Describing the Fate of Autochthonous Lactic Acid Bacteria in Artisanal Goat’s Raw Milk Cheeses during Storage: An Omnibus Modelling Approach †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lab-Scale Cheese Manufacturing
2.2. Validation Experiments
2.3. Microbial Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. First-Order Modelling
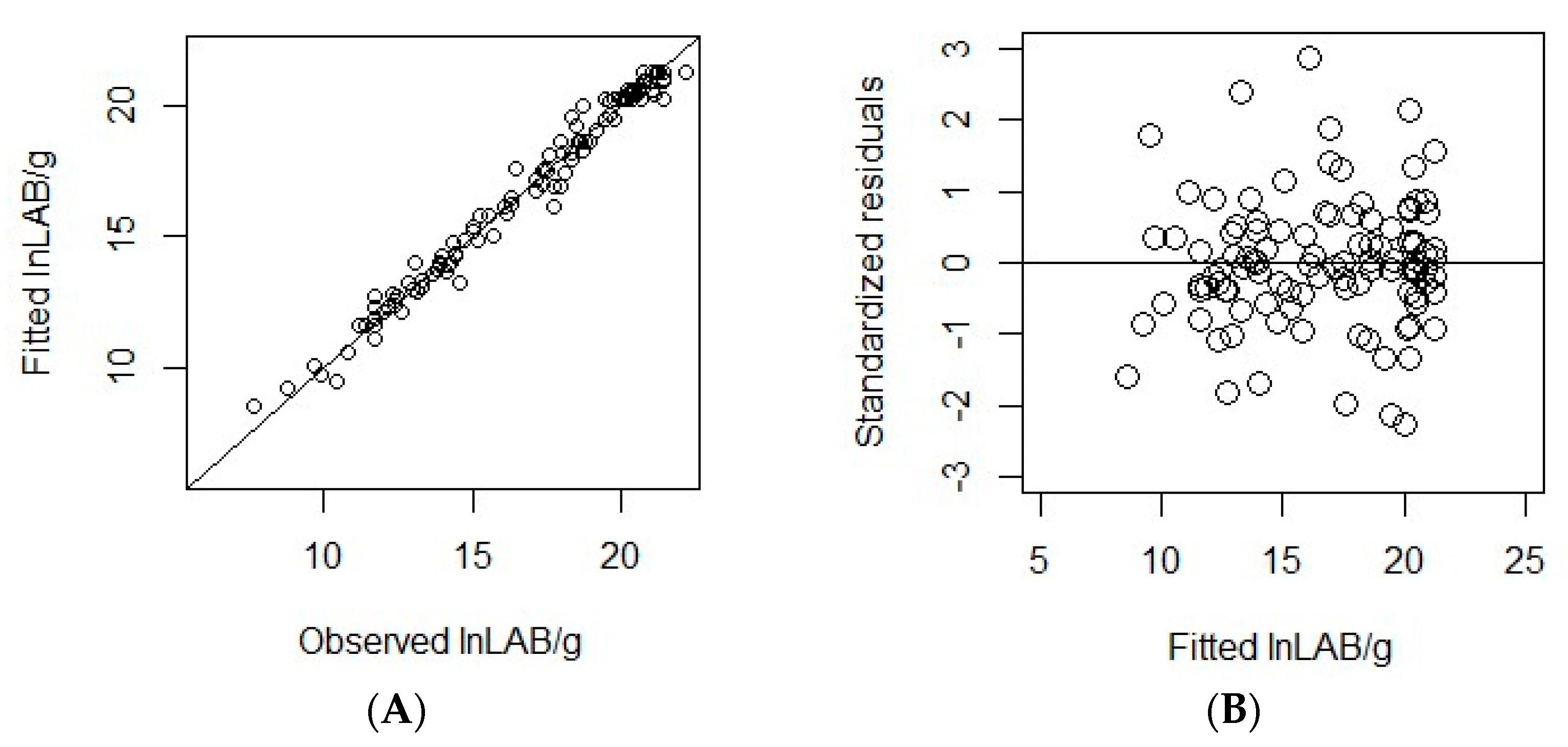
3.2. Omnibus Modelling
3.3. Internal Validation
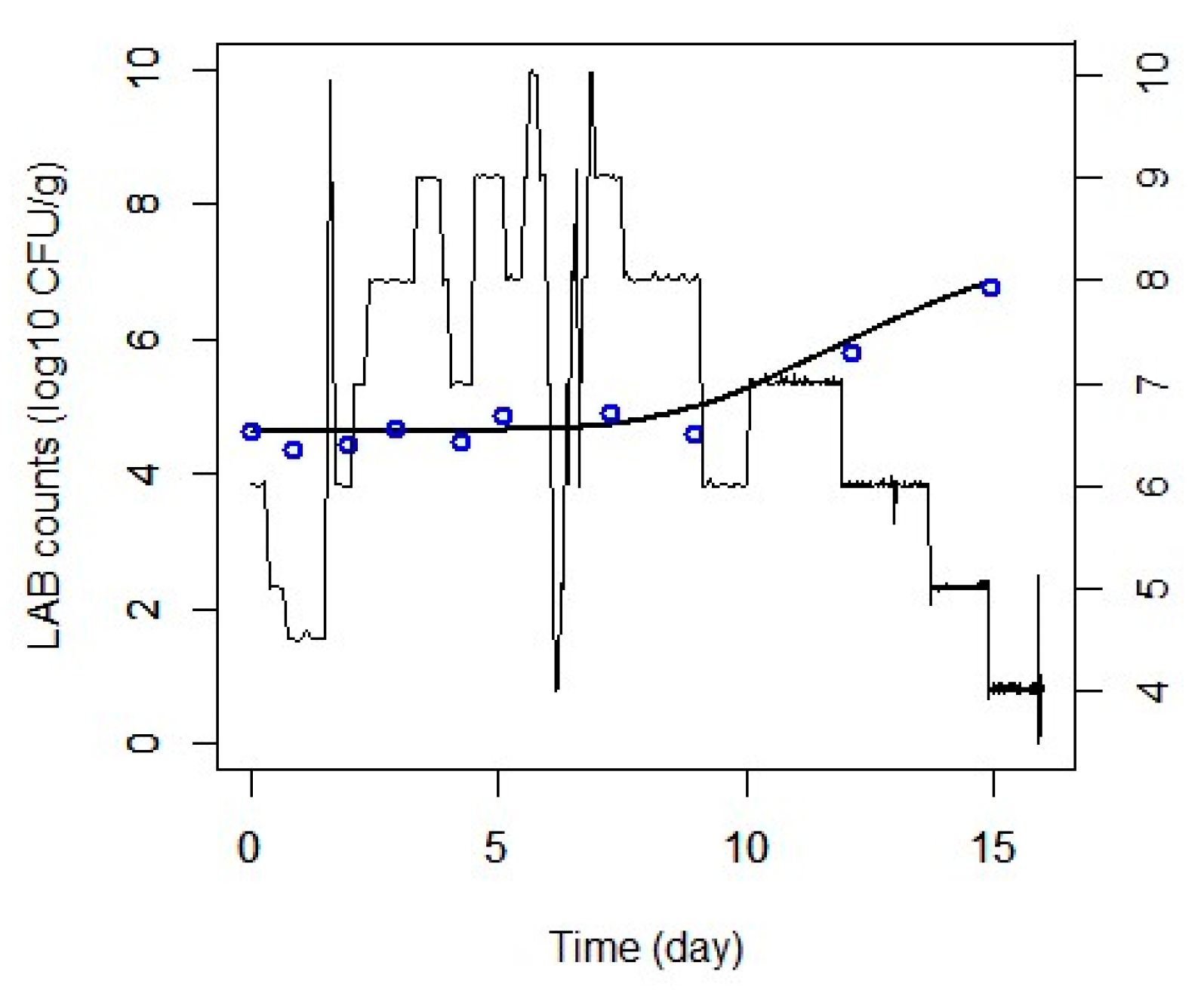
3.4. External Validation
3.4.1. Fixed Temperatures
3.4.2. Dynamic Temperatures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Alerta por presencia de Listeria monocytogenes en salmón ahumado marinado procedente de España. 2021. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/ampliacion/2021_146.htm (accessed on 2 September 2023).
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Alerta por presencia de Salmonella en FUET/SECALLONA procedentes de España. 2021. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/ampliacion/2021_243.htm (accessed on 1 September 2023).
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Alerta por presencia de Listeria monocytogenes en carne de cabeza de cerdo cocida procedente de España. 2022. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/ampliacion/cabeza_cerdo.htm (accessed on 2 September 2023).
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Alerta por presencia de Listeria monocytogenes en morcilla (embutido de hígado) procedente de España. 2022. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/ampliacion/2022_275.htm (accessed on 2 September 2023).
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Alerta por presencia de Salmonella en chistorra procedente de España. 2022. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/ampliacion/Salmonella_Chistorra.htm (accessed on 2 September 2023).
