A Clinical Review of a Polyvalent F(ab’)2 Antivenom (InoserpTM PAN-AFRICA) in the Management of Snakebite Envenomation in Sub-Saharan Africa: Clinical Studies and Actual Use since Its Introduction in 2012 †
Abstract
:1. Introduction and Background
1.1. Objective of This Review
1.2. Use of InoserpTM PAN-AFRICA in Sub-Saharan Africa
1.3. Specificities of InoserpTM PAN-AFRICA
2. Methodology
- -
- Evaluation of a new polyvalent antivenom against snakebite envenomation (InoserpTM PAN-AFRICA) in two different epidemiological settings—Northern Benin and Maritime Guinea (Chippaux et al., 2015) [5]: This is the first clinical study performed with 209 patients treated overall. The study objective was to evaluate the clinical efficacy and safety of InoserpTM PAN-AFRICA in 2 countries with different SBE profiles: North Benin, where Echis ocellatus represents 75% of envenomation, and Maritime Guinea, which is well known for a high incidence of elapids bites (Naja and Dendroaspis gender).
- -
- Antivenom serotherapy in Mali: experience at Kati reference health center, Kouligoro region (Coulibaly et al., 2018) [6]: This single-center observational study was performed on 154 patients in a health center in west Mali with the objective of assessing the clinical efficacy and safety of InoserpTM PAN-AFRICA in a region with high incidence of Echis ocellatus bites.
- -
- Evaluation of the efficacy and tolerance of InoserpTM PAN-AFRICA in Senegal (Lam et al., 2019) [7]: This cohort multicenter study was performed on 63 patients in Senegal. The objective was to evaluate the safety and efficacy of InoserpTM PAN-AFRICA under realistic conditions at rural health facilities experiencing cytotoxic, hemotoxic, and neurotoxic envenomation.
- -
- Snakebites in Cameroon: evaluation of snake antivenom in Africa (ESAA) and real-life conditions (Chippaux et al., 2022) [8]: This is the largest multicenter clinical study performed with InoserpTM PAN-AFRICA. Overall, 250 patients have been treated in 14 clinical sites in Cameroon. The main objective was to study the short- and mid-term safety and efficacy profile of the antivenom in real conditions.
3. Results
3.1. Clinical Studies
3.1.1. Demographic Characteristics and SBE Syndromes
3.1.2. Efficacy and Safety Profile
3.2. Supporting Data
3.2.1. Published Patient Cases
3.2.2. Pharmacovigilance Surveillance
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chippaux, J.P. Estimate of the burden of snakebites in sub-Saharan Africa: A meta-analytic approach. Toxicon 2011, 57, 586–599. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO: Geneva, Switzerland, 2017. Available online: https://iris.who.int/bitstream/handle/10665/255657/9789241210133-eng.pdf?isAllowed=y&sequence=1#page=217 (accessed on 17 September 2019).
