Abstract
Krill oil (KO) and the impregnation process to enrich food matrices with bioactive compounds have become relevant. The impregnation process of KO microcapsules into Golden apple tissue was evaluated in this study. A KO emulsion was used at different temperatures to impregnate Golden apple slices. The results show that an increase in temperature causes greater removal of water and penetration of the KO microcapsules. At 60 °C, the volume changes of the samples were evident, obtaining the highest concentration of astaxanthin. Through the impregnation process, it is possible to produce a new food product with potential functional properties.
1. Introduction
In recent years, people have been interested in new and better food products that incorporate bioactive compounds in their formulation, encouraging the development of new techniques aimed at the development of functional foods.
Microencapsulation is a process where certain biomolecules are coated with or protected by a material so that they do not participate in detrimental reactions with the surrounding environment. Some other benefits of microencapsulation include storage stability, controlled or retarded release of compounds, and volatile stabilization [1]. It has been shown that some functional compounds, such as oils, have beneficial health properties; however, their lipophilic nature makes it difficult to incorporate them into water-rich food matrices. One way to achieve the above is through impregnation—this process employs the osmotic pressure to introduce solutes from the hypertonic solution into the intercellular tissue of a food, partially eliminating the water contained in the food while at the same time impregnating it with a solute of interest (antioxidants, probiotics, oils, etc.), without affecting the integrity of the food [2]. Krill oil (KO) has been widely used and it has gained great relevance since it contains astaxanthin (AST), which is a biomolecule with great antioxidant power. Additionally, it contains eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), with proved beneficial effects on health [3]. The aim of this work was to evaluate the effect of temperature during the impregnation process of krill oil microcapsules into Golden apple slices and generate a new food product with potential effects on human health.
2. Materials and Methods
2.1. Materials
Golden apples of commercial ripeness and sucrose were purchased from a local supermarket in Orizaba, Veracruz, Mexico. Whey protein concentrate was obtained from Droguería Cosmopólita, Mexico, CDMX. Krill oil was purchased from Natura Extracta, Mexico, Guadalajara. Ethyl acetate was analytical grade.
2.2. Sample and Emulsion Preparation
A solution (30% solids w/w) of whey protein concentrate (WPC) was homogenized at 5000 rpm for 10 min; emulsions were made at a proportion of 1:4 (KO:WPC) by adding drop-wise KO and dispersing it into the first solution. Then, the solid concentration of the emulsion was adjusted to 60% (w/w) with sucrose. Finally, the emulsion was mixed for 30 min before being employed. Golden apples were manually peeled and axially sliced with dimensions as follows: a 36 mm diameter and 3.5 mm thick [2].
2.3. Impregnation Process
Each Golden apple slice was weighed and immersed into the sucrose-emulsion solution at 35, 45, and 60 °C, keeping a 1:20 (w/w) slice/solution ratio for 120 min. Samples were withdrawn at regular time intervals, and the excess of solution from the surface was blot-dried using paper towels. The method reported by Azuara et al. [4] was employed to evaluate the kinetics of the process; water loss (WL in g/g fresh fruit) and solid gain (SG in g/g fresh fruit) were calculated with the following equations:
where t represents the time of the impregnation process, s1 is a constant related to the water lost, WL∞ is the amount of water lost when equilibrium is reached, and, ML is the mass lost. WL∞ and s1 can be estimated with Equations (3) and (4):
WL = s1tWL∞/(1 + s1t)
SG = WL − ML
WL∞ = 1/[p(1 − (SG/WL)m)]
S1 = 1/[bWFL∞(1 − (SG/WL)m)]
The constants p and b are the slope and intercept, respectively, of the t/ML vs. t plot, while the subindex m means that WL and SG were calculated at the last point of each experiment using Equations (5) and (6), where M0 and Mf are the initial and final sample weight (g), respectively, and X0 and Xf are the initial and final moisture content (wet basis).
WL/M0 = (M0X0 − MfXf)/M0
SG/M0 = (M0(X0 − 1) − Mf(Xf − 1))/M0
2.4. Analysis of Samples Impregnated with KO Microcapsules
Initial and final moisture contents were obtained using the AOAC method (23.003:2003) [5]. For AST content, apple slices were withdrawn at 90 and 180 min, ground, and mixed manually with 15 mL of ethyl acetate for 10 min, and then subjected to vortexing for 10 min at 5000 rpm. The solvent phase was recuperated and its absorbance was recorded at 480 nm using a UV-Vis spectrophotometer (Thermo Scientific, MA, USA), and the astaxanthin concentration (μg/g) was determined using the following equation:
where A is the absorbance in nm; V corresponds to the total extract volume in mL; w stands for the sample weight in grams; and A(1%, 1cm) is the astaxanthin-specific absorbance in ethyl acetate (2150 nm) [6].
