Instrumental Evaluation of Selected Properties of Oil Extracted from Walnuts before and after the Roasting Process †
Abstract
:1. Introduction
2. Methods
2.1. Fatty Acid Composition
2.2. Oxidative Stability
2.3. Acid Value
2.4. Peroxide Value
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krajewska, M.; Zdybel, B.; Andrejko, D.; Śląska-Grzywna, B.; Tańska, M. Właściwości chemiczne wybranych olejów tłoczonych na zimno. Acta Agrophysica 2017, 24, 579–590. [Google Scholar]
- Ferreira, I.J.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M. Green emerging extraction technologies to obtain high-quality vegetable oils from nuts: A review. Innov. Food Sci. Emerg. Technol. 2022, 76, 102931. [Google Scholar] [CrossRef]
- Garcia-Aloy, M.; Hulshof, P.J.M.; Estruel-Amades, S.; Osté, M.C.J.; Lankinen, M.; Geleijnse, J.M.; de Goede, J.; Ulaszewska, M.; Mattivi, F.; Bakker, S.J.L.; et al. Biomarkers of food intake for nuts and vegetable oils: An extensive literature search. Genes Nutr. 2019, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Cofán, M.; Sala-Vila, A.; Haddad, E.; Serra-Mir, M.; Bitok, E.; Roth, I.; Freitas-Simoes, T.M.; Kaur, A.; Valls-Pedret, C.; et al. Effects of Walnut Consumption for 2 Years on Lipoprotein Subclasses among Healthy Elders: Findings from the WAHA Randomized Controlled Trial. Circulation 2021, 144, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Cao, Y.; Liu, R.; Jin, Q.; Wang, X. Phytochemical content, minor-constituent compositions, and antioxidant capacity of screw-pressed walnut oil obtained from roasted kernels. Eur. J. Lipid Sci. Technol. 2019, 121, 1800292. [Google Scholar] [CrossRef]
- Masoodi, L.; Gull, A.; Masoodi, F.A.; Gani, A.; Nissar, J.; Ahad, T.; Nayik, G.A.; Mukarram, S.A.; Kovács, B.; Prokisch, J.; et al. An Overview on Traditional vs. Green Technology of Extraction Methods for Producing High Quality Walnut Oil. Agronomy 2022, 12, 2258. [Google Scholar] [CrossRef]
- Babiker, E.E.; Uslu, N.; Al Juhaimi, F.; Ahmed, I.A.M.; Ghafoor, K.; Özcan, M.M.; Almusallam, I.A. Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT 2021, 139, 110537. [Google Scholar] [CrossRef]
- ISO 5509:2000; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2000.
- Bryś, J.; Vaz Flores, I.F.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC methods to characterize human milk fat substitutes obtained from lard and milk thistle oil mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the oxidative stability of linseed (Linum usitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef] [PubMed]
