Microcrystals and Microfibers of Cellulose from Acrocomia aculeata (Arecaceae) Characterization †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Compositional Analysis
2.3. Cellulose Preparation
Microfibrillated and Microcrystal Cellulose Preparation
2.4. Characterization of MCC and MFC
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. X-ray Diffraction (XRD)
3. Results and Discussion
3.1. Compositional Analysis
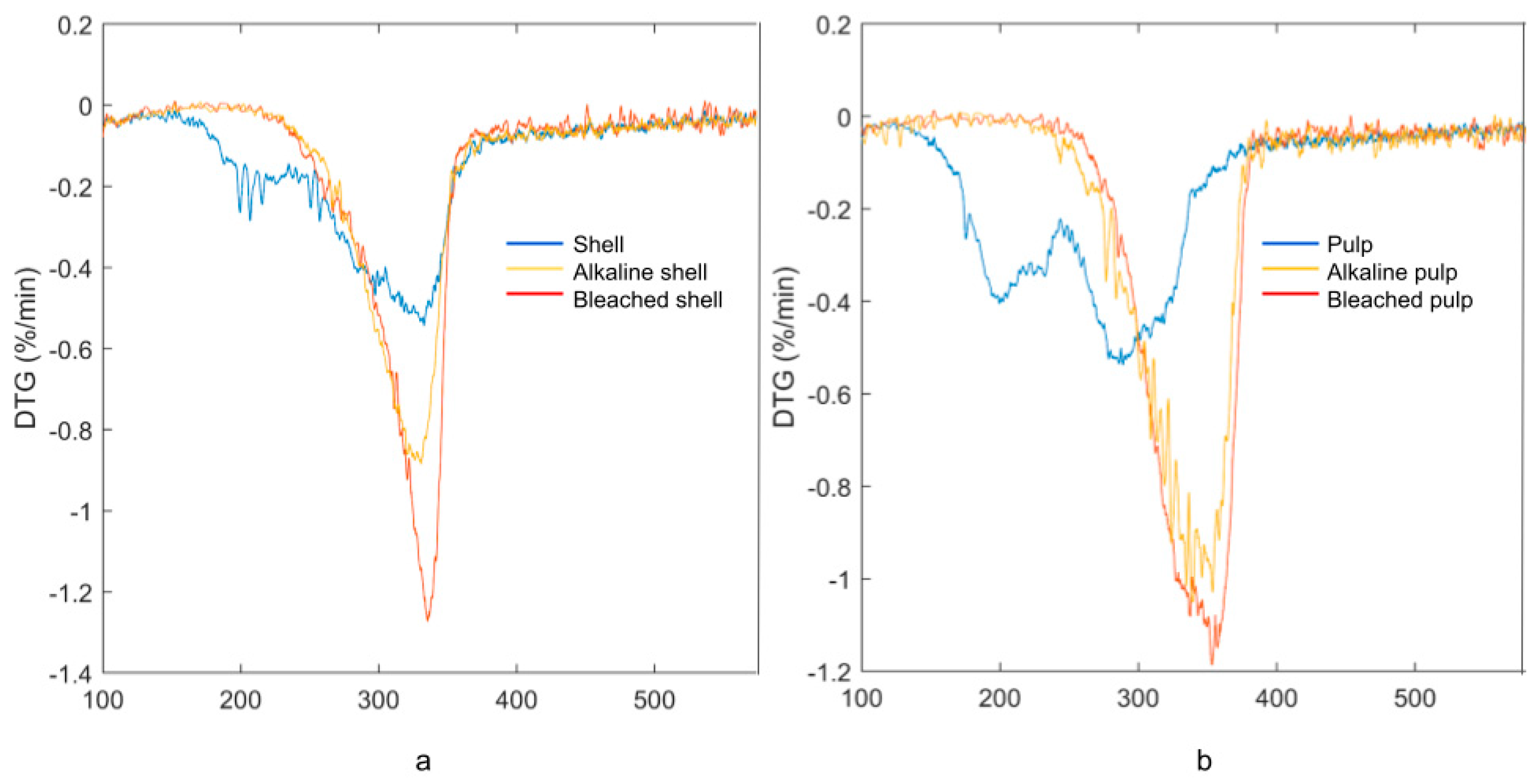
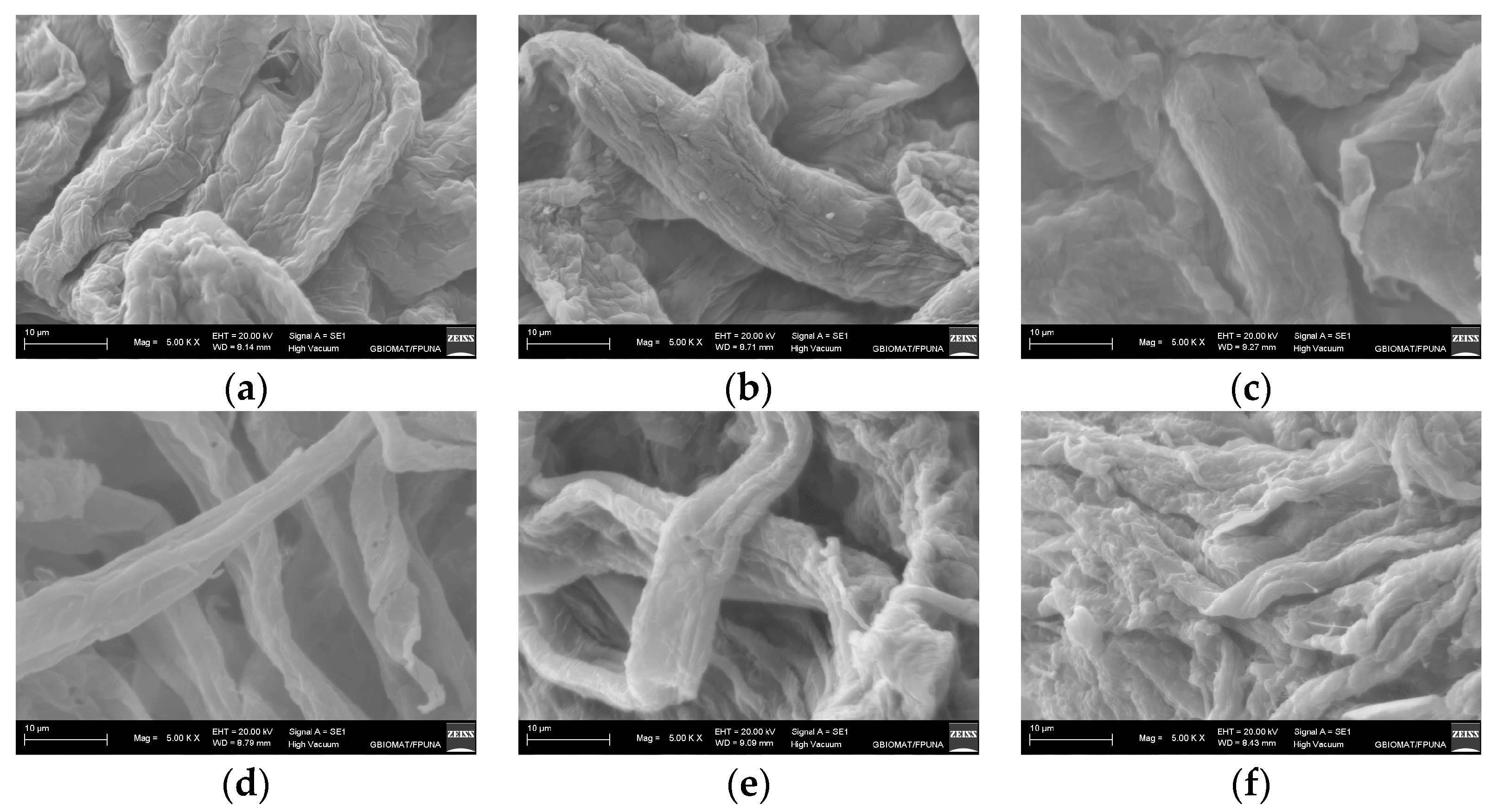
3.2. Cellulose Preparation and Purification
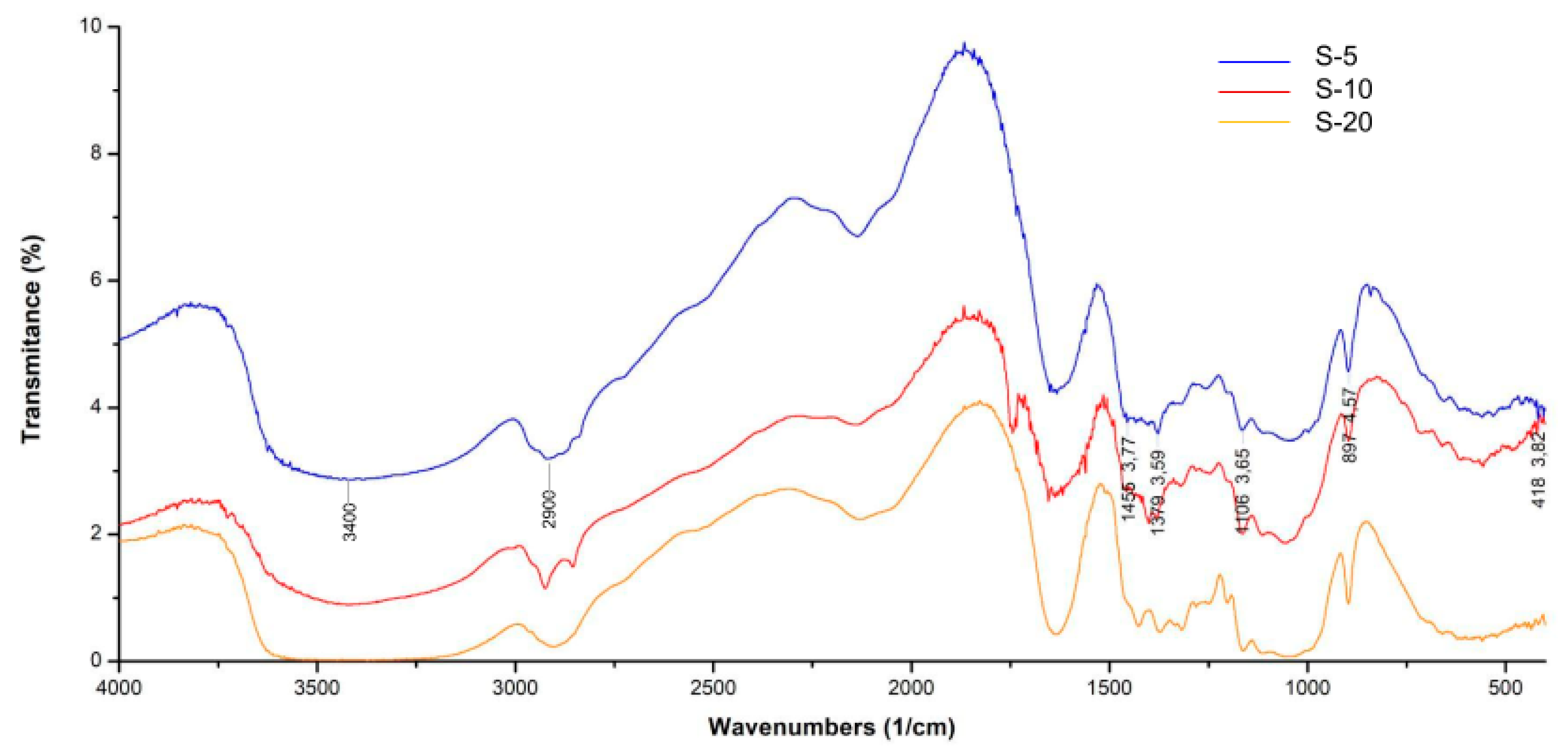
3.3. Characterization of MCC and MFC
Fourier Transform Infrared Spectroscopy (FTIR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evaristo, A.B.; Grossi, J.A.S.; Carneiro, A.d.C.O.; Pimentel, L.D.; Motoike, S.Y.; Kuki, K.N. Actual and putative potentials of macauba palm as feedstock for solid biofuel production from residues. Biomass-Bioenergy 2016, 85, 18–24. [Google Scholar] [CrossRef]
- Reis, S.B.; Mercadante-Simões, M.O.; Ribeiro, L.M. Pericarp development in the macaw palm Acrocomia aculeata (Arecaceae). Rodriguésia 2012, 63, 541–549. [Google Scholar] [CrossRef]
- Plath, M.; Moser, C.; Bailis, R.; Brandt, P.; Hirsch, H.; Klein, A.-M.; Walmsley, D.; von Wehrden, H. A novel bioenergy feedstock in Latin America? Cultivation potential of Acrocomia aculeata under current and future climate conditions. Biomass-Bioenergy 2016, 91, 186–195. [Google Scholar] [CrossRef]