AST = AV104/w A(1%, 1cm)
3. Results and Discussion
The kinetics of WL during the osmotic dehydration/impregnation process are shown in Figure 1A; as can be seen, there is a greater WL as temperature increases, and a similar behavior was observed by Jiménez-Hernández et al. [7] in mango impregnated with inulin and piquin-pepper oleoresin. They argued that cell swelling and an increase in thermal energy due to temperature enhance water removal. The WL of apple slices was 0.713 ± 0.006, 0.696 ± 0.011, and 0.723 ± 0.002 g water/g fresh fruit, respectively, after 180 min of impregnation. Flores-Andrade et al. [8] reported a WL value of 0.75 during the vacuum impregnation of L. rhamnosus microcapsules into apple slices, and they observed that WL appeared to increase exponentially over the course of the process because of the differences in osmotic pressure between the fruit and the sucrose solution.
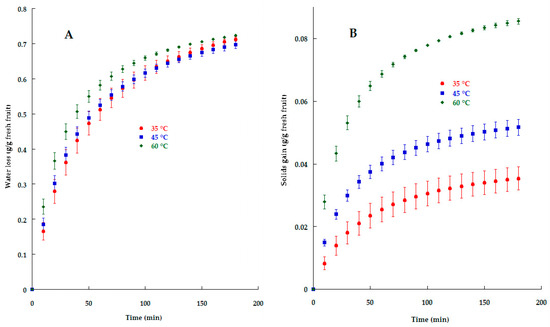
Figure 1.
Water loss (A) and solid gain (B) kinetics during KO microcapsules’ impregnation into apple slices at different temperatures.
The solid gain kinetics can be observed in Figure 1B, and there is a remarkable difference in SG when temperature increases. Some reasons for this phenomenon are the changes in cell membrane permeability and structural changes (compaction in the surface layers) that lead to a reduction in SG as well as WL.
Ahmed et al. [9] explained that during the solute impregnation process, a cell submerged in a hypertonic medium will lose water due to plasmolysis, which starts with dehydration of the protoplasm causing cell shrinkage, leading to plasmalemma’s separation from the cell wall; finally, the volume between the plasmalemma and cell wall gets filled with the osmotic solution due to the permeability of cell wall.
SG values for 35, 45, and 60 °C were 0.035 ± 0.003, 0.051 ± 0.002 and 0.085 ± 0.001 g water/g fresh fruit, respectively. Similar values were reported by Jiménez-Hernández et al. [7], who obtained 0.03 and 0.05 g water/g fresh fruit when the temperature of impregnation process was 30 and 40 °C, respectively; they observed that over the course of the process, the particle size of the impregnation solution has an influence, as large particles block pores on the fruit surface, diminishing solid gain and producing lower internal resistance, which favors WL by diffusion. Salazar López et al. [2] explained that fruit cells change their shape and reduce their size due to liquid loss, and they observed that the impregnation of oleoresin into pineapple tissue was a consequence of the internal gas/liquid replacement in open pores by the external liquid phase as a result of hydrodynamic mechanisms promoted by pressure changes.
Figure 2 shows the changes in volume and moisture content during the impregnation process. Three stages can be observed: during stage one, the water contained in the extra and intercellular tissue is lost, which causes that apple slice to reduce their size; in the second stage, intracellular water is released, leading to a protoplasm and vacuole size reduction, and during this stage, solute gain increases due the new intercellular spaces created among cells with a consequent porosity increase; and finally, the structure of the food starts to collapse and a volume reduction occurs.
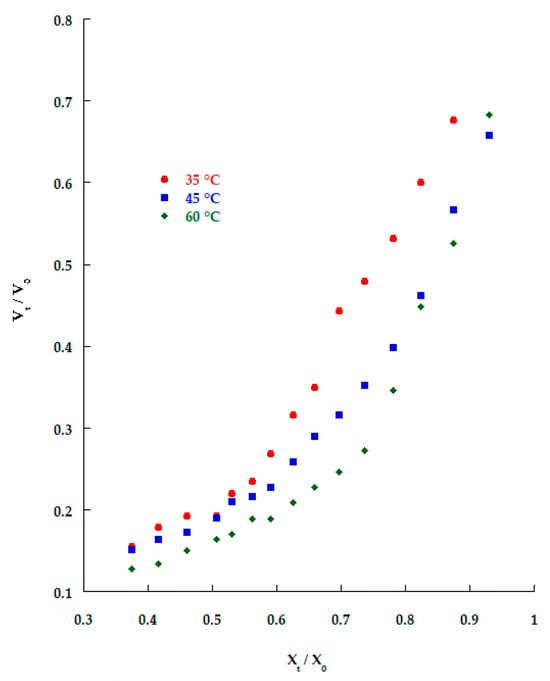
Figure 2.
