Effects of Salinity on Edible Marigold Flowers (Tagetes patula L.) †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Determination of Mineral Contents, Total Phenols, and Proteins
2.3. Statistical Analysis
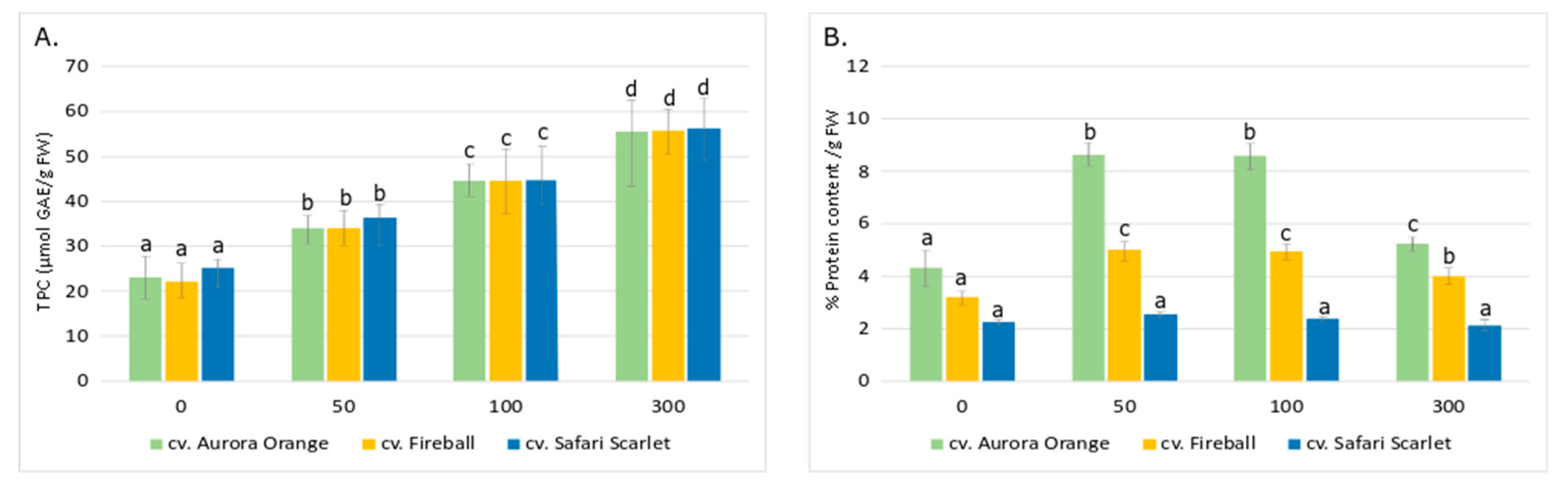
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guiné, R.; Florença, S.; Ferrão, A.C.; Correia, P. Study about the use of edible flowers for gastronomic purposes In Portugal. In Proceedings of the International Conference on Mediterranean Diet and Gastronomy, Evora, Portugal, 15–16 October 2018; p. 41. [Google Scholar]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional composition and antioxidant capacity in edible flowers: Characterisation of phenolic compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2015, 16, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E. What Nutritional Contribution Do Edible Flowers Make? J. Acad. Nutr. Diet. 2015, 115, 856. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Mazzoncini, M. The Biodiversity of Edible Flowers: Discovering New Tastes and New Health Benefits. Front. Plant Sci. 2021, 11, 569499. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Pelvan, E.; Özdemir, K.S.; Kocadagìli, T.; Mogol, B.A.; Pasli, A.A.; Özcan, N.; Özçelik, B.; Gökmen, V. Compositional, nutritional, and functional characteristics of instant teas produced from low- and high-quality black teas. J. Agric. Food Chem. 2013, 61, 7529–7536. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Tzionis, A.; Xylia, P.; Tzortzakis, N. Effects of salinity on tagetes growth, physiology, and shelf life of edible flowers stored in passive modified atmosphere packaging or treated with ethanol. Front. Plant Sci. 2018, 871, 1765. [Google Scholar] [CrossRef] [PubMed]
- Cicevan, R.; Al Hassan, M.; Sestras, A.F.; Prohens, J.; Vicente, O.; Sestras, R.E.; Boscaiu, M. Screening for drought tolerance in cultivars of the ornamental genus Tagetes (Asteraceae). PeerJ 2016, 2016, e2133. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are antioxidants helpful for disease prevention? Res. Pharm. Sci. 2010, 5, 5–12. [Google Scholar]
- Moliner, C.; Barros, L.; Dias, M.I.; López, V.; Langa, E.; Ferreira, I.C.F.R.; Gómez-Rincón, C. Edible flowers of tagetes erecta l. As functional ingredients: Phenolic composition, antioxidant and protective effects on caenorhabditis elegans. Nutrients 2018, 10, 2002. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M. Plant Responses and Tolerance to Salt Stress: Physiological and Molecular Interventions. Int. J. Mol. Sci. 2022, 23, 4810. [Google Scholar] [CrossRef] [PubMed]
- Laamari, I.; Marques, I.; Ribeiro-Barros, A.I.; Zoubeir, B.; Abassi, M. Can saline preconditioning enhance plant survival in degraded soils? Physiological, biochemical, and molecular responses in Casuarina glauca saplings. Plant Ecol. 2023, 224, 905–919. [Google Scholar] [CrossRef]
- Yue, L.J.; Li, S.X.; Ma, Q.; Zhou, X.R.; Wu, G.Q.; Bao, A.K.; Zhang, J.L.; Wang, S.M. NaCl stimulates growth and alleviates water stress in the xerophyte Zygophyllum xanthoxylum. J. Arid Environ. 2012, 87, 153–160. [Google Scholar] [CrossRef]