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Alerta por presencia de Listeria monocytogenes en salmón ahumado procedente de España. 2023. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/ampliacion/2023_19.htm (accessed on 2 September 2023).
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Alerta por presencia de Listeria monocytogenes en cecina en lonchas con aceite de oliva procedente de España. 2023. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/ampliacion/2023_03.htm (accessed on 2 September 2023).
- Junta de Andalucía. Alerta alimentaria por presencia de Listeria monocytogenes en queso fresco de cabra y mezcla de Andalucía. 2022. Available online: https://www.juntadeandalucia.es/organismos/saludyconsumo/areas/seguridad-alimentaria/alertas-alimentarias/paginas/listeria-queso-2022.html (accessed on 2 September 2023).
- Bondi, M.; Lauková, A.; De Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural preservatives to improve food quality and safety. J. Food Qual. 2017, 2017, 1090932. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; D’Opazo, V.; Quiles, J.M.; Romano, R.; Mañes, J.; Meca, G. Biopreservation of tomatoes using fermented media by lactic acid bacteria. LWT 2020, 130, 109618. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Converti, A.; Cotter, P.D.; De Souza Oliveira, R.P. Bacteriocin production by Bifidobacterium spp. A review. Biotechnol. Adv. 2013, 31, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Pennone, V.; Gonzales-Barron, U.; Hunt, K.; Cadavez, V.; McAuliffe, O.; Butler, F. Omnibus modeling of Listeria monocytogenes growth rates at low temperatures. Foods 2021, 10, 1099. [Google Scholar] [CrossRef] [PubMed]
- FRISBEE Project. Available online: https://www.frisbee-project.eu (accessed on 16 August 2023).
- R Studio (Version 4.0.0). Computer Software, R Studio, PBC. R Studio Team. 2023. Available online: https://www.rstudio.com/ (accessed on 15 April 2023).
- Cadavez, V.; Gonzales-Barron, U. “Predmicror”: Fitting Predictive Microbiology Models in R. Available online: https://github.com/fsqanalytics/predmicror (accessed on 20 April 2023).
