Abstract
Platelet function is closely linked to cardiovascular health. Functional foods such as yogurt and oily fish are enriched in bioactive polar lipids, which can reduce platelet activation and the incidence of cardiovascular disease. This pilot project investigated the effect of fermented ovine yogurt on platelet sensitivity in human plasma. Overall, in vitro studies established that yogurt lipid fractions inhibit platelet aggregation. Preliminary dietary intervention data indicate that yogurt intake may reduce platelet activation against the thrombin pathway, compared to the placebo. However, larger studies are required to robustly establish the effect of yogurt intake on platelet sensitivity, following this interim analysis.
1. Introduction
While a measured immune response is a necessary protective physiological reaction in response to tissue damage or pathogenic insults, unresolved inflammation is implicated in the pathology of many chronic diseases [1]. The initiation of cardiovascular diseases (CVD) and other conditions have been linked to low-grade systemic inflammation [2,3]. Platelets are important effectors of these immune responses via mechanisms such as the recruitment of neutrophils to the site of inflammation and by the release of proinflammatory factors and chemokines that further activate leukocytes and intensify the immune response [4,5]. Platelet activity can be modulated by stimuli such as platelet agonists which contribute to platelet aggregation, or platelet inhibitors, which deactivate platelets and discourage the formation of thromboses [6]. Thrombin receptor activator peptide-6 (TRAP-6) is a peptide fragment that selectively activates the thrombin receptor protease-activated receptor 1 (PAR-1), thus inducing platelet activation similar to thrombin. Thrombin is a serine protease that is a key enzyme in the coagulatory pathway. It signals by binding to the protease-activated receptors (PARs), which are a family of transmembrane peptide receptors [7,8]. Platelet-activating factor (PAF) is a phospholipid mediator of inflammation that also induces platelet activation and aggregation. PAF functions by binding to the PAF receptor (PAF-R), a G-protein coupled receptor (GPCR) located on cells involved in immune function [9,10].
The consumption of foods or nutraceuticals rich in bioactive lipids (dietary polar lipids) that target platelet signalling pathways such as thrombin and PAF may be beneficial against inflammatory diseases [10]. However, the research is still at a preliminary stage to date, which necessitates the need for further study. Numerous studies have been conducted to investigate the cardioprotective properties of polar lipids such as marine and dairy-derived lipids in vitro [11,12,13,14]. These dietary polar lipids appear to inhibit platelet aggregation induced by agonists such as PAF and TRAP-6.
Ovine or sheep’s milk has been noted for its many potential health benefits besides its rich nutritional content, such as its anti-inflammatory, antiplatelet, and antimicrobial properties [15,16]. The fermentation of ovine milk has been shown to further enhance its antithrombotic properties by altering the fatty acid profile of monounsaturated fatty acids (MUFA) [13,14].
The aim of this conference paper was to conduct an interim analysis on our pilot study that aims to assess the antiplatelet properties of an ovine yogurt drink (YD) enriched in polar lipids using platelet aggregometry in vitro and to assess the effect of its long-term consumption on platelet sensitivity in a randomized controlled trial.
2. Materials and Methods
2.1. Materials and Instrumentation
All consumables and solvents were purchased from Fisher Scientific Ltd. (Dublin, Ireland). 22G safety needles and evacuated S-monovettes were purchased from Sarstedt Ltd (Wexford, Ireland). Platelet aggregometry consumables were purchased from Labmedics LLP (Abingdon on Thames, U.K.). Other reagents and chemicals were purchased from Sigma Aldrich (Wicklow, Ireland). Platelet aggregation testing was performed on a Chronolog-490 two-channel turbidimetric platelet aggregometer and its accompanying AGGRO/LINK software (version 5.2.5).
2.2. Production of the Yogurt Drink and Placebo Yogurt Drink
Ewe’s whole milk was obtained from Velvet Cloud Ltd. (Claremorris, Co Mayo, Ireland), from the Friesland and Lacaune breeds of dairy ewes. The percentage of fat in the raw milk was approximately 7%. The milk was pasteurised on site by heating to 91 °C for 15 s, and then cooled to 42 °C before being packaged and refrigerated (4 °C ± 1 °C) F.