- Mathé, H.; Soria, R. A new generation of F(ab’)2 antivenoms made of highly purified and specific immunoglobulin fragments. In Proceedings of the Venoms and Toxins 2020, Oxford, UK, 16–17 September 2020. [Google Scholar]
- Martinez, M.; Almaguer, J.; Saldivar, A.; Soria, R.; Mathé, H. Preclinical evaluation of the polyspecific antivenom InoserpTM PAN-AFRICA against the venoms of elapids and viperids of Sub-Saharan Africa region: Neutralization of toxic activities. In Proceedings of the IST Congress 2019, Buenos Aires, Argentina, 8–13 September 2019. [Google Scholar]
- Chippaux, J.P.; Balde, M.C.; Sessinou, E.; Boiro, M.Y. Evaluation d’un nouvel antivenin polyvalent contre les envenimations ophidiennes (Inoserp Panafricain) dans deux contextes épidémiologiques: Le Nord Bénin et la Guinée Maritime. Med. Sante Trop. 2015, 25, 56–64. [Google Scholar] [PubMed]
- Coulibaly, S.K.; Diallo, T.; Diara, A.; Soulaymani, A.; Maiga, A.I. Sérothérapie antivenimeuse au Mali: Expérience du centre de santé de référence de Kati région de Koulikoro. Toxicol. Anal. Clin. 2018, 30, 165. [Google Scholar]
- Lam, A.; Cabral, M.; Touré, A.; Ba, F.; Camara, B.; Kane, O.; Fall, M.; Diouf, A.; Chippaux, J.-P. Evaluation de l’efficacité et la tolérance de Inoserp Panafricain au Sénégal. Toxicol. Anal. Clin. 2019, 31, 18–29. [Google Scholar]
- ESAA France and Cameroon Group. Snakebites in cameroon: Evaluation of snake antivenom in Africa (esaa) and real life conditions. In Proceedings of the Abstract at Venom Week Conference, Scottsdale, AZ, USA, 18–21 July 2022. [Google Scholar]
- Baldé, M.C.; Diallo, M.C.; Baldé, M.A.; Millimouno, E.; Chippaux, J.-P.; Bolro, M.Y. Défis et perspectives de la prise en charge des envenimations par élapidés en Guinée. Cas d’un patient traité avec Inoserp PAN-AFRICA. In Proceedings of the Poster at Actualités du Pharo, Marseille, France, 3–4 October 2019. [Google Scholar]
- Surugue, L. Why Is It so Hard to Stop People Dying from Snakebite? Online Publication. Mosaïcs in Science, 2019. Available online: https://web.archive.org/web/20190921045013/https://mosaicscience.com/story/snakebite-antivenom-crisis-Africa-Togo/ (accessed on 17 September 2019).
- Nicolon, T. Snakebites Are ‘Neglected’ Health Crisis in Africa; National Geographic: Washington, DC, USA, 2020. [Google Scholar]
