Abstract
Pleurotus ostreatus has potent antimicrobial properties. In this study, bioactive compounds were extracted from P. ostreatus and screened against bacterial and fungal phytopathogens. In terms of the antibacterial activity, the n-hexane extract of P. ostreatuse exhibited a significant inhibition zone of 88.55 mm against Xanthomonas axonopodis, while the highest antifungal activity of 83% was against Fusarium oxysporum. It was observed that the highest level of concentrations, i.e., 25 mg mL−1, caused a 76, 82, 82, 83, and 60% decrease in fungal biomass over the control against the fungal strains, i.e., A. alternata, A. flavus, D. australiensis, F. oxysporum and M. phaseolina, respectively. GC-MS analysis was performed on the n-hexane extract depicting the presence of 26 compounds. A compound identified as Toluene (Molecular weight = 92) exhibited a peak area of 91% followed by another compound named Cyclopentane, methyl- (Molecular weight = 84) showing a peak area of 56%. A well-known antimicrobial compound Aziridine (Mol. Weight = 99) was identified and showed a maximum hit of 84%, with a peak area of 56%. P. ostreatus could be a potent biocontrol antagonist against the plant pathogens.
1. Introduction
Bacteria and fungi are major phytopathogens that affect crop yields worldwide [1]. With a growing global population, a well-organized disease management and control program is essential for ensuring food security and safety [2,3]. Using agrochemicals to control crop diseases impacts the environment and humans [4]. Biocontrol is an eco-friendly solution to this problem [5]. The numbers of tested biocontrol agents (BCAs) and commercial products are not positively connected [3]. Identification of novel BCAs is essential to commercial biocontrol product development and needs a reliable screening procedure [6]. According to research, fungi produce various bioactive volatile organic compounds (VOCs) [7]. Specifically, mushroom-derived compounds exhibit promising potential for biological activity [8,9]. By considering the aforementioned characteristics of macro-fungus, our study investigated the antimicrobial efficacy of P. ostreatus against phytopathogens.
2. Experiments
2.1. Pathogens and Antagonist
P. ostreatus was purchased from BioTech, Sahiwal, Pakistan. Bacterial and fungal pathogens were purchased from the Culture Bank of Pakistan, Institute of Agricultural Sciences Punjab University, located at the main campus in Lahore. The bacterial plant pathogens, Erwinia carotovora, Pseudomonas syringae, Ralstonia solanacearum, and Xanthomonas axonopodis were subcultured on malt extract agar, while the fungal pathogens, Alternaria alternata, Aspergillus flavus, Drechslera australiensis, Fusarium oxysporum, and Macrophomina phaseolina were subcultured on PDA medium. Then, these grown cultures were stored in a refrigerator at 4 °C.
2.2. Extraction of the Bioactive Compounds
P. ostreatus bioactive components were extracted following [10], with minor changes. We used a piston mortar to crush the sun-dried fruiting bodies into powder. 500 g of dry P. ostreatus powder was steeped in 1000 mL methanol for 5 days. After filtering using Whattman paper, the extract was evaporated in a rotary evaporator at 45 °C for one hour. The dry methanolic extract was remeasured and re-suspended in 200 mL of distilled water to yield 9 g. We stored it aseptically for future use.
Additional solvent extraction was performed using n-hexane. We added 200 mL of the re-suspended extract to a 500 mL separating funnel and added 200 mL of the n-hexane organic solvent at a 1:1 ratio. We let it sit overnight. The flask had clear layers, which were carefully transferred to an electrically pre-weighted beaker for evaporation. This was performed three times to remove everything. The extract was re-evaporated using a rotary evaporator to pure fractions.
2.3. Gas Chromatography Mass Spectrometry Analysis
The biochemical components of P. ostreatus n-hexane extract were evaluated using a GC/MS with column number HP-5MS (30 m × 250 µm × 0.25 µm). GC/MS analysis was conducted on a Thermo GC Trace ultra-version 5.0, Thermo MS DSQ II, using a fused silica column and Elite ZB 5 MS capillary standard nonpolar column (30 m, 0.25 mm, 0.25 µm). The components were separated using helium at 1 mL/min. The Thermo MS DSQ II identified the 1 µL sample extract supplied to the instrument. The oven was set at 260 °C for 38.50 min (mass analyzer). After 40 min of MS detection, the relative amounts of all components were computed.