- Huang, L. IPMP Global Fit—A one-step direct data analysis tool for predictive microbiology. Int. J. Food Microbiol. 2017, 262, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Hernandez, M.; De Cesare, A.; Manfreda, G.; González-García, P.; Rodríguez-Lázaro, D. Survival kinetics of Listeria monocytogenes on raw sheep milk cured cheese under different storage temperatures. Int. J. Food Microbiol. 2014, 184, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ačai, P.; Valík, L.; Medved’ová, A. One-and two-step kinetic data analysis applied for single and co-culture growth of Staphylococcus aureus, Escherichia coli, and lactic acid bacteria in milk. Appl. Sci. 2021, 11, 8673. [Google Scholar] [CrossRef]
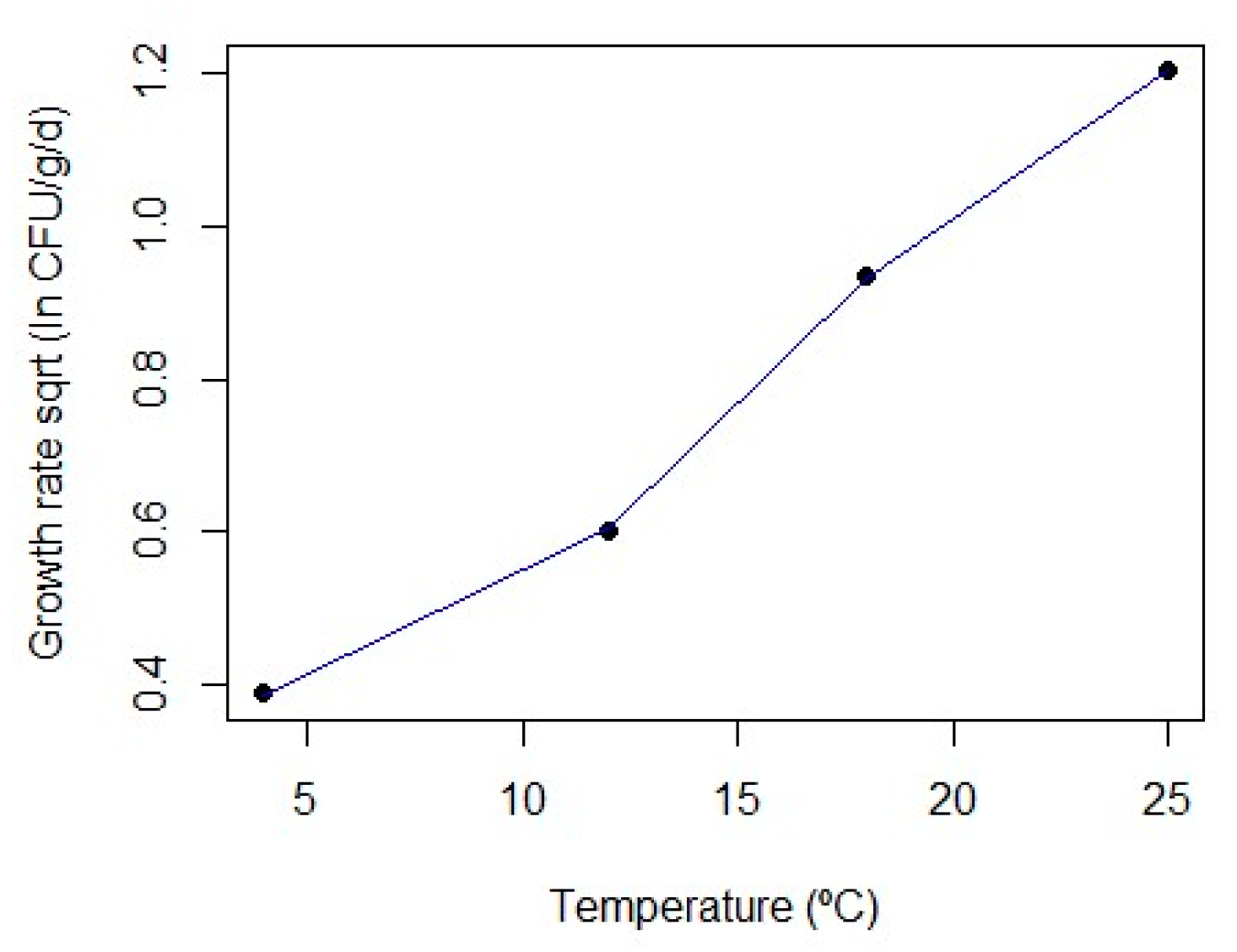
| Temp (°C) | µmax | Ymax | ∆Y |
|---|---|---|---|
| 4 | 0.345 (0.162) | 13.085 (2.412) | 3.388 (0.203) |
| 12 | 0.833 (0.411) | 18.820 (1.998) | 7.870 (2.333) |
| 18 | 2.011 (0.690) | 19.907 (1.201) | 7.917 (0.952) |
| 25 | 3.338 (0.907) | 20.749 (0.493) | 7.877 (1.480) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla-Luque, O.M.; Possas, A.; Gonzales-Barron, Ú.; Cadavez, V.; Ezzaky, Y.; Valero, A. Describing the Fate of Autochthonous Lactic Acid Bacteria in Artisanal Goat’s Raw Milk Cheeses during Storage: An Omnibus Modelling Approach. Biol. Life Sci. Forum 2023, 26, 47. https://doi.org/10.3390/Foods2023-15100
Bonilla-Luque OM, Possas A, Gonzales-Barron Ú, Cadavez V, Ezzaky Y, Valero A. Describing the Fate of Autochthonous Lactic Acid Bacteria in Artisanal Goat’s Raw Milk Cheeses during Storage: An Omnibus Modelling Approach. Biology and Life Sciences Forum. 2023; 26(1):47. https://doi.org/10.3390/Foods2023-15100
Chicago/Turabian StyleBonilla-Luque, Olga María, Arícia Possas, Úrsula Gonzales-Barron, Vasco Cadavez, Youssef Ezzaky, and Antonio Valero. 2023. "Describing the Fate of Autochthonous Lactic Acid Bacteria in Artisanal Goat’s Raw Milk Cheeses during Storage: An Omnibus Modelling Approach" Biology and Life Sciences Forum 26, no. 1: 47. https://doi.org/10.3390/Foods2023-15100
APA StyleBonilla-Luque, O. M., Possas, A., Gonzales-Barron, Ú., Cadavez, V., Ezzaky, Y., & Valero, A. (2023). Describing the Fate of Autochthonous Lactic Acid Bacteria in Artisanal Goat’s Raw Milk Cheeses during Storage: An Omnibus Modelling Approach. Biology and Life Sciences Forum, 26(1), 47. https://doi.org/10.3390/Foods2023-15100