After pasteurization, the milk was divided into two smaller batches and refrigerated. From one of these batches, skimmed milk was produced by double centrifugation: initially by centrifugation at 6900 × g for 30 min at 40 °C and afterwards at 7440× g for 30 min also at 40 °C. After both centrifugations, the settled layer of fat was removed to produce skimmed milk. Both milk batches were then inoculated with commercially available YO-MIX 205 LYO 250 DCU starter culture (Danisco France SAS, France; 5 mg/100 mL whole milk) to induce fermentation at a temperature of 40.5 °C for a period of 7 h. The milk batches were placed in a water bath to ferment and reach a pH = 4.6 and desired consistency (thick and creamy). The cultures contained the following probiotics: Streptococcus thermophilus, Lactococcus delbrueckii subsp. Bulgaricus, Lactobacillus acidophilus, and Bifidobacterium lactis. After fermentation, the milk was then poured into 250 mL bottles and sealed.
2.3. Extraction of Lipids and Determination of Nutrient Content
The total lipids (TL) of a yogurt drink (YD) and a placebo drink (PD) were extracted from 100 g samples according to the Bligh and Dyer technique [17] (Table 1, Section 3.1). Total neutral lipids (TNL) and total polar lipids (TPL) were further isolated from TL with the counter-current distribution method [18]. Samples were stored under nitrogen at −20 °C.
2.4. In Vitro Assessment of Antithrombotic Properties of Lipid Fractions against TRAP-6 and PAF-Induced Platelet Aggregation
Platelet inhibition was assessed for each lipid fraction using platelet aggregometry, as detailed in Table 2 (Section 3.2). Various subclasses of TPL were isolated using preparative thin-layer chromatography (TLC) and the IC50 of these was determined similarly (Table 3, Section 3.3). The half-maximal inhibitory concentration (IC50) was expressed as the mean mass of lipid fraction (µg) in the aggregometer cuvette ± standard deviation (SD). All experiments were performed in triplicate.
2.5. Dietary Intervention Study of Irish Ovine Yogurt Drink
The dietary intervention was designed as a randomized double-blind crossover study, with the control group provided a placebo drink containing significantly lower amounts of polar lipids (n = 18), produced as described in Section 2.2. Two study phases were organized with equally divided groups, and each phase of trial was conducted for 28 days. Figure 1 is a schematic representation of the study design.
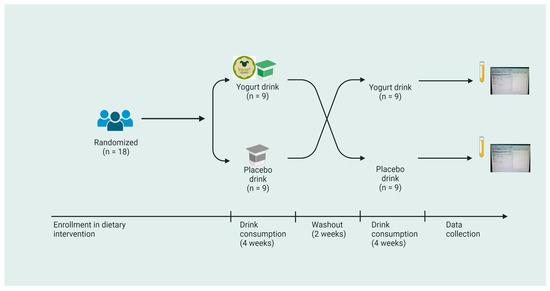
Figure 1.
Schematic representation of the design of the crossover dietary intervention study. Eighteen participants were recruited in the randomized study, with equal numbers assigned 250 gm of either the yogurt drink or placebo drink. Blood sampling was performed at four timepoints with a washout period of two weeks prior to the crossover phase.
3. Results
3.1. Yield of Lipid Fractions Isolated from Ovine Yogurt Drink
A side-by-side comparison of the ovine yogurt drink and placebo drink with respect to their nutritional content is given in Table 1. The yogurt drink was significantly higher in fat content compared to the placebo (prepared from skimmed milk).

Table 1.
Yield of the lipid fractions (TL, total lipid; PL, polar lipids; NL, neutral lipids) from raw sheep’s milk, skimmed milk-derived placebo drink, and full fat yogurt drink (g/100 mL). Data are presented as mean ± SD.
Table 1.
Yield of the lipid fractions (TL, total lipid; PL, polar lipids; NL, neutral lipids) from raw sheep’s milk, skimmed milk-derived placebo drink, and full fat yogurt drink (g/100 mL). Data are presented as mean ± SD.
| Samples (100 mL) | Total Lipids (TL) in g | Polar lipids (PL) in g | Neutral lipids (NL) in g |
|---|---|---|---|
| Raw ovine milk | 9.96 ± 0.30 a | 0.16 ± 0.01 a | 8.24 ± 0.23 a |
| Yogurt drink | 8.17 ± 0.22 a | 0.15 ± 0.004 a | 7.75 ± 0.19 a |
| Skimmed milk-derived yogurt drink (Placebo) | 0.36 ± 0.01 b | 0.06 ± 0.002 b | 0.29 ± 0.01 b |
a,b, Different superscripts in the same column indicate significant differences among different lipid extracts within the same lipid class (p < 0.05) when means are compared using a Tukey’s HSD multiple comparison test. Polar lipids (PL) are amphiphilic and possess a hydrophilic head and hydrophobic tail. Neutral lipids (NL) have a hydrophobic structure.
3.2. In Vitro Assessment of Antithrombotic Properties of Lipid Fractions against TRAP-6 and PAF-Induced Platelet Aggregation
Lipid fractions (TL, TNL, and TPL) were extracted and assessed for bioactivity against PAF and TRAP-6 (Table 2).