2.4. Antibacterial Assays
The disk diffusion technique was employed for antibacterial trials. The treatment included dissolving 50 mg of mushroom extract in n-hexane solution and 140 µL of DMSO and adding distilled water to reach 500 µL. To generate the negative control solution, we combined 140 µL of DMSO with 360 µL of distilled water. For the positive control, we dissolved 50 mg of Penicillin in 140 µL of DMSO and added distilled water to achieve a volume of 500 µL. All bacteria were cultured on Lysogeny agar.
2.5. Antifungal Assays
Antifungal screening of the isolated compounds was carried out by dilution technique. We prepared Potato Dextrose Broth (PDB) in a conical flask and autoclaved it at 121 °C for 20 min. We mixed 300 mg of the mushroom extract in n-hexane solution; then, we dissolved it in 333 µL of DMSO and add distilled water to reach 1000 µL for the stock solutions. The control solution with DMSO was made similarly. To promote fungal development, mycelial spores of each species were introduced to growth media and incubated at 27 °C for 6 days. Following filtering using pre-weighted filter paper, the filtered material was oven-dried for 2 days at 65 °C to extract the dry fungal biomass.
2.6. Statistical Analysis
For the statistical analysis, two way ANOVA was applied using Fisher’s Least Significant Difference (LSD) test to elucidate the treatment means. In ANOVA, two variable factors were analyzed, i.e., Extracts over Control treatments. This analysis was performed utilizing the Minitab-17 statistical software.
3. Results and Discussion
The results showed that P. ostreatus has potent antimicrobial properties against bacterial and fungal phytopathogens. This indicated that P. ostreatus could be an efficient antagonistic agent. Various species of basidiomycetes, including the edible mushroom P. ostreatus, have the ability to inhibit the growth of plant pathogens [11].
3.1. Antibacterial Activity of the n-Hexane Extract of P. ostreatus
The results showed that the n-hexane extract of P. ostreatus showed the highest zone of inhibition of 88.55 mm against X. axonopodis. However, E. carotovora, was the only pathogenic strain that was not inhibited by this extract (Figure 1). Even the control showed lower bioactivity against E. carotovora. This extract also exhibited significant bioactivity against other pathogenic bacteria, such as P. syringae and R. solanacearum, with inhibition zones of 69.18 mm and 59.79 mm (Figure 1).
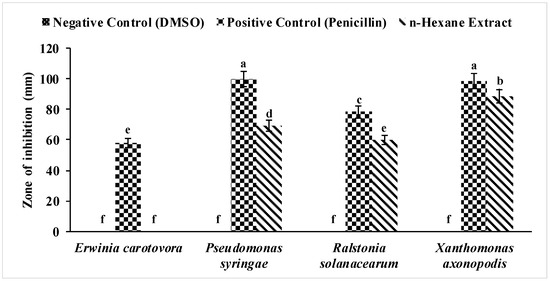
Figure 1.
Effect of the n-hexane extract of P. ostreatus against E. carotovora, P. syringae, R. solanacearum, and X. axonopodis. The vertical bars show the standard error of the means of three replicates. Values with different letters show a significant difference (p ≤ 0.05) as determined by ANOVA followed by Fisher’s LSD Test, using Minitab 17.
The positive control (penicillin) formed significant inhibition zones in all bacterial strains. In E. carotovora a 58.02 mm, in P. syringae a 99.87 mm, in R. solanacearum a 78.34 mm and in X. axonopodis a 98.61 mm zone of inhibition was formed. Penicillin is a synthetic antibacterial capsule, which contains a pure compound, which is why it showed maximum activity against all bacterial strains.
The negative control did not show any antibacterial activity against any bacterial strains. This confirmed that the DMSO did not harm any plant pathogenic bacteria. According to this experimental result, this extract was further analyzed by GCMS, and its chemical composition was observed. Heleno et al. [12] studied the antibacterial properties of P. ostreatus acidic extract against Pseudomonas aeruginosa and found similar results to our experiment. Other information against bacterial strains was not available and was firstly reported in our experiment. n-hexane was significantly active against bacteria. The broad spectrum antibacterial activity of Aziridine, 1-(2-aminoethyl)-, was optimized by a one-variable-at-a-time system coupled with the response surface methodology, which led to a 45% enhancement in antibacterial activity [13].