- Ciconini, G.; Favaro, S.; Roscoe, R.; Miranda, C.; Tapeti, C.; Miyahira, M.; Bearari, L.; Galvani, F.; Borsato, A.; Colnago, L.; et al. Biometry and oil contents of Acrocomia aculeata fruits from the Cerrados and Pantanal biomes in Mato Grosso do Sul, Brazil. Ind. Crop. Prod. 2013, 45, 208–214. [Google Scholar] [CrossRef]
- Ovelar, R.L.; Ortellado, J.; Echauri, C.; Aguero, J.; Galeano, M. Residuos de “acrocomia aculeata” como fuente de biomasa: Una revisión sistemática. Extensionismo Innov. Y Transf. Tecnol. 2019, 5, 326–330. [Google Scholar] [CrossRef]
- Duarte, S.; Lv, P.; Almeida, G.; Rolón, J.C.; Perre, P. Alteration of physico-chemical characteristics of coconut endocarp—Acrocomia aculeata—By isothermal pyrolysis in the range 250–550 °C. J. Anal. Appl. Pyrolysis 2017, 126, 88–98. [Google Scholar] [CrossRef]
- Oviedo Ch, A.; Vinueza, G.J. Lignocellulosic waste and its uses, a review. infoANALÍTICA 2020, 8 (Extra 1), 133–147. [Google Scholar]
- Hasan, M.; Lai, T.K.; Gopakumar, D.A.; Jawaid, M.; Owolabi, F.A.T.; Mistar, E.M.; Alfatah, T.; Noriman, N.Z.; Haafiz, M.K.M.; Khalil, H.P.S.A. Micro Crystalline Bamboo Cellulose Based Seaweed Biodegradable Composite Films for Sustainable Packaging Material. J. Polym. Environ. 2019, 27, 1602–1612. [Google Scholar] [CrossRef]