Table 2.
Inhibitory effects (IC50 values) of polar lipids (PL), neutral lipids (NL), and total lipids (TL) isolated from the ovine yogurt drink against human platelet aggregation induced by PAF and TRAP-6 in vitro. Data are expressed as mean ± SD.
Table 2.
Inhibitory effects (IC50 values) of polar lipids (PL), neutral lipids (NL), and total lipids (TL) isolated from the ovine yogurt drink against human platelet aggregation induced by PAF and TRAP-6 in vitro. Data are expressed as mean ± SD.
| IC50 against TRAP-6 (µg) | IC50 against PAF (µg) | ||
|---|---|---|---|
| Yogurt drink | YPL | 312.95 ± 15.04 | 156.72 ± 5.88 |
| YNL | 259.41 ± 13.56 | 729.15 ± 35.079 | |
| YTL | 209.57 ± 10.11 | 248.23 ± 11.39 | |
| Placebo (skimmed milk) | YPL | 45.8 ± 2.05 | 44.0 ± 1.98 |
| YNL | 47.37 ± 2.14 | 47.20 ± 2.13 | |
| YTL | 59.16 ± 3.13 | 53.84 ± 2.85 |
3.3. In Vitro Assessment of Antithrombotic Properties of Each PL Subclass towards TRAP-6 and PAF-Induced Platelet Aggregation in PRP, Expressed as Mean ± SD
Table 3 depicts the in vitro inhibition of the subclasses of yogurt TPL in response to PAF and TRAP-6-induced platelet aggregation, represented as IC50 values for each TLC fraction. The lipid fractions corresponding to PC (phosphatidylcholine) had the lowest IC50 values against PAF and TRAP and consequently the highest biological activity, closely followed by the PE fraction against PAF.

Table 3.
Antiplatelet activity of each PL subclass towards PAF-induced platelet aggregation in PRP, expressed as mean ± SD.
Table 3.
Antiplatelet activity of each PL subclass towards PAF-induced platelet aggregation in PRP, expressed as mean ± SD.
| Yogurt Drink Polar Lipid Fractions | EC50 (−) or IC50 against PAF (µg) | IC50 against TRAP-6 (µg) | |
|---|---|---|---|
| 1 | LPC | −48.1 ± 3.9 | 348.6 ± 7.9 |
| 2 | SM | 1120 ± 54 | 250.6 ± 8.3 |
| 3 | PC | 169.5 ± 8.3 | 205.3 ± 8.9 |
| 4 | CL | ND | 208.0 ± 10.8 |
| 5 | PE | 196.7 ± 6.4 | 592.1 ± 8.8 |
Abbreviations: CL, cardiolipin; LPC, lyso-phosphatidylcholine; ND, not determined; PAF, platelet-activating factor; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin; TRAP-6, thrombin receptor activator peptide 6.
3.4. Assessment of the Impact of Dietary Yogurt Intake on Platelet Sensitivity against TRAP-6
Table 4 depicts the effect of yogurt consumption on TRAP-6 induced platelet activity, compared to placebo, at different timepoints during the study. Overall, a trend of increased EC50 in the YD cohort was observed, which would indicate reduced platelet activation status, although these data were not statistically significant. Further particpants are required to draw conclusive evidence from this study.

Table 4.
The effect of yogurt (YD) consumption on platelet activation by TRAP-6, expressed as the mean ± SD ΔEC50 at different timepoints.
4. Discussion
Research has shown that fermented ovine dairy has potent antiplatelet effects against the thrombin and pathway of platelet activation in vitro in human PRP [19]. The effect of the daily intake of yogurt on the PAF pathway of platelet activation has also been demonstrated as a dietary intervention study, although no intervention studies so far have demonstrated the ex vivo effect of yogurt on platelet activation through the thrombin pathway. Due to funding constraints, the present study did not investigate the effect of non-fermented ovine milk on the TRAP-6 platelet activation pathway. However, in vitro data suggest that there is less potent activity of non-fermented milk vs. fermented yogurt. Thus, the present dietary intervention study sought to determine the effect of consumption of an ovine yogurt drink (YD) enriched with polar lipids over 28 days on baseline platelet reactivity, through the thrombin pathway of activation. In vitro analyses found that yogurt lipids inhibit platelet activation through both the PAF and thrombin pathways. While we observed a trend indicating that yogurt intake may reduce platelet activation through the thrombin pathway in human volunteers, studies at a larger scale are required to establish the impact of yogurt lipids more robustly on platelet activity.