3.2. Antifungal Activity
The antifungal activity of the n-hexane extract of P. ostreatus against Alternaria alternata, Aspergillus flavus, Drechslera australiensis, Fusarium oxysporum, and Macrophomina phaseolina was examined. It was observed that organic extracts of n-hexane and its different concentrations increased from 5 to 25 mg mL−1; the fungal growth was inhibited, which resulted in the decrease in the fungal biomass (Figure 2). The maximum growth reduction occurred in the extracts with a 25 mg mL−1 concentration. In these bioassays, the n-hexane extracts showed pronounced inhibitory effects on the tested fungal pathogen. The maximum growth was seen in the control to which no extract was added; as the organic extracts were added the fungal biomass decreased. All the extracts were prepared in DMSO; so, it was used as the control. The DMSO had no activity against any plant pathogenic fungal strains.
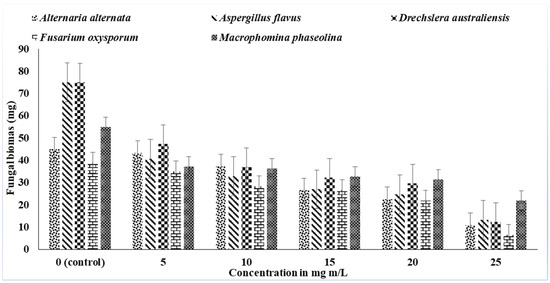
Figure 2.
Effect of the n-hexane extract of P. ostreatus against Alternaria alternata, Aspergillus flavus, Drechslera australiensis, Fusarium oxysporum, and Macrophomina phaseolina. Vertical bars show the standard error of the means of three replicates. Values with different letters show a significant difference (p ≤ 0.05) as determined by ANOVA followed by Fisher’s LSD test using Minitab 17.
The maximum antifungal activity was observed in the n-hexane extracts of 25 mg mL−1 concentration on the fungal pathogen A. flavus which decreased the fungal biomass by 82% and 81% over the control, respectively. Among all the fungal strains examined in this experiment, the growth of F. oxysporum was the most reduced, which confirmed that these extracts were very effective against this strain. In a previous investigation, an antifungal component, obtained from Pleurotus sajorcaju, showed bioactivity against Fusarium and Mycosphaerella species [14]. The isolation of antifungal constituents from Pleurotus spp. with activity on these fungi can be fruitful from an industrial point of view [15].
3.3. GC/MS Analysis of the n-Hexane Extract of P. ostreatus
A total of 26 compounds were identified in the n-hexane extract of P. ostreatus mushroom, as presented in Table S1. Among the 26 compounds, 7 compounds exhibited a higher frequency of occurrence and demonstrated a greater number of successful outcomes. Aziridine, 2-methyl-3-(1-methylethyl)-, trans- Aziridine, 2-isopropyl-3-methyl-, trans 2-Isopropyl-3-Methyl (Mol. Weight = 99) was identified only one time and showed a maximum hit of 84%. The potential structure is depicted in (Figure 3A,B). Santra et al. [13] first reported the Aziridine, 1-(2-aminoethyl)-, from any endophytic source. Cochliobolus sp. APS1 possesses industrial importance for the production of bioactive alkaloids with broad spectrum bactericidal action. Kowalczyk et al. [16] reported that Aziridines were widely used as building blocks in the multi-step syntheses of more complex molecules; however, due to the presence of the aziridine ring, their derivatives exhibit significant biological activity. Toluene (Mol. Weight = 92) showed a maximum hit of 91%, and hexane (Mol. Weight = 86) showed a maximum hit of 51%. The other prominent compounds were pentane, specifically the isomer 3-methylpentane, with a molecular weight of 86, which exhibited a maximum yield of 57%. In the same way, the compounds pentane, 2-methyl-Isohexane, 2-methylpentane, and methyl pentane (CH3)2CH(CH2)2CH3, with a molecular weight of 86, were all identified. P. ostreatus contains bioactive compounds such as polysaccharides (glucans and chitin), as well as secondary metabolites (phenolic compounds, terpenoids, and lectins). These compounds have been found to possess antibacterial, antiviral, and antifungal properties [17].
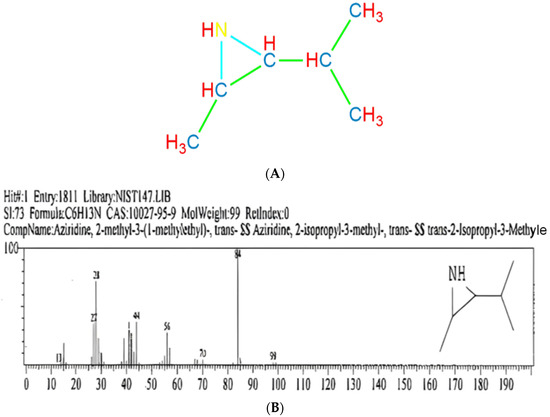
Figure 3.