Volume changes during KO microcapsules’ impregnation of apple slices at different temperatures.
According to Chiralt and Fito [10], cell water loss not only influences volume changes but also the cell membrane, since the lipid bilayer shows lyotropic and thermotropic mesomorphism, so a rupture of the bilayer structure of the membrane is observed and solutes diffuse freely to all parts of the tissue, not just to the open cellular spaces. Volume changes are also affected by the type of cellular packaging; in high-porosity materials, the total volume reduction may be lower than a compact structure because a smaller reduction in the gas phase volume mitigates the total volume change.
Nieto et al. [11] reported a decrease in shape and volume in apple slices during glucose and sucrose dehydration, and they observed that early in the osmotic process, the steep loss of water resulted in a decrease in the initially fully turgid cell volume and folding and deformation of cell walls, while other modifications at intercellular level include cell lumen deformation and shrinkage. As the osmotic process advances, slow relaxation of stressed cell walls may occur, and the shape of the cells may become spherical.
Changes in volume may also be attributed to a reduction in the internal gases of the food material; these gases are mainly located in pores and apple tissue, accounting for around 20% of the total food material [8].
Figure 3 shows the amount of astaxanthin impregnated into apple plates, and it can be seen that as the temperature and time increased, AST content increased as well, with the process performed at 60 °C providing the greatest AST content (1.4 μg/g).
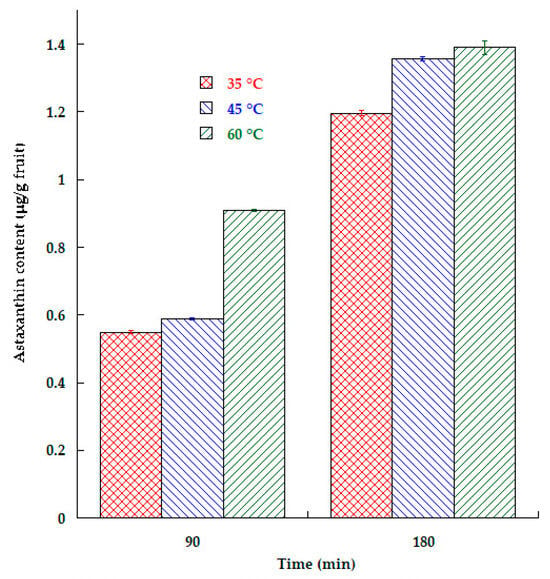
Figure 3.
Astaxanthin content in impregnated apple slices at different temperatures and time process.
Ertek et al. [12] observed that process temperature positively affects the total soluble solid content during the impregnation of strawberries with phenolic extracts. They argued that the most important factors that trigger mass transfer are concentration difference and process temperature.
Salazar et al. [2] reported 8.0 ± 0.4 g/kg of piquin-pepper microcapsules impregnated in pineapple rings, and later, Rascón et al. [13] reported 109 CFU/g of L. rhamnosus impregnated in banana tissue. Differences in the amount of bioactive molecules incorporated into cellular tissue could be attributed to the porosity of the food material, as when sample porosity is low, cells are densely packed, and intercellular spaces are reduced, making solute uptake difficult [10]. Apple pore diameter has been reported to be around 12 μm and the intercellular spaces are in the range of 172–2297 μm; due to these characteristics, it can host different bioactive compounds [14].
Osorio Gutierrez et al. [15] evaluated the impregnation process on apple slices, and they compared the effect of an emulsion or a solution of sucrose-jamaica for solid intake. Their results showed that solids intake was significantly increased when an emulsion was employed, and therefore the final amount of bioactive compounds impregnated was higher. At the end of the 120 min process, they incorporated 4.33 and 0.6 g of solids/100 g of dried fruit using an emulsion and a sucrose-jamaica solution, respectively. They also observed that, regardless of the osmotic solution used, a smooth surface was observed in the food and their cells displayed a reduction in shape and size because of the loss of water.
KO microcapsules’ impregnation into apple tissue can represent a new approach for developing a food product rich in antioxidants as well as essential fatty acids.
4. Conclusions
The impregnation of krill oil microcapsules using osmotic dehydration is a feasible method that can help to incorporate lipophilic biomolecules into a hydrophilic medium. The temperature of the impregnation process positively augmented solid gain and water loss, leading to higher astaxanthin incorporation into apple tissue. The information about the process evaluated in this work can help in the development of enriched food matrices with possible health benefits for the human population. Further studies need to be performed to evaluate the final product in terms of sensory acceptance and storage stability.
