Determination of Critical Storage Conditions for Spray-Dried Habanero Pepper (Capsicum chinense) Extracts by Coupling Water Adsorption Isotherms and Glass Transition Temperature †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microparticles of Habanero Pepper Ethanolic Extract
2.2. Water Vapor Adsorption Isotherms
2.3. Calorimetric Analysis
2.4. Changes in Surface Color
3. Results and Discussions
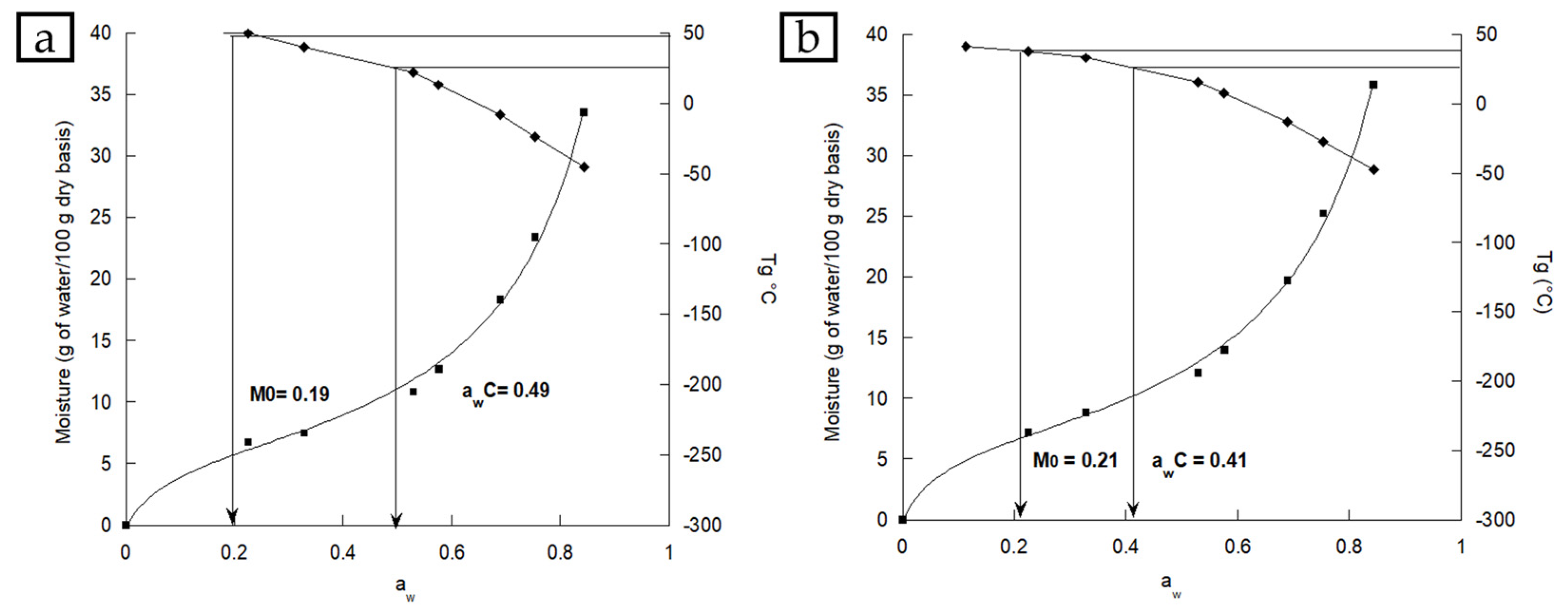
Adsorption Isotherms and Critical Storage Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olguín Rojas, J.A.; Vázquez-León, L.A.; Salgado-Cervantes, M.A.; Fernandez-Barbero, G.; Díaz-Pacheco, A.; García-Alvarado, M.A.; Rodriguez-Jimenes, G.C. Water and Phytochemicals Dynamic during Drying of Red Habanero Chili Pepper (Capsicum chinense) Slices. Rev. Mex. De Ing. Quim. 2019, 18, 851–864. [Google Scholar] [CrossRef]
- Fabela-Morón, M.F.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T.; Pacheco, N. Trends in Capsaicinoids Extraction from Habanero Chili Pepper (Capsicum chinense Jacq.): Recent Advanced Techniques. Food Rev. Int. 2020, 36, 105–134. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioproc. Tech. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of Capsaicin and Its Implications for Drug Delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Roos, Y.H. Water Activity and Physical State Effects on Amorphous Food Stability. J. Food Process Preserv. 1993, 16, 433–447. [Google Scholar] [CrossRef]
- Pascual-Pineda, L.A.; Rascón, M.P.; Quintanilla-Carvajal, M.X.; Castillo-Morales, M.; Marín, U.R.; Flores-Andrade, E. Effect of Porous Structure and Spreading Pressure on the Storage Stability of Red Onion Microcapsules Produced by Spray Freezing into Liquid Cryogenic and Spray Drying. J. Food Eng. 2019, 245, 65–72. [Google Scholar] [CrossRef]
- Rahman, M.S.; Labuza, T.P. Water Activity and Food Preservation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007; pp. 465–494. [Google Scholar]
- Flores-Andrade, E.; Bonilla, E.; Luna-Solano, G.; Marín, U.R.; González-Arnao, M.T.; Rascón, M.P. Effect of the Microstructure on the Stability of Red Onion Microcapsules. Dry. Technol. 2019, 37, 223–231. [Google Scholar] [CrossRef]
- Lang, K.W.; McCune, T.D.; Steinberg, M.P. A Proximity Equilibration Cell for Rapid Determination of Sorption Isotherms. J. Food Sci. 1981, 46, 936–938. [Google Scholar] [CrossRef]