- ISO 660:2009; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 3960:2007; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. International Organization for Standardization: Geneva, Switzerland, 2007.
- Krygier, K.; Ratusz, K.; Supeł, B. Jakość i stabilność olejów tłoczonych na zimno. Rośliny Oleiste-Oilseed Crops 1995, 16, 307–313. [Google Scholar]
- Poggetti, L.; Ferfuia, C.; Chiabà, C.; Testolin, R.; Baldini, M. Kernel oil content and oil composition in walnut (Juglans regia L.) accessions from north-eastern Italy. J. Sci. Food Agric. 2018, 98, 955–962. [Google Scholar] [CrossRef] [PubMed]
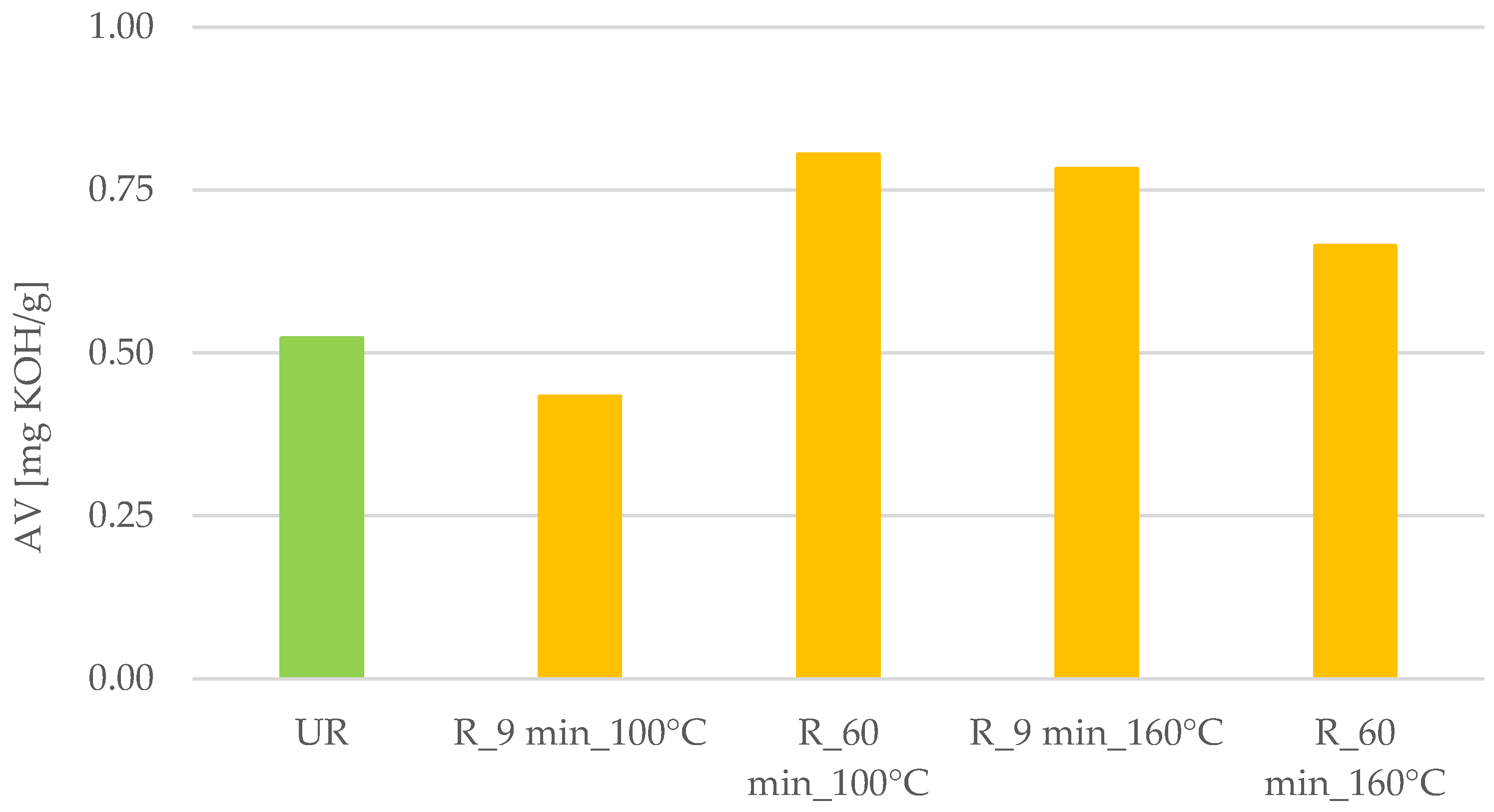


| Fatty Acid | UR | R 9 min_100 °C | R 60 min_100 °C | R 9 min_160 °C | R 60 min_160 °C |
|---|---|---|---|---|---|
| C16:0 | 7.50 ± 1.22 | 6.90 ± 0.37 | 7.55 ± 1.05 | 7.51 ± 0.61 | 6.96 ± 0.11 |
| C16:1 | 0.12 ± 0.02 | 0.14 ± 0.06 | 0.13 ± 0.02 | 0.13 ± 0.03 | 0.12 ± 0.01 |
| C17:0 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.06 | 0.08 ± 0.01 |
| C17:1 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.05 | 0.07 ± 0.02 |
| C18:0 | 2.15 ± 0.08 | 2.54 ± 0.05 | 2.42 ± 0.02 | 2.27 ± 0.11 | 2.34 ± 0.11 |
| C18:1 n-9 | 18.52 ± 0.25 | 19.43 ± 0.03 | 18.70 ± 0.06 | 18.77 ± 0.35 | 18.21 ± 0.52 |
| C18:2 n-6 | 59.01 ± 0.82 | 58.22 ± 0.18 | 58.43 ± 0.93 | 58.36 ± 0.08 | 58.93 ± 0.42 |
| C18:3 n-3 | 12.12 ± 0.17 | 12.26 ± 0.01 | 12.21 ± 0.08 | 12.00 ± 0.68 | 12.51 ± 0.33 |
| C20:0 | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.03 | 0.21 ± 0.14 | 0.14 ± 0.04 |
| C20:1 | 0.18 ± 0.03 | 0.25 ± 0.04 | 0.28 ± 0.04 | 0.36 ± 0.13 | 0.31 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryś, J.; Stańczak, A.; Skwierczyńska, A.; Adamczuk, U. Instrumental Evaluation of Selected Properties of Oil Extracted from Walnuts before and after the Roasting Process. Biol. Life Sci. Forum 2023, 26, 100. https://doi.org/10.3390/Foods2023-15161
Bryś J, Stańczak A, Skwierczyńska A, Adamczuk U. Instrumental Evaluation of Selected Properties of Oil Extracted from Walnuts before and after the Roasting Process. Biology and Life Sciences Forum. 2023; 26(1):100. https://doi.org/10.3390/Foods2023-15161
Chicago/Turabian StyleBryś, Joanna, Artur Stańczak, Aneta Skwierczyńska, and Urszula Adamczuk. 2023. "Instrumental Evaluation of Selected Properties of Oil Extracted from Walnuts before and after the Roasting Process" Biology and Life Sciences Forum 26, no. 1: 100. https://doi.org/10.3390/Foods2023-15161
APA StyleBryś, J., Stańczak, A., Skwierczyńska, A., & Adamczuk, U. (2023). Instrumental Evaluation of Selected Properties of Oil Extracted from Walnuts before and after the Roasting Process. Biology and Life Sciences Forum, 26(1), 100. https://doi.org/10.3390/Foods2023-15161







