate primers that specifically target the KS domain. The sequence of this gene appears to correlate with both PKSs in an OTA-producing strain of A. niger and A. tubingensis. Here, the new molecular approach used in this study offers new possibilities to study PKSs genes in both species A. niger and A. tubingensis by using (KSLB-LC6) and (Afl1F-LC2c), respectively. Sequencing the products of these reactions revealed that they were part of Pks gene clusters that had not yet been identified. The pieces could be employed in blotting assays with fungal genomic DNA that is homologous or heterologous. These PKS genes exhibited a great deal of overlap with other PKS genes that encoded the enzymes necessary for the production of secondary metabolites made of polyketides. Here, three potential PKSs genes were our main focus [2,23].
Previous research indicates that the functional domains’ highly conserved nature made it easier to clone and characterise the genes encoding fungal PKSs at the molecular level. This made it possible to extract the gene segments encoding the PKS sequences by designing gene probes and using degenerate primers [3,5,6,7,11,12,14,15,16,21,24,25,26,27,28,29,30,31,32].
Furthermore, additional molecular research has concentrated on identifying the PKS genes in A. carbonarius that are involved in OTA production. In this regard, Lebrihi and his colleagues described the cloning of five distinct and highly varied ketosynthase (KS) domain sequences of potential polyketide synthase genes in A. carbonarius [19,33].
This work represents a significant step in expanding our understanding of the genetic mechanism of OTA biosynthesis in A. carbonarius, which is important given its relevance as the primary fungi responsible for OTA contamination in grapes, followed by A. niger and A. tubingensis. In line with other research projects [3,4,5,6,7,9,11,12,14,15,16,17,18,21,26,27,28,29,30,31,32,34,35], there has been less information about the screening of PKSs in A. niger and A. tubingensis up until this point.
Martinez-Culebras et al. [29] have, nevertheless, also developed primers that are appropriate for amplifying AT domain sequences in strains that are part of the A. niger aggregate. These primers also demonstrated broad application for additional Aspergillus species that are part of section Nigri. These advancements meet a critical demand for the creation of tests for high specificity toxigenic property detection. The molecular approach used to target PKSs genes in A. niger and A. tubingensis using primers (KSLB-LC6; AFL1F-LC2c) may be a useful molecular tool to facilitate the cloning of novel fungal polyketide synthase genes. Additionally, the sequencing of the products generated by these PCR reactions revealed that the inferred proteins were segments of previously unidentified PKS genes.
Similarities between these amino acid sequences and PKS genes implicated in the production pathway of numerous mycotoxins, including aflatoxins, were found to range from 68% to 97% [30,35,36].
The main goal of this study was to identify PKSs by the cloning of genes related to OTA synthesis in fungi. The ultimate objective will be to identify the genes that regulate the production of these chemicals and to fully comprehend how the genes regulate the way in which the compounds cluster together from the basic elements present in fungal cells. Furthermore, we are interested in doing logical genetic engineering research to modify genes and create novel compounds that may be applied as new herbicides and fungicides in agriculture.
Author Contributions
Conceptualization, S.B.F.M. and A.M.; methodology, S.B.F.M.; software, S.B.F.M.; validation, S.B.F.M., A.M. and A.G.; formal analysis, S.B.F.M.; investigation, S.B.F.M.; resources, S.B.F.M.; data curation, S.B.F.M.; writing—original draft preparation, S.B.F.M.; writing—review and editing, S.B.F.M.; visualization, S.B.F.M.; supervision, A.M.; project administration, A.M.; funding acquisition, S.B.F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Unesco-l’Oréal for Women in Sciences granted for the first author in collaboration with the Ministry of Research in Tunisia.
Institutional Review Board Statement
This statement acknowledges the absence of human subjects in the research and emphasizes the organization’s commitment to ethical conduct across all research activities. It also encourages researchers to consider ethical considerations and seek guidance as needed, even in the absence of IRB oversight.
Informed Consent Statement
This statement ensures that participants are fully informed about the study before consenting to participate, thereby upholding ethical standards and protecting the rights and welfare of the participants.