- Staudt, P.B.; Kechinski, C.P.; Tessaro, I.C.; Marczak, L.D.F.; Soares, R.d.P.; Cardozo, N.S.M. A New Method for Predicting Sorption Isotherms at Different Temperatures Using the BET Model. J. Food Eng. 2013, 114, 139–145. [Google Scholar] [CrossRef]
- Oswin, C.R. The Kinetics of Package Life. III. The Isotherm. J. Soc. Chem. Ind. 1946, 65, 419–421. [Google Scholar] [CrossRef]
- Lewicki, P.P. A Three Parameter Equation for Food Moisture Sorption Isotherms. J. Food Process Eng. 1998, 21, 127–144. [Google Scholar] [CrossRef]
- Lomauro, C.J.; Bakshi, A.S.; Labuza, T.P. Evaluation of Food Moisture Sorption Isotherm Equations Part II: Milk, Coffee, Tea, Nuts, Oilseeds, Spices and Starchy Foods. LWT-Food Sci. Technol. 1985, 18, 118–124. [Google Scholar]
- Gordon, M.; Taylor, J.S. Ideal Copolymers and the Second-order Transitions of Synthetic Rubbers. I. Non-crystalline Copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of Colour Change of Kiwifruits during Hot Air and Microwave Drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Kaymak-Ertekin, F.; Gedik, A. Sorption Isotherms and Isosteric Heat of Sorption for Grapes, Apricots, Apples and Potatoes. LWT-Food Sci. Technol. 2004, 37, 429–438. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van Der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Tonon, R.V.; Baroni, A.F.; Brabet, C.; Gibert, O.; Pallet, D.; Hubinger, M.D. Water Sorption and Glass Transition Temperature of Spray Dried Açai (Euterpe oleracea Mart.) Juice. J. Food Eng. 2009, 94, 215–221. [Google Scholar] [CrossRef]
- Díaz, D.I.; Lugo, E.; Pascual-Pineda, L.A.; Jiménez-Fernández, M. Encapsulation of Carotenoid-Rich Paprika Oleoresin through Traditional and Nano Spray Drying. Ital. J. Food Sci. 2019, 31, 125–138. [Google Scholar] [CrossRef]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-López, J.A. Production of a Red–Purple Food Colorant from Opuntia stricta Fruits by Spray Drying and Its Application in Food Model Systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Shirkole, S.S.; Sutar, P.P. Modeling Sorption Phenomena and Moisture Migration Rates in Paprika (Capsicum annuum L.) Using Physicochemical Characteristics. J. Food Sci. Technol. 2018, 55, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, S.; Hong, Y.K.; Qu, Z.; Sablani, S.S. State Diagram, Water Sorption Isotherms and Color Stability of Pumpkin (Cucurbita pepo L.). J. Food Eng. 2020, 273, 109820. [Google Scholar] [CrossRef]