Author Contributions
Conceptualization, U.R.M.-C. and C.A.O.-S.; methodology, E.F.-A.; validation, M.C.M.; formal analysis, R.U.S.; investigation, F.E.G.J.; resources, N.G.C.; data curation, U.R.M.-C. and E.F.-A.; writing—original draft preparation, C.A.O.-S.; writing—review and editing, C.A.O.-S. and U.R.M.-C.; visualization, N.G.C.; supervision, R.U.S. and F.E.G.J.; project administration, M.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mazza, K.E.L.; Costa, A.M.M.; da Silva, J.P.L.; Alviano, D.S.; Bizzo, H.R.; Tonon, R.V. Microencapsulation of Marjoram Essential Oil as a Food Additive Using Sodium Alginate and Whey Protein Isolate. Int. J. Biol. Macromol. 2023, 233, 123478. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López, E.I.; Jiménez, M.; Salazar, R.; Azuara, E. Incorporation of Microcapsules in Pineapple Intercellular Tissue Using Osmotic Dehydration and Microencapsulation Method. Food Bioprocess Technol. 2015, 8, 1699–1706. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia Superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Azuara, E.; Beristain, C.I.; Gutiérrez, G.F. A Method for Continuous Kinetic Evaluation of Osmotic Dehydration. LWT-Food Sci. Technol. 1998, 31, 317–321. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemistry (AOAC). Official Methods of Analysis; AOAC: Arlington, VA, USA, 2023. [Google Scholar]
- Vakarelova, M.; Zanoni, F.; Lardo, P.; Rossin, G.; Mainente, F.; Chignola, R.; Menin, A.; Rizzi, C.; Zoccatelli, G. Production of Stable Food-Grade Microencapsulated Astaxanthin by Vibrating Nozzle Technology. Food Chem. 2017, 221, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Hernández, J.; Estrada-Bahena, E.B.; Maldonado-Astudillo, Y.I.; Talavera-Mendoza, Ó.; Arámbula-Villa, G.; Azuara, E.; Álvarez-Fitz, P.; Ramírez, M.; Salazar, R. Osmotic Dehydration of Mango with Impregnation of Inulin and Piquin-Pepper Oleoresin. LWT-Food Sci. Technol. 2017, 79, 609–615. [Google Scholar] [CrossRef]
- Flores-Andrade, E.; Pascual-Pineda, L.A.; Alarcón-Elvira, F.G.; Rascón-Díaz, M.P.; Pimentel-González, D.J.; Beristain, C.I. Effect of Vacuum on the Impregnation of Lactobacillus Rhamnosus Microcapsules in Apple Slices Using Double Emulsion. J. Food Eng. 2017, 202, 18–24. [Google Scholar] [CrossRef]
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in Osmotic Dehydration Technique for the Preservation of Fruits and Vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Chiralt, A.; Fito, P. Transport Mechanisms in Osmotic Dehydration: The Role of the Structure. Food Sci. Technol. Int. 2003, 9, 179–186. [Google Scholar] [CrossRef]
- Nieto, A.B.; Salvatori, D.M.; Castro, M.A.; Alzamora, S.M. Structural Changes in Apple Tissue during Glucose and Sucrose Osmotic Dehydration: Shrinkage, Porosity, Density and Microscopic Features. J. Food Eng. 2004, 61, 269–278. [Google Scholar] [CrossRef]
- Ertek, G.; Taştan, Ö.; Baysal, T. Combined Use of Vacuum Impregnation and Encapsulation Technologies for Phenolic Enrichment of Strawberries. Food Chem. 2023, 398, 133853. [Google Scholar] [CrossRef] [PubMed]
- Rascón, M.P.; Huerta-Vera, K.; Pascual-Pineda, L.A.; Contreras-Oliva, A.; Flores-Andrade, E.; Castillo-Morales, M.; Bonilla, E.; González-Morales, I. Osmotic Dehydration Assisted Impregnation of Lactobacillus Rhamnosus in Banana and Effect of Water Activity on the Storage Stability of Probiotic in the Freeze-Dried Product. LWT 2018, 92, 490–496. [Google Scholar] [CrossRef]
- Caballero, M.C.S.; Muñiz, C.H.A.; Leal, V.C.; Aguilar, G.G.; Hernández, C.G.S.; Quezada, G.D.A. Enrichment of Sliced Apple Fruit with Bacillus Coagulans. Emirates J. Food Agric. 2021, 33, 12. [Google Scholar] [CrossRef]
- Osorio Gutiérrez, F.X.; Peñaloza Ortiz, A.; Maldonado Astudillo, Y.I.; Jiménez Hernández, J.; Salazar, R.; Osorio Gutiérrez, F.X.; Peñaloza Ortiz, A.; Maldonado Astudillo, Y.I.; Jiménez Hernández, J.; Salazar, R. Evaluation of Osmotic Dehydration as a Tool for Enrichment with Bioactive. Rev. Mex. Cienc. Agrícolas 2019, 10, 1151–1156. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).