A typical chemical structure of Aziridine derivatives (A). Chromatogram of Aziridine derivatives (B).
4. Conclusions
This study revealed that the extract derived from P. ostreatus exhibits significant antibacterial and antifungal activities against crop pathogens. The GC-MS analysis revealed the presence of aziridine and its derivatives in the extract of P. ostreatus. This study could be useful to develop an eco-friendly and sustainable biocontrol agent for crop disease management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/IECAG2023-16378/s1, Table S1: GC/MS Analysis of the n-hexane extract of Pleurotus ostreatus.
Author Contributions
Conceptualization, M.U. and M.A.; methodology, M.A.; software, M.H.; validation, M.A., T.A. and M.U.; formal analysis, M.U.; investigation, M.U.; resources, M.A.; data curation, M.A. and M.H.; writing—original draft preparation, T.A.; writing—review and editing, M.U. and M.A.; visualization, M.A.; supervision, M.A.; project administration, M.A.; funding acquisition, T.A. All authors have read and agreed to the published version of the manuscript.
Funding
Basic scientific research business expenses from Liaoning Academy of Agricultural Sciences (2022XTCX0502003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author on reasonable request.
Acknowledgments
A very special thanks to Taswar Ahsan for providing the funds.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Bacterial disease management: Challenges, ssexperience, innovation and future prospects: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Vannacci, G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Kanjanasopa, D.; Aiedhet, W.; Thitithanakul, S.; Paungfoo-Lonhienne, C. Plant growth promoting rhizobacteria as biological control agent in rice. Agric. Sci. 2021, 12, 1. [Google Scholar] [CrossRef]
- Ahsan, T.; Liang, C.; Yu, S.; Pei, X.; Xie, J.; Lin, Y.; Liu, X.; Umair, M.; Zang, C. Screening and Optimization of Fermentation Medium for Bacillus velezensis BP-1 and Its Biocontrol Effects against Peyronellaea arachidicola. Appl. Sci. 2023, 13, 4653. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P. Screening for novel biocontrol agents applicable in plant disease management–a review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Guo, Y.; Jud, W.; Weikl, F.; Ghirardo, A.; Junker, R.R.; Polle, A.; Rosenkranz, M. Volatile organic compound patterns predict fungal trophic mode and lifestyle. Commun. Biol. 2021, 4, 673. [Google Scholar] [CrossRef] [PubMed]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An important source of natural bioactive compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar] [CrossRef]
- Waithaka, P.N.; Gathuru, E.M.; Githaiga, B.M.; Onkoba, K.M. Antimicrobial activity of mushroom (Agaricus bisporus) and fungal (Trametes gibbosa) extracts from mushrooms and fungi of Egerton main campus, Njoro Kenya. J. Biomed. Sci. 2017, 6, 1. [Google Scholar] [CrossRef]
- Ocimati, W.; Were, E.; Tazuba, A.F.; Dita, M.; Zheng, S.J.; Blomme, G. Spent Pleurotus ostreatus Substrate Has Potential for Managing Fusarium Wilt of Banana. J. Fungi 2021, 7, 946. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, I.C.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, phydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Santra, H.K.; Maity, S.; Banerjee, D. Production of bioactive compounds with broad spectrum bactericidal action, bio-film inhibition and antilarval potential by the secondary metabolites of the endophytic fungus Cochliobolus sp. APS1 isolated from the Indian medicinal herb Andrographis paniculata. Molecules 2022, 27, 1459. [Google Scholar] [PubMed]
- Han, E.H.; Hwang, Y.P.; Kim, H.G.; Choi, J.H.; Im, J.H.; Yang, J.H. Inhibitory effect of Pleurotus eryngii extracts on the activities of allergic mediators in antigen stimulated mast cells. Food Chem. Toxicol. 2011, 49, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.H.; Ng, T. A ribonuclease with antimicrobial, antimitogenic and antiproliferative activities from the edible mushroom Pleurotus sajor-caju. Peptides 2004, 25, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Pieczonka, A.M.; Rachwalski, M.; Leśniak, S.; Stączek, P. Synthesis and Evaluation of Biological Activities of Aziridine Derivatives of Urea and Thiourea. Molecules 2018, 23, 45. [Google Scholar] [CrossRef]
- Törős, G.; El-Ramady, H.; Prokisch, J.; Velasco, F.; Llanaj, X.; Nguyen, D.H.H.; Peles, F. Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus ostreatus Mushroom. Foods 2023, 12, 2010. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).