- Huang, X.; Xie, F.; Xiong, X. Surface-modified microcrystalline cellulose for reinforcement of chitosan film. Carbohydr. Polym. 2018, 201, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, J.; Qin, Z.; Chen, H.; Gao, Q.; Li, J. Mechanical and thermal properties of microcrystalline cellulose-reinforced soy protein isolate–gelatin eco-friendly films. RSC Adv. 2015, 5, 56518–56525. [Google Scholar] [CrossRef]
- Wypych, G. Fillers—Origin, Chemical Composition, Properties, And Morphology. In Handbook of Fillers; ChemTec Publishing, Science Direct: Toronto, ON, Canada, 2016; pp. 13–266. [Google Scholar]
- Ventura-Cruz, S.; Tecante, A. Nanocellulose and microcrystalline cellulose from agricultural waste: Review on isolation and application as reinforcement in polymeric matrices. Food Hydrocoll. 2021, 118, 106771. [Google Scholar] [CrossRef]
- Álvarez, A.; Cachero, S.; González-Sánchez, C.; Montejo-Bernardo, J.; Pizarro, C.; Bueno, J.L. Novel method for holocellulose analysis of non-woody biomass wastes. Carbohydr. Polym. 2018, 189, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Browning, B.L. Methods of Wood Chemistry Vol I & II; John Wiley & Sons: New York, NY, USA, 1967. [Google Scholar]
- Bhawna, S.; El Barbary, H.; Barakat, M. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar]
- Moharram, M.A.; Mahmoud, O.M. FTIR spectroscopic study of the effect of microwave heating on the transformation of cellulose I into cellulose II during mercerization. J. Appl. Polym. Sci. 2008, 107, 30–36. [Google Scholar] [CrossRef]