Data Availability Statement
Data are available in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Pks (Polyketides Syntase); AC Pks (Aspergillus carbonarius Polyketides Syntase); AN Pks (Aspergillus niger Polyketides Syntase); AT Pks (Aspergillus tubingesis Polyketides Syntase)
References
- Abarca, M.L.; Bragulat, M.R.; Castellá, G.; Cabañes, F.J. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 1994, 60, 2650–2652. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Rigó, K.; Kocsubé, S.; Farkas, B.; Pál, K. Diversity of polyketide synthase gene sequences in Aspergillus species. Res. Microbiol. 2003, 154, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Abbas, D.A. Lactogenic and Cytogenetic Effects of Ochratoxin A in Adult Male Rats and Pups. Br. J. Pharmacol. Toxicol. 2013, 4, 101–105. [Google Scholar] [CrossRef]
- Petziner, E.; Ziegler, K. Ochratoxin A from a toxicological perspective. J. Veter- Pharmacol. Ther. 2000, 23, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.P.; Mantle, P.G. Biosynthesis of ochratoxins by Aspergillus ochraceus. Phytochemistry 2001, 58, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.-L.; Hocking, A.D.; Pitt, J. Occurrence of fruit rot fungi (Aspergillus section Nigri) on some drying varieties of irrigated grapes. Aust. J. Grape Wine Res. 2004, 10, 83–88. [Google Scholar] [CrossRef]
- Melki Ben Fredj, S.; Gautier, A.; Brygoo, Y.; Mliki, A. Molecular strategy to discrim inate between two ochratoxin A producing fungal species in Aspergillus niger ag gregate group isolated from fresh and dried grapes. Ann. Microbiol. 2009, 50, 1–7. [Google Scholar]
- Chang, P.K.; Ehrlich, K.C.; Linz, J.E.; Bhatnagar, D.; Cleveland, T.E.; Bennett, J.W. Characterization of the Aspergillus parasiticus niaD and niiA gene cluster. Curr. Genet. 1996, 30, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Brown, D.W.; Plattner, R.D.; Desjardins, A.E. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet. Biol. 2003, 38, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Suzuki, T.; Ono, C.; Iwamoto, K.; Hosobuchi, M.; Yoshikawa, H. Molecular cloning and characterization of an ML-236B (compactin) biosynthetic gene cluster in Penicillium citrinum. Mol. Genet. Genom. 2002, 267, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Hohn, T.M. Metabolic pathway gene clusters in filamentous fungi. Fungal Genet. Biol. 1997, 21, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.E.; Hamilton, P. Mycotoxins—Their Biosynthesis in Fungi: Ochratoxins—Metabolites of Combined Pathways. J. Food Prot. 1979, 42, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.; Donovan, F. Studies in relation to biosynthesis I. Some possible routes to derivatives of Orcinol and Phloroglucinol. Aust. J. Chem. 1953, 6, 360–368. [Google Scholar] [CrossRef]
- Kao, C.M.; Katz, L.; Khosla, C. Engineered biosynthesis of a complete macrolactone in a heterologous host. Science 1994, 265, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.G.; Roessler, P.; Facciotti, D.; Levering, C.; Dittrich, F.; Lassner, M.; Valentine, R.; Lardizabal, K.; Domergue, F.; Yamada, A.; et al. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 2001, 293, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Graziani, S.; Vasnier, C.; Daboussi, M.J. Novel polyketide synthase from Nectria haematococca. Appl. Environ. Microbiol. 2004, 70, 2984–2988. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.; Caddick, M.X.; Dobson, A.D.W. A polyketide synthase gene required for ochratoxin A biosynthesis in Aspergillus ochraceus. Microbiology 2003, 149, 3485–3491. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Atoui, A.; Dao, H.P.; Mathieu, F.; Lebrihi, A. Amplification and diversity analysis of ketosynthase domains of putative polyketide synthase genes in Aspergillus ochraceus and Aspergillus carbonarius producers of ochratoxin A. Mol. Nutr. Food Res. 2006, 50, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Bingle, L.E.; Simpson, T.J.; Lazarus, C.M. Ketosynthase domain probes identify two subclasses of fungal polyketide synthase genes. Fungal Genet. Biol. 1999, 26, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.P.; Rudd, B.A.; Dawson, M.; Lazarus, C.M.; Simpson, T.J.; Cox, R.J. Design and utility of oligonucleotide gene probes for fungal polyketide synthases. Chem. Biol. 2001, 8, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Téren, J.; Varga, J.; Hamari, Z.; Rinyu, E.; Kevei, F. Immunochemical detection of ochratoxin A in black Aspergillus strains. Mycopathologia 1996, 134, 171–176. [Google Scholar] [CrossRef]
- Duncombe, J.U. Infrared navigation—Part I: An assessment of feasibility. IEEE Trans. Electron Devices 1959, 11, 34–39. [Google Scholar]
- Cox, R.J.; Glod, F.; Hurley, D.; Lazarus, C.M.; Nicholson, T.P.; Rudd, B.A.M.; Simpson, T.J.; Wilkinson, B.; Zhang, Y. Rapid cloning and expression of a fungal polyketide synthase gene involved in squalestatin biosynthesis. Chem. Commun. 2004, 20, 2260–2261. [Google Scholar] [CrossRef] [PubMed]
- Dalcero, A.; Magnoli, C.; Hallak, C.; Chiacchiera, S.M.; Palacio, G.; Rosa, C.A. Detection of ochratoxin A in animal feeds and capacity to produce this mycotoxin by Aspergillus section Nigri in Argentina. Food Addit. Contam. 2002, 19, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, L.; Davis, C.R.; Roach, C.; Nguyen, D.K.; Aldrich, T.; McAda, P.C.; Reeves, C.D. Lovastatin biosynthesis in Aspergillus terreus: Characterization of blocked mutants, enzyme activities and a multifunctional polyketide synthase gene. Chem. Biol. 1999, 6, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Yun, S.-H.; Hodge, K.T.; Humber, R.A.; Krasnoff, S.B.; Turgeon, G.B.; Yoder, O.C.; Gibson, D.M. Polyketide synthase genes in insect- and nematode associated fungi. Appl. Microbiol. Biotechnol. 2001, 56, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.F.; Khosla, C. Building-block selectivity of polyketide synthases. Curr. Opin. Chem. Biol. 2003, 7, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Culebras, P.V.; Crespo-Sempere, A.; Gil, J.V.; Ramón, D. Acyl Transferase Domains of Putative Polyketide Synthase (PKS) Genes in Aspergillus and Penicillium Producers of Ochratoxin A and the Evaluation of PCR Primers to Amplify PKS Sequences in Black Aspergillus Species. Food Sci. Technol. Int. 2009, 15, 97–105. [Google Scholar] [CrossRef]
- Mbah, M.C.; Akueshi, C.O. Aflatoxin in mould infested sesame seeds. Afr. J. Biotech. 2009, 8, 391–394. [Google Scholar]
- Fredj, S.M.B.; Chebil, S.; Lebrihi, A.; Lasram, S.; Ghorbel, A.; Mliki, A. Occurrence of pathogenic fungal species in Tunisian vineyards. Int. J. Food Microbiol. 2007, 113, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Fredj, S.M.B.; Chebil, S.; Mliki, A. Isolation and characterization of ochratoxin A and aflatoxin B1 producing fungi infecting grapevines cultivated in Tunisia. Afr. J. Microbiol. Res. 2009, 3, 523–527. [Google Scholar]
- Lin, X.; Huang, Y.J.; Zheng, Z.H.; Su, W.J.; Qian, X.M.; Shen, Y.M. Endophytes from the pharmaceutical plant, Annona squamosa: Isolation, bioactivity, identification and diversity of its polyketide synthase gene. Fungal Div. 2010, 41, 41–51. [Google Scholar] [CrossRef]
- Schümann, J.; Hertweck, C. Advances in cloning, functional analysis and heterologous expression of fungal polyketide synthase genes. J. Biotechnol. 2006, 124, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Recep, K.; Neslihan, D.; Hidayet, B. Biological control of post-harvest disease caused by Aspergillus flavus on stored lemon fruits. Afr. J. Biotech. 2009, 8, 209–214. [Google Scholar]
- Lin, C.Y.; Wu, M.; Bloom, J.A.; Cox, I.J.; Miller, M.; Lui, Y.M. Rotation, scale, and translation resilient watermarking for images. IEEE Trans. Image Process. 2001, 10, 767–782. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).