- Chippaux, J.P. Prise en charge des morsures de serpent en Afrique subsaharienne. Med. Sante. Trop. 2015, 25, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Potet, J.; Smith, J.; Mclver, L. Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial. PLoS Negl. Trop. Dis. 2019, 13, e7551. [Google Scholar] [CrossRef] [PubMed]
| West Africa: Benin, Burkina Faso, Ghana, Guinea, Ivory Coast, Mali, Niger, Senegal, Sierra Leone, Togo | 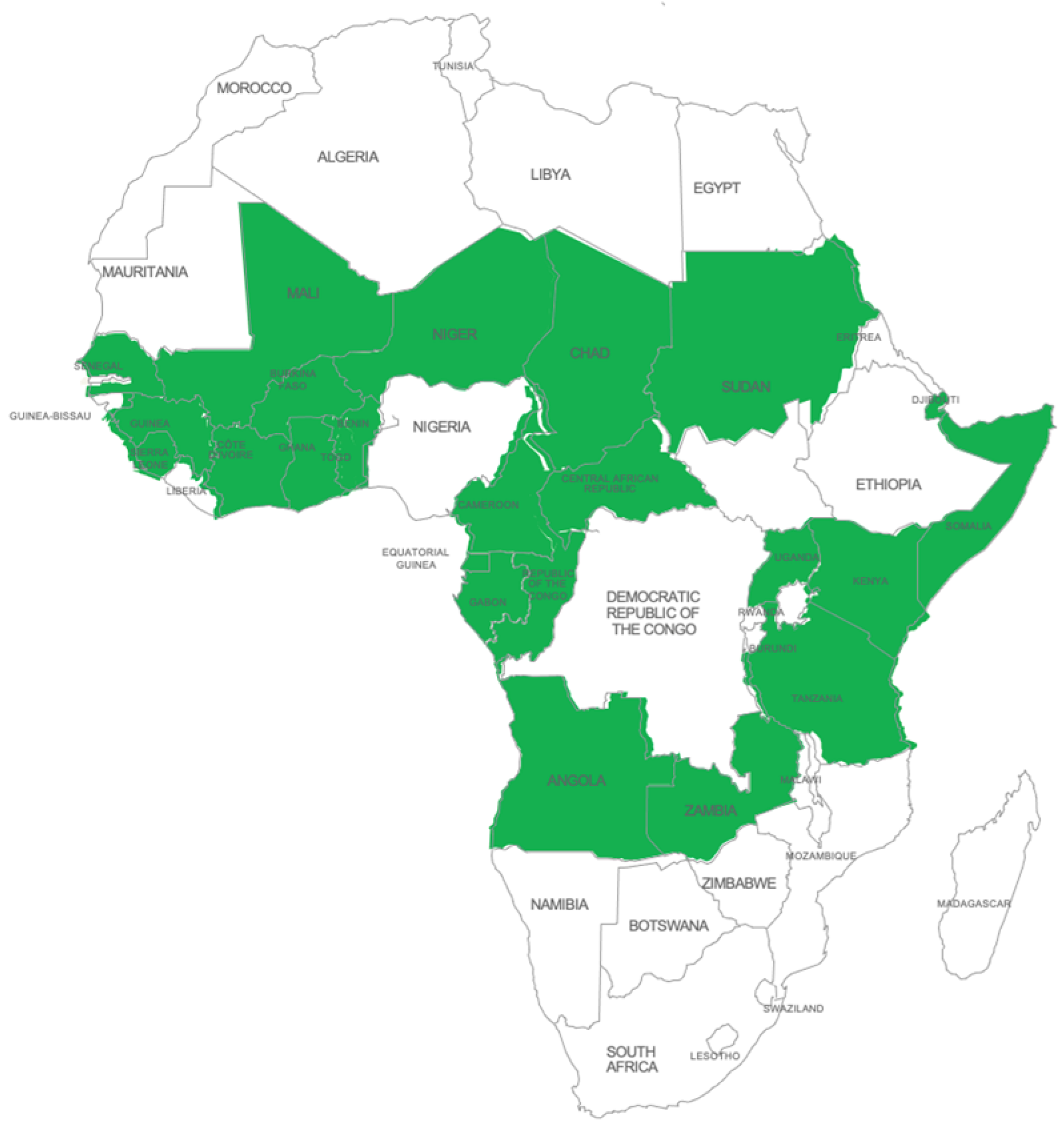 |
| Central Africa: Cameroon, Central African Republic, Chad, Equatorial Guinea, Gabon, Republic of Congo | |
| East Africa: Djibouti, Kenya, Sudan, Tanzania, Uganda | |
| Southern Africa: Angola, Zambia |
| IgG Type | F(ab’)2 (Equine) |
|---|---|
| F(ab’)2 | Over 95% |
| Protein | Less than 10% |
| Preservatives | None |
| Species coverage | Viperidae: Cerastes cerastes, Echis ocellatus, Echis leucogaster, Echis pyramidum, Bitis arietans, Bitis rhinoceros, Bitis nasicornis, Bitis gabonica Elapidae: Dendroaspis polylepis, Dendroaspis viridis, Dendroaspis angusticeps, Dendroaspis jamesoni, Naja anchieta, Naja annulifera, Naja ashei, Naja nigricollis, Naja haje, Naja katiensis, Naja melanoleuca, Naja mossambica, Naja nubiae, Naja pallida and Naja senegalensis Atractaspididae: Atractaspis irregularis Colubridae: Dispolidus typus |
| Formulation | Lyophilized |
| Storage | Up to 30 °C with possible excursions up to 40 °C for a reduced period |
| Study Reference | Design | Countries | Number of Clinical Sites | Nb of Patients Treated |
|---|---|---|---|---|
| Chippaux et al., 2015 [5] | Multicenter prospective clinical study | Benin Guinea | 3 | 209 |
| Coulibaly et al., 2018 [6] | Monocenter prospective observational study | Mali | 1 | 154 |
| Lam et al., 2019 [7] | Multicenter prospective cohort study | Senegal | 4 | 63 |
| Chippaux et al., 2022 [8] | Multicenter prospective clinical study | Cameroon | 14 | 250 |
| Overall statistics on clinical studies | 5 countries | 22 clinical sites | 676 patients | |
| Study Reference | Country | Age Median (Q25%–Q75%) | Sex-Ratio (M/F) | SBE Syndromes % Hemorrhagic (Including Positive WBCT) (1) | SBE Syndromes % Neurotoxic | Time from Snake Bite to Admission (Median) |
|---|---|---|---|---|---|---|
