Optimization of Enzymatic Extraction of Melanoidins from Bread by-Products: Bioactivity and Microbiota Modulation †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction, Isolation, Digestion and Colonic Fermentation of Melanoidins
2.2. Microbiota Analysis
2.3. Determination of Total Antioxidant Capacity (TAC)
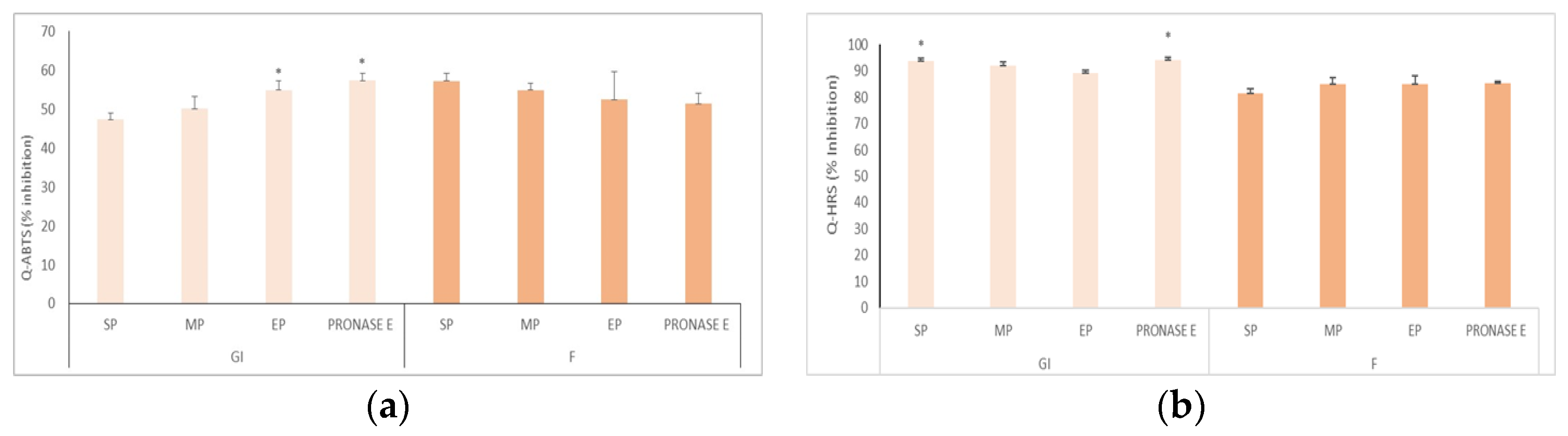
2.4. Determination of Radical Scavenger Activity (Q-HRS)
2.5. Determination of Metal Chelating Capacity
2.6. Cell Culture and Exposure Conditions
2.7. MTT Assay
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verni, M.; Minisci, A.; Convertino, S.; Nionelli, L.; Rizzello, C.G. Wasted Bread as Substrate for the Cultivation of Starters for the Food Industry. Front. Microbiol. 2020, 11, 293. [Google Scholar] [CrossRef]
- Pastoriza, S.; Rufián-Henares, J.A. Contribution of melanoidins to the antioxidant capacity of the Spanish diet. Food Chem. 2014, 164, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, I.; Delgado-Andrade, C.; Haro, A. Effects of dietary bread crust Maillard reaction products on calcium and bone metabolism in rats. Amino Acids 2013, 44, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- González-Mateo, S.; González-Sanjosé, M.L.; Muñiz, P. Presence of Maillard products in Spanish muffins and evaluation of colour and antioxidant potential. Food Chem. Toxicol. 2009, 47, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Brodkorb, A. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Modulation of Akt-p38-MAPK/Nrf2/SIRT1 and NF-κB pathways by wine pomace product in hyperglycemic endothelial cell line. J. Funct. Foods 2019, 58, 255–265. [Google Scholar] [CrossRef]
- Del Pino-Garcia, R.; Garcia-Lomillo, J.; Rivero-Perez, M.D.; Gonzalez San Jose, M.L.; Muniz, P. Adaptation and validation of quick, easy, new, cheap, and reproducible (QUENCHER) antioxidant capacity assays in model products obtained from residual wine pomace. J. Agric. Food Chem. 2015, 63, 6922–6931. [Google Scholar] [CrossRef] [PubMed]
- Yesiloglu, Y.; Aydin, H.; Kilic, I. In Vitro Antioxidant Activity of Various Extracts of Ginger (Zingiber officinale L.) Seed. Asian J. Chem. 2013, 25, 3573–3578. [Google Scholar] [CrossRef]
- Celik, E.E.; Rubio, J.M.A.; Andersen, M.L.; Gokmen, V. Interactions of coffee and bread crust melanoidins with hydroxycinnamic and hydroxybenzoic acids in aqueous radical environment. Food Res. Int. 2018, 108, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Wang, H.; Qian, H.; Yao, W. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Diaz-Morales, N.; Cavia-Saiz, M.; Salazar-Mardones, G.; Rivero-Perez, M.D.; Muñiz, P. Cytotoxicity study of bakery product melanoidins on intestinal and endothelial cell lines. Food Chem. 2021, 343, 128405. [Google Scholar] [CrossRef] [PubMed]
- Cossu, A.; Posadino, A.M.; Giordo, R.; Emanueli, C.; Sanguinetti, A.M.; Piscopo, A.; Pintus, G. Apricot melanoidins prevent oxidative endothelial cell death by counteracting mitochondrial oxidation and membrane depolarization. PLoS ONE 2012, 7, e48817. [Google Scholar] [CrossRef] [PubMed]