- Lu, Y.; Lei, J.Q.; Zeng, F.J.; Zhang, B.; Liu, G.J.; Liu, B.; Li, X.Y. Effect of NaCl-induced changes in growth, photosynthetic characteristics, water status and enzymatic antioxidant system of Calligonum caput-medusae seedlings. Photosynthetica 2017, 55, 96–106. [Google Scholar] [CrossRef]
- Green, T.G.A.; Sancho, L.G.; Pintado, A.; Saco, D.; Martín, S.; Arróniz-Crespo, M.; Casermeiro, M.A.; de la Cruz Caravaca, M.T.; Cameron, S.; Rozzi, R. Sodium chloride accumulation in glycophyte plants with cyanobacterial symbionts. AoB Plants 2017, 9, plx053. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Myrzabayeva, M.; Alikulov, Z.; Omarov, R.; Khozin-Goldberg, I.; Sagi, M. Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants 2014, 6, plu053. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Koprowska, K.; Gottschling, A.; Janda-milczarek, K. Edible Flowers as a Source of Dietary Fibre (Total, Insoluble and Soluble) as a Potential Athlete’s Dietary Supplement. Nutrients 2022, 14, 2470. [Google Scholar] [CrossRef] [PubMed]
| Minerals | Cultivars | 0 | 50 | 100 | 300 |
|---|---|---|---|---|---|
| N | cv. Aurora Orange | 11.11 ± 1.22 a | 16.31 ± 1.22 b | 18.25 ± 1.12 c | 19.37 ± 1.56 d |
| cv. Fireball | 10.13 ± 1.31 a | 16.44 ± 1.18 b | 18.24 ± 1.35 c | 19.20 ± 1.33 d | |
| cv. Safari Scarlet | 14.21 ± 1.65 a | 17.52 ± 1.11 b | 19.22 ± 1.56 c | 22.11 ± 1.56 d | |
| K | cv. Aurora Orange | 11.09 ± 1.17 a | 12.22 ± 1.26 b | 13.25 ± 1.56 c | 12.15 ± 1.68 b |
| cv. Fireball | 11.11 ± 1.21 b | 11.59 ± 1.12 d | 11.16 ± 1.65 c | 10.23 ± 2.55 a | |
| cv. Safari Scarlet | 11.01 ± 1.13 c | 11.55 ± 1.10 d | 10.20 ± 1.32 b | 10.11 ± 2.15 a | |
| Ca | cv. Aurora Orange | 3.11 ± 1.12 b | 9.55 ± 1.57 d | 3.21 ± 1.99 c | 3.01 ± 2.95 a |
| cv. Fireball | 3.15 ± 1.26 c | 9.50 ± 1.44 d | 3.10 ± 2.60 b | 3.00 ± 2.78 a | |
| cv. Safari Scarlet | 4.03 ± 1.19 b | 8.52 ± 1.35 d | 4.22 ± 2.24 c | 3.99 ± 2.67 a | |
| Mg | cv. Aurora Orange | 0.18 ± 0.04 b | 1.55 ± 0.57 d | 0.21 ± 0.05 c | 0.11 ± 0.01 a |
| cv. Fireball | 0.14 ± 0.05 b | 1.50 ± 0.57 d | 0.10 ± 0.08 a | 0.20 ± 0.02 c | |
| cv. Safari Scarlet | 0.28 ± 0.04 c | 1.52 ± 0.61 d | 0.22 ± 0.06 b | 0.19 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzman, M.R.; Marques, I. Effects of Salinity on Edible Marigold Flowers (Tagetes patula L.). Biol. Life Sci. Forum 2023, 27, 38. https://doi.org/10.3390/IECAG2023-15986
Guzman MR, Marques I. Effects of Salinity on Edible Marigold Flowers (Tagetes patula L.). Biology and Life Sciences Forum. 2023; 27(1):38. https://doi.org/10.3390/IECAG2023-15986
Chicago/Turabian StyleGuzman, María Rita, and Isabel Marques. 2023. "Effects of Salinity on Edible Marigold Flowers (Tagetes patula L.)" Biology and Life Sciences Forum 27, no. 1: 38. https://doi.org/10.3390/IECAG2023-15986
APA StyleGuzman, M. R., & Marques, I. (2023). Effects of Salinity on Edible Marigold Flowers (Tagetes patula L.). Biology and Life Sciences Forum, 27(1), 38. https://doi.org/10.3390/IECAG2023-15986