- Mosquera, L.H.; Moraga, G.; de Córdoba, P.F.; Martínez-Navarrete, N. Water Content–Water Activity–Glass Transition Temperature Relationships of Spray-Dried Borojó as Related to Changes in Color and Mechanical Properties. Food Biophys. 2011, 6, 397–406. [Google Scholar] [CrossRef]

| Model | Parameter | MD | MDSP |
|---|---|---|---|
| GAB | (g of H2O/100 g) d.s.) | 6.17 | 6.79 |
| 12.21 | 14.64 | ||
| 0.97 | 0.96 | ||
| R2 | 0.99 | 0.99 | |
| E% | 4.57 | 3.17 | |
| LEWICKI | A | 11.07 | 12.28 |
| B | 0.34 | 0.36 | |
| R2 | 0.99 | 0.99 | |
| E% | 21.74 | 7.75 | |
| OSWIN | A | 11.07 | 12.28 |
| B | 0.65 | 0.63 | |
| R2 | 0.99 | 0.99 | |
| E% | 7.86 | 21.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín Castro, U.R.; Cansino Jácome, F.; Olguín-Rojas, J.A.; Rodríguez-Jimenes, G.d.C.; González Arnao, M.T.; Flores Andrade, E.; Rascón Díaz, M.P. Determination of Critical Storage Conditions for Spray-Dried Habanero Pepper (Capsicum chinense) Extracts by Coupling Water Adsorption Isotherms and Glass Transition Temperature. Biol. Life Sci. Forum 2023, 26, 97. https://doi.org/10.3390/Foods2023-151
Marín Castro UR, Cansino Jácome F, Olguín-Rojas JA, Rodríguez-Jimenes GdC, González Arnao MT, Flores Andrade E, Rascón Díaz MP. Determination of Critical Storage Conditions for Spray-Dried Habanero Pepper (Capsicum chinense) Extracts by Coupling Water Adsorption Isotherms and Glass Transition Temperature. Biology and Life Sciences Forum. 2023; 26(1):97. https://doi.org/10.3390/Foods2023-151
Chicago/Turabian StyleMarín Castro, Ubaldo Richard, Fernando Cansino Jácome, José Arturo Olguín-Rojas, Guadalupe del Carmen Rodríguez-Jimenes, María Teresa González Arnao, Enrique Flores Andrade, and Martha Paola Rascón Díaz. 2023. "Determination of Critical Storage Conditions for Spray-Dried Habanero Pepper (Capsicum chinense) Extracts by Coupling Water Adsorption Isotherms and Glass Transition Temperature" Biology and Life Sciences Forum 26, no. 1: 97. https://doi.org/10.3390/Foods2023-151
APA StyleMarín Castro, U. R., Cansino Jácome, F., Olguín-Rojas, J. A., Rodríguez-Jimenes, G. d. C., González Arnao, M. T., Flores Andrade, E., & Rascón Díaz, M. P. (2023). Determination of Critical Storage Conditions for Spray-Dried Habanero Pepper (Capsicum chinense) Extracts by Coupling Water Adsorption Isotherms and Glass Transition Temperature. Biology and Life Sciences Forum, 26(1), 97. https://doi.org/10.3390/Foods2023-151