Author Contributions
Conceptualization, I.Z., A.T. and R.L.; methodology, I.Z., S.H., R.L. and H.R.; formal analysis, S.H., H.R. and R.L.; investigation, S.H., H.R., K.S. and S.K.S.; resources, I.Z.; data curation, H.R. and S.H.; writing—original draft preparation, S.H. and R.L.; writing—review and editing, R.L., S.H. and I.Z; visualization, S.H. and H.R.; supervision, I.Z.; project administration, I.Z., H.R., R.L. and S.H.; funding acquisition, I.Z., A.T. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Enterprise Ireland, grant number IP-2021-0972.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of University of Limerick (ethical approval code 2022_01_01_S&E, approved 16 February 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to the volunteers who took part in the study and to Elaine Ahern for her phlebotomy support. The authors acknowledge the financial support of Enterprise Ireland (study grant reference: IP-2021-0972), the Velvet Cloud private company for their donation and help preparing the yogurt drinks and placebo drinks. We also thank the Department of Physical Education and Sports Science and the Department of Biological Sciences at the University of Limerick, Ireland, for their continued support and use of their facilities.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Neher, M.D.; Weckbach, S.; Flierl, M.A.; Huber-Lang, M.S.; Stahel, P.F. Molecular mechanisms of inflammation and tissue injury after major trauma--is complement the “bad guy”? J. Biomed. Sci. 2011, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Zotova, E.; Nicoll, J.A.; Kalaria, R.; Holmes, C.; Boche, D. Inflammation in Alzheimer’s disease: Relevance to pathogenesis and therapy. Alzheimers Res. Ther. 2010, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.Y.; Granger, D.N. Platelets: A critical link between inflammation and microvascular dysfunction. J. Physiol. 2012, 590, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Ollivier, V.; Ho-Tin-Noé, B. Platelets in Inflammation: Regulation of Leukocyte Activities and Vascular Repair. Front. Immunol. 2015, 5, 678. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, S.; Holmes, A.; Loscalzo, J. Platelets and cardiovascular disease. Eur. J. Cardiovasc. Nurs. 2002, 1, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 2000, 407, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Derhaschnig, U.; Spiel, A.; Keen, P.; Cardona, F.; Mayr, F.; Jilma, B. Regulation of protease-activated receptor 1 (PAR1) on platelets and responsiveness to thrombin receptor activating peptide (TRAP) during systemic inflammation in humans. Thromb. Haemost. 2003, 90, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.C.; Weyrich, A.S.; Zimmerman, G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie 2010, 92, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Harishkumar, R.; Hans, S.; Stanton, J.E.; Grabrucker, A.M.; Lordan, R.; Zabetakis, I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients 2022, 14, 4414. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Nasopoulou, C.; Tsoupras, A.; Zabetakis, I. The Anti-inflammatory Properties of Food Polar Lipids. In Bioactive Molecules in Food; Reference Series in Phytochemistry; Springer: Cham, Swizerland, 2018; pp. 1–34. [Google Scholar]
- Lordan, R.; Walsh, A.M.; Crispie, F.; Finnegan, L.; Cotter, P.D.; Zabetakis, I. The effect of ovine milk fermentation on the antithrombotic properties of polar lipids. J. Funct. Foods 2019, 54, 289–300. [Google Scholar] [CrossRef]
- Glenn-Davi, K.; Hurley, A.; Brennan, E.; Coughlan, J.; Shiels, K.; Moran, D.; Saha, S.K.; Zabetakis, I.; Tsoupras, A. Fermentation Enhances the Anti-Inflammatory and Anti-Platelet Properties of Both Bovine Dairy and Plant-Derived Dairy Alternatives. Fermentation 2022, 8, 292. [Google Scholar] [CrossRef]
- Lordan, R.; Walsh, A.; Crispie, F.; Finnegan, L.; Demuru, M.; Tsoupras, A.; Cotter, P.D.; Zabetakis, I. Caprine milk fermentation enhances the antithrombotic properties of cheese polar lipids. J. Funct. Foods 2019, 61, 103507. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Shinde, A.K.; Singh, R. Sheep milk: A pertinent functional food. Small Rumin. Res. 2019, 181, 6–11. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Galanos, D.S.; Kapoulas, V.M. Isolation of polar lipids from triglyceride mixtures. J. Lipid Res. 1962, 3, 134–136. [Google Scholar] [CrossRef]
- Lordan, R.; Vidal, N.P.; Huong Pham, T.; Tsoupras, A.; Thomas, R.H.; Zabetakis, I. Yoghurt fermentation alters the composition and antiplatelet properties of milk polar lipids. Food Chem. 2020, 332, 127384. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).