| Chippaux et al., 2015 [5] | Benin (n = 100) | 18 (13–29) | 4.5 | 90% | 2% | 24 h |
| Guinea (n = 109) | 30 (19–41) | 3 | 88% | 12% | 6 h | |
| Coulibaly et al., 2018 [6] | Mali (n = 154) | 38 NA | 3.7 | 82% | NA (2) | NA (2) |
| Lam et al., 2019 [7] | Senegal (n = 63) | 22 (13–35) | 2.7 | 38% | 13% | 6 h (Health Unit) 24 h (Hospital) |
| Chippaux et al., 2022 [8] | Cameroon (n = 250) | 25 (14–50) | NA (2) | 93% (3) | 9% (3) | 4 h 50 |
| Study Reference | Country | Number of Vials per Patient (Mean) | Length (Days) of Hospitalization (Mean) | Lethality | Adverse Events |
|---|---|---|---|---|---|
| Chippaux et al., 2015 [5] | Benin (n = 100) | 1.8 | 5.8 | 3 (3%) | 11 (11%) |
| Guinea (n = 109) | 1.4 | 4.5 | 1 (1%) | 6 (5.5%) | |
| Coulibaly et al., 2018 [6] | Mali (n = 154) | 2 | 1 to 5 (Min–Max) | 0 (0%) | 14 (6.5%) |
| Lam et al., 2019 [7] | Senegal (n = 63) | 1.4 | 1.6 | 2 (3.2%) | 3 (4.8%) |
| Chippaux et al., 2022 [8] | Cameroon (n = 250) | 3.2 | NA | 11 (4.4%) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathé, H. A Clinical Review of a Polyvalent F(ab’)2 Antivenom (InoserpTM PAN-AFRICA) in the Management of Snakebite Envenomation in Sub-Saharan Africa: Clinical Studies and Actual Use since Its Introduction in 2012. Biol. Life Sci. Forum 2023, 24, 13. https://doi.org/10.3390/IECT2023-14812
Mathé H. A Clinical Review of a Polyvalent F(ab’)2 Antivenom (InoserpTM PAN-AFRICA) in the Management of Snakebite Envenomation in Sub-Saharan Africa: Clinical Studies and Actual Use since Its Introduction in 2012. Biology and Life Sciences Forum. 2023; 24(1):13. https://doi.org/10.3390/IECT2023-14812
Chicago/Turabian StyleMathé, Henri. 2023. "A Clinical Review of a Polyvalent F(ab’)2 Antivenom (InoserpTM PAN-AFRICA) in the Management of Snakebite Envenomation in Sub-Saharan Africa: Clinical Studies and Actual Use since Its Introduction in 2012" Biology and Life Sciences Forum 24, no. 1: 13. https://doi.org/10.3390/IECT2023-14812
APA StyleMathé, H. (2023). A Clinical Review of a Polyvalent F(ab’)2 Antivenom (InoserpTM PAN-AFRICA) in the Management of Snakebite Envenomation in Sub-Saharan Africa: Clinical Studies and Actual Use since Its Introduction in 2012. Biology and Life Sciences Forum, 24(1), 13. https://doi.org/10.3390/IECT2023-14812





