Divergent Impacts of Moderate and Severe Drought on the Antioxidant Response of Calendula officinalis L. Leaves and Flowers †
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Experimental Design
2.2. Non-Enzymatic Activities in Leaves and Flowers
2.3. Antioxidative Enzyme Activities in Leaves and Flowers
2.4. Impacts of Drought on the Production of Flowers
2.5. Statistical Analysis
3. Results and Discussion
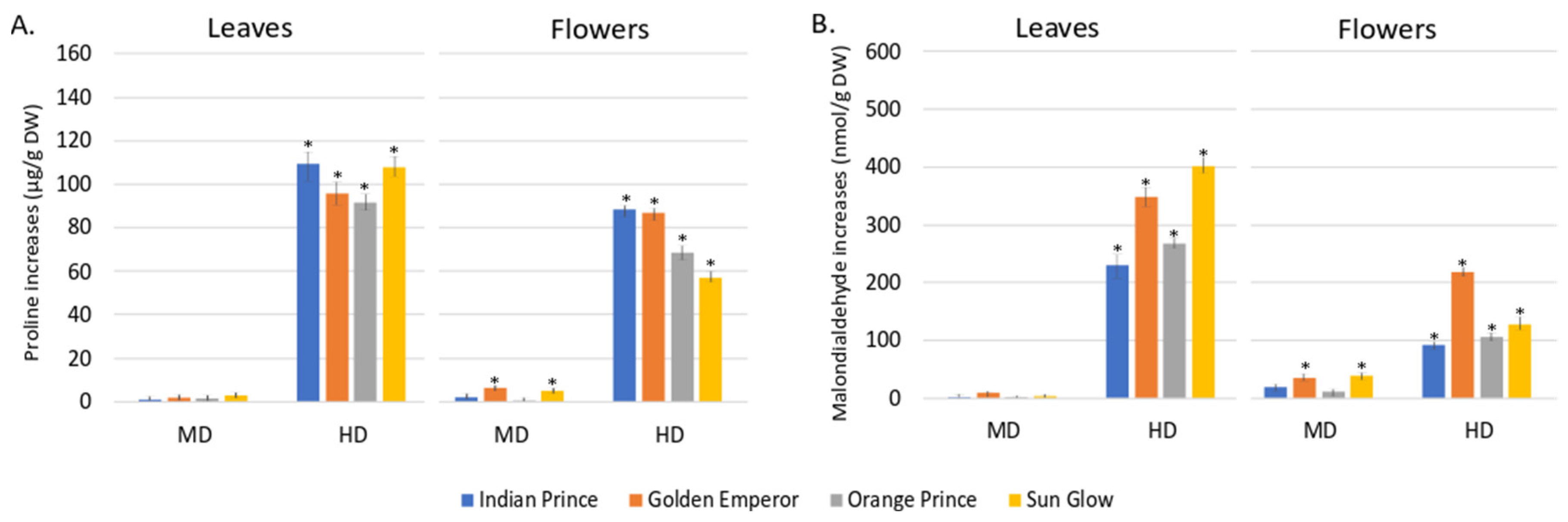
3.1. Non-Enzymatic Activities in Leaves and Flowers
3.2. Antioxidative Enzyme Activities in Leaves and Flowers
3.3. Impacts of Drought on the Production of Flowers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, I.; Marques, I.; Paulo, O.S.; Batista, D.; Partelli, F.L.; Lidon, F.C.; Damatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Understanding the impact of drought in Coffea genotypes: Transcriptomic analysis supports a common high resilience to moderate water deficit but a genotype dependent sensitivity to severe water deficit. Agronomy 2021, 11, 2255. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Kuppler, J.; Wieland, J.; Junker, R.R.; Ayasse, M. Drought-induced reduction in flower size and abundance correlates with reduced flower visits by bumble bees. AoB Plants 2021, 13, plab001. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [PubMed]
- Lawas, L.M.F.; Shi, W.; Yoshimoto, M.; Hasegawa, T.; Hincha, D.K.; Zuther, E.; Jagadish, S.V.K. Combined drought and heat stress impact during flowering and grain filling in contrasting rice cultivars grown under field conditions. Field Crop. Res. 2018, 229, 66–77. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A major locus controlling malondialdehyde content under water stress is associated with Fusarium crown rot resistance in wheat. Mol. Genet. Genom. 2015, 290, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Vinci, G.; Marques, I.; Rodrigues, A.P.; Martins, S.; Leitão, A.E.; Semedo, M.C.; Silva, M.J.; Lidon, F.C.; DaMatta, F.M.; Ribeiro-Barros, A.I.; et al. Protective Responses at the Biochemical and Molecular Level Differ between a Coffea arabica L. Hybrid and Its Parental Genotypes to Supra-Optimal Temperatures and Elevated Air [CO2]. Plants 2022, 11, 2702. [Google Scholar] [CrossRef]
- Marques, I.; Fernandes, I.; Paulo, O.S.; Batista, D.; Lidon, F.C.; Partelli, F.; DaMatta, F.M.; Ribeiro-Barros, A.I.; Ramalho, J.C. Overexpression of Water-Responsive Genes Promoted by Elevated CO2 Reduces ROS and Enhances Drought Tolerance in Coffea Species. Int. J. Mol. Sci. 2023, 24, 3210. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmed, W.; Mihoub, A.; Jamal, A.; Farhan Saeed, M.; Masood, N.; Radicetti, E.; Fawad, M.; Nicola, S. Developmental, Phytochemical and Enzymatic Changes in Pot Marigold (Calendula officinalis L.) cvs. Hybrid and French with Salicylic Acid (SA) and Polyamine Spermidine (SP) Foliar Spray. Agronomy 2023, 13, 191. [Google Scholar] [CrossRef]
- Ghadyeh Zarrinabadi, I.; Razmjoo, J.; Abdali Mashhadi, A.; Karim mojeni, H.; Boroomand, A. Physiological response and productivity of pot marigold (Calendula officinalis) genotypes under water deficit. Ind. Crops Prod. 2019, 139, 111488. [Google Scholar] [CrossRef]
- Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S.; et al. Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. [Google Scholar] [CrossRef]