- Temiño, V.; Porras, D.; Salazar-Mardones, G.; Gerardi, G.; Muñiz, P.; Cavia-Sáiz, M. Melanoidins mitigate oxidative distress induced by CoCl2 in neuronal cells (SH-SYY5Y). Free. Radic. Biol. Med. 2023, 201, 63. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Fogliano, V. Bread crust melanoidins as potential prebiotic ingredients. Mol. Nutr. Food Res. 2005, 49, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Angel Rufian-Henares, J. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef] [PubMed]

| A | CACO-2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| % Viability | NT | SP | MP | EP | Pronase E | ||||
| Fractions | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | |
| GI | 100 ± 3.59 | 102 ± 9.26 | 93.41 ± 6.64 | 96.46 ± 5.01 | 98.73 ± 4.37 | 94.14 ± 4.07 | 95.47 ± 4.91 | 99.30 ± 3.61 | 97.88 ± 2.63 |
| F | 100 ± 9.11 | 95.86 ± 3.98 | 96.95 ± 2.67 | 92.02 ± 2.66 | 94.09 ± 6.94 | 86.12 ± 5.65 | 84.46 ± 6.57 * | 93.99 ± 2.43 | 92.74 ± 4.27 |
| B | HUVEC | ||||||||
| % Viability | NT | SP | MP | EP | Pronase E | ||||
| Fractions | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | |
| GI | 100 ± 6.58 | 91.94 ± 8.73 | 94.52 ± 9.84 | 106 ± 0.98 | 92.86 ± 11.39 | 82.12 ± 5.26 * | 75.42 ± 1.28 * | 91.77 ± 9.54 | 81.45 ± 10.53 |
| F | 100 ± 6.58 | 90.83 ± 3.43 | 83.41± 5.73 | 105.85 ± 5.01 | 105.32 ± 3.61 | 85.95 ± 10.88 | 88.72 ± 10.61 | 97.67 ± 9.29 | 95.24 ± 1.24 |
| C | SHSY5Y | ||||||||
| % Viability | NT | SP | MP | EP | Pronase E | ||||
| Fractions | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | |
| GI | 100 ± 9.55 | 88.5 ± 5.07 | 91.60 ± 6.15 * | 91.78 ± 7.25 | 85.05 ± 8.0 | 93.87 ± 1.72 | 98.52 ± 9.06 | 100 ± 1.88 | 95.5 ± 5.27 |
| F | 100 ± 6.55 | 90.30± 5.44 | 90.29 ± 12.05 | 92.39 ± 9.25 | 89.34 ±14.43 | 93.87 ± 4.86 | 94.49 ± 11.6 | 96.28 ±12.01 | 94.76 ± 9.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temiño, V.; Gerardi, G.; Cavia-Saiz, M.; Muñiz, P.; Salazar, G. Optimization of Enzymatic Extraction of Melanoidins from Bread by-Products: Bioactivity and Microbiota Modulation. Biol. Life Sci. Forum 2023, 26, 25. https://doi.org/10.3390/Foods2023-15102
Temiño V, Gerardi G, Cavia-Saiz M, Muñiz P, Salazar G. Optimization of Enzymatic Extraction of Melanoidins from Bread by-Products: Bioactivity and Microbiota Modulation. Biology and Life Sciences Forum. 2023; 26(1):25. https://doi.org/10.3390/Foods2023-15102
Chicago/Turabian StyleTemiño, Virginia, Gisela Gerardi, Monica Cavia-Saiz, Pilar Muñiz, and Gonzalo Salazar. 2023. "Optimization of Enzymatic Extraction of Melanoidins from Bread by-Products: Bioactivity and Microbiota Modulation" Biology and Life Sciences Forum 26, no. 1: 25. https://doi.org/10.3390/Foods2023-15102
APA StyleTemiño, V., Gerardi, G., Cavia-Saiz, M., Muñiz, P., & Salazar, G. (2023). Optimization of Enzymatic Extraction of Melanoidins from Bread by-Products: Bioactivity and Microbiota Modulation. Biology and Life Sciences Forum, 26(1), 25. https://doi.org/10.3390/Foods2023-15102