- Brahim, M.M.; El-Zawawy, W.K.; Jüttke, Y.; Koschella, A.; Heinze, T. Cellulose and microcrystalline cellulose from rice straw and banana plant waste: Preparation and characterization. Cellulose 2013, 20, 2403–2416. [Google Scholar] [CrossRef]
- Razali, N.; Hossain, M.S.; Taiwo, O.A.; Ibrahim, M. Influence of Acid Hydrolysis Reaction Time on the Isolation of Cellulose Nanowhiskers from Oil Palm Empty Fruit Bunch Microcrystalline Cellulose. BioResources 2017, 12, 6773–6788. [Google Scholar] [CrossRef]
- Adel, A.M.; El-Gendy, A.A.; Diab, M.A.; Abou-Zeid, R.E.; El-Zawawy, W.K.; Dufresne, A. Microfibrillated cellulose from agricultural residues. Part I: Papermaking application. Ind. Crop. Prod. 2016, 93, 161–174. [Google Scholar] [CrossRef]
- Vora, R.; Shah, Y. Extraction, characterization of micro crystalline cellulose obtained from corn husk using different acid alkali treatment methods. Indo Am. J. Pharm. Sci. 2017, 4, 2399–2408. [Google Scholar]
- Martinez-Pavetti, M.B.; Medina, L.; Espínola, M.; Monteiro, M. Study on two eco-friendly surface treatments on Luffa cylindrica for development of reinforcement and processing materials. J. Mater. Res. Technol. 2021, 14, 420–2427. [Google Scholar] [CrossRef]
| Composition | Shell (%w/w) | Pulp (%w/w) |
|---|---|---|
| Extractives | 6.27 | 17.13 |
| Cellulose | 39.65 | 45.42 |
| Hemicellulose | 19.22 | 15.89 |
| Lignin | 30.80 | 17.90 |
| Ash | 4.06 | 3.66 |