- Khedr, A.H.A.; Abbas, M.A.; Abdel Wahid, A.A.; Quick, W.P.; Abogadallah, G.M. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003, 54, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzionis, A.; Xylia, P.; Tzortzakis, N. Effects of salinity on tagetes growth, physiology, and shelf life of edible flowers stored in passive modified atmosphere packaging or treated with ethanol. Front. Plant Sci. 2018, 871, 1765. [Google Scholar] [CrossRef] [PubMed]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaal, M.; Ouerghi, Z.; Navari-Izzo, F. Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol. Biochem. PPB 2010, 48, 772–777. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Elsayed, A.I.; El-Hamahmy, M.A.M.; Rafudeen, M.S.; Mohamed, A.H.; Omar, A.A. The impact of drought stress on antioxidant responses and accumulation of flavonolignans in milk thistle (Silybum marianum (L.) gaertn). Plants 2019, 8, 611. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Descamps, C.; Boubnan, N.; Jacquemart, A.L.; Quinet, M. Growing and Flowering in a Changing Climate: Effects of Higher Temperatures and Drought Stress on the Bee-Pollinated Species Impatiens glandulifera Royle. Plants 2021, 10, 988. [Google Scholar] [CrossRef] [PubMed]
| Control | MD | SD | |
|---|---|---|---|
| CAT | |||
| Indian Prince | 11.23 ± 2.21 a/8.14 ± 1.17 a | 11.66 ± 3.44 a/8.11 ± 1.19 a | 28.37 ± 4.22 b/10.14 ± 1.29 a |
| Golden Emperor | 14.25 ± 2.74 a/7.89 ± 1.15 a | 14.23 ± 3.01 a/8.55 ± 2.20 b | 21.99 ± 4.05 b/10.31 ± 2.17 c |
| Orange Prince | 12.07 ± 2.22 a/7.99 ± 1.11 a | 12.03 ± 2.66 a/9.01 ± 1.25 b | 16.28 ± 3.31 b/14.20 ± 1.99 c |
| Sun Glow | 10.03 ± 2.00 a/6.45 ± 1.11 a | 10.20 ± 2.11 a/7.25 ± 1.19 b | 17.25 ± 3.07 b/9.29 ± 2.33 c |
| POX | |||
| Indian Prince | 1.24 ± 0.22 a/0.33 ± 0.07 a | 1.51 ± 0.44 a/1.03 ± 0.98 b | 3.25 ± 0.22 b/2.07 ± 0.01 c |
| Golden Emperor | 0.98 ± 0.21 a/0.41 ± 0.11 a | 1.03 ± 0.11 a/0.48 ± 0.67 a | 2.71 ± 0.56 b/1.06 ± 0.23 b |
| Orange Prince | 1.08 ± 0.22 a/0.37 ± 0.13 a | 1.13 ± 0.66 a/0.41 ± 0.21 a | 2.08 ± 0.71 b/1.03 ± 0.01 b |
| Sun Glow | 1.12 ± 0.19 a/0.56 ± 0.212 a | 1.18 ± 0.18 a/0.23 ± 2.21 a | 2.01 ± 0.98 b/1.20 ± 0.21 b |
| APX | |||
| Indian Prince | 9.44 ± 2.01 a/4.55 ± 1.04 a | 9.51 ± 2.03 a/4.23 ± 2.01 a | 18.23 ± 3.05 b/5.20 ± 2.24 b |
| Golden Emperor | 10.21 ± 2.33 a/3.03 ± 1.89 a | 10.25 ± 2.26 a/2.99 ± 2.28 a | 16.39 ± 2.56 b/4.56 ± 1.98 b |
| Orange Prince | 11.23 ± 2.04 a/6.21 ± 2.23 a | 10.99 ± 3.01 a/6.15 ± 1.99 a | 14.99 ± 2.27 b/8.05 ± 2.01 b |
| Sun Glow | 8.05 ± 2.31 a/4.02 ± 1.89 a | 8.12 ± 2.01 a/4.11 ± 2.17 a | 11.13 ± 2.27 b/6.73 ± 2.35 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzman, M.R.; Marques, I. Divergent Impacts of Moderate and Severe Drought on the Antioxidant Response of Calendula officinalis L. Leaves and Flowers. Biol. Life Sci. Forum 2023, 27, 53. https://doi.org/10.3390/IECAG2023-16636
Guzman MR, Marques I. Divergent Impacts of Moderate and Severe Drought on the Antioxidant Response of Calendula officinalis L. Leaves and Flowers. Biology and Life Sciences Forum. 2023; 27(1):53. https://doi.org/10.3390/IECAG2023-16636
Chicago/Turabian StyleGuzman, María Rita, and Isabel Marques. 2023. "Divergent Impacts of Moderate and Severe Drought on the Antioxidant Response of Calendula officinalis L. Leaves and Flowers" Biology and Life Sciences Forum 27, no. 1: 53. https://doi.org/10.3390/IECAG2023-16636
APA StyleGuzman, M. R., & Marques, I. (2023). Divergent Impacts of Moderate and Severe Drought on the Antioxidant Response of Calendula officinalis L. Leaves and Flowers. Biology and Life Sciences Forum, 27(1), 53. https://doi.org/10.3390/IECAG2023-16636