| Time (min) | Pulp (%w/w) | Shell (% w/w) | |
|---|---|---|---|
| Alkaline treatment | 240 | 20.52 | 30.88 |
| Bleaching treatment | 120 | 45.56 | 58.31 |
| Sample | %CrI | Sample | %CrI | |
|---|---|---|---|---|
| MCC | S15′ | 42.42 | P15′ | 46.40 |
| S30′ | 68.19 | P30′ | 64.36 | |
| S60′ | 69.74 | P60′ | 67.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, S.; Monteiro, M.; Campuzano, P.A.; Giménez, N.; Penayo, M.C. Microcrystals and Microfibers of Cellulose from Acrocomia aculeata (Arecaceae) Characterization. Biol. Life Sci. Forum 2023, 28, 8. https://doi.org/10.3390/blsf2023028008
Duarte S, Monteiro M, Campuzano PA, Giménez N, Penayo MC. Microcrystals and Microfibers of Cellulose from Acrocomia aculeata (Arecaceae) Characterization. Biology and Life Sciences Forum. 2023; 28(1):8. https://doi.org/10.3390/blsf2023028008
Chicago/Turabian StyleDuarte, Shirley, Magna Monteiro, Porfirio Andrés Campuzano, Natalia Giménez, and María Cristina Penayo. 2023. "Microcrystals and Microfibers of Cellulose from Acrocomia aculeata (Arecaceae) Characterization" Biology and Life Sciences Forum 28, no. 1: 8. https://doi.org/10.3390/blsf2023028008
APA StyleDuarte, S., Monteiro, M., Campuzano, P. A., Giménez, N., & Penayo, M. C. (2023). Microcrystals and Microfibers of Cellulose from Acrocomia aculeata (Arecaceae) Characterization. Biology and Life Sciences Forum, 28(1), 8. https://doi.org/10.3390/blsf2023028008






