Abstract
Films and coatings fabricated with renewable biopolymers and antimicrobial agents have attracted research interest owing to their contribution to food safety and biodegradability. The study aimed to determine the effect of natural plant extracts from the leaves of curry tree, neem, tulsi, and Mexican mint in developing and characterizing biodegradable composite films of talipot starch and carboxymethyl cellulose (CMC) matrices. Talipot starch isolated from the stem pith of talipot palm (Corypha umbraculifera L.) is an underutilized source of starch with a high yield (76%). All composite films were prepared using the solution blending-casting method. The dominant properties of biodegradable films such as structural, morphological, barrier, and antimicrobial properties were studied. The relative crystallinity (RC) of composite films comparatively decreased with native talipot starch film. The surface of the talipot starch film made with CMC and plant extracts showed higher roughness and opacity. Incorporation of plant extracts into talipot starch and CMC matrices decreased water vapor permeability (WVP) and oxygen permeability (OP), indicating the improved barrier properties of the films. Antimicrobial activity as assessed by the inhibition zone method showed that composite films exhibited excellent antimicrobial activity against Staphylococcus aureus and Escherichia coli. These results revealed that biodegradable composite films from the non-conventional starch of talipot palm can possibly be used as a substitute for the one- time use petroleum-based films and can be used as a bioactive packaging material for food applications.
1. Introduction
The usage of synthetic polymers by the food packaging industries has exponentially increased because of their economic feasibility and long durability. However, their use causes augmentation in waste generation and environmental pollution due to their non-degradability and generation of harmful components during their disposal [1]. To combat this problem, the packaging industries have encouraged the development and use of novel environmental friendly non-toxic biodegradable materials [2]. Biodegradable films fabricated with natural biopolymers is a promising sustainable method to substitute plastic food packaging materials due to their biodegradability and biocompatibility [3]. Amongst the biodegradable polymers, starch is found to be a promising material owing to its abundance, low cost, biodegradability, edibility, and good film-forming properties [4]. The utilization of starch from non-conventional sources such as rhizomes, legumes, seeds, and palms with a greater yield than commercially exploited starches has been promoted recently [5]. In that context, talipot starch is an underutilized starch from talipot palm (Corypha umbraculifera L.) of the Arecaceae family native to India, Sri Lanka, Malaysia, and Myanmar. The talipot starch, yielding 76% starch with an appreciable amylose content of 28%, makes it a suitable candidate for preparing biobased starch films [6].
However, the biodegradable nature of starch is insufficient to promote the production of starch-based packaging materials in the industries for commercialization. Lately, starch-based films started gaining attraction when researchers enriched them with nutrients, antioxidants, antimicrobials, and color change indicators for the development of active and intelligent packaging materials to increase the shelf life of food [2]. The addition of natural additives possessing antioxidants and antimicrobial activity are preferred over synthetic additives in biobased food packaging materials concerning food safety and consumers’ health [4]. Natural plant extracts are abundant sources of active compounds that mainly exhibit antioxidant and antimicrobial activities. Plant extracts majorly have the presence of phytochemicals such as organic acids, fatty acids, flavonoids, phenolic compounds, carotenoids, and terpenoids that might act as antioxidants and antimicrobial agents and react with free radicals and inhibit microbial growth, respectively [1]. The plant extracts acquired from different sources such as leaf, barks, shoots, and seeds are used in varied polysaccharide-based films [3].
Among the plant extracts, the extracts from the leaves of the curry tree, neem, tulsi, and Mexican mint can be considered for developing active films due to their effectual bioactive characteristics. Curry leaves (Murraya koenigii), which belong to the family of Rutaceae, are ubiquitous aromatic small trees or deciduous shrubs of medicinal importance that grow vastly in the Indian subcontinent and other South-East Asian countries [7]. Neem (Azadirachta indica), comprising approximately 140 bioactive constituents where azadirachtin is the primal constituent, from the family of Maliaceae cultivated mainly in the tropics of the Indian subcontinent, is an extensively used plant for treating infections, inflammation, skin diseases, and dental problems [8]. Tulsi (Ocimum tenuiflorum) also regarded as holy basil of Lamiaceae family is a vital aromatic medicinally important plant native to the tropical regions of Africa and Asia [9]. The leaves of this plant contain essential oils comprising carvacrol, eugenol, limatrol, caryophylline, methyl-chavicol, etc., possessing medicinal value and exhibiting cogent antimicrobial activity against S. aureus and E. coli [10]. Plectranthus amboinicus (Lour.) Spreng., commonly called Mexican mint or country borage and belongs to the Lamiaceae family, is a fleshy, succulent aromatic perennial herb found in the warm tropics of Africa and Asia. Mexican mint shows therapeutic and analgesic effects, and the bioactivity of the plant is good because of its main constituents, germacrene, carvacrol, and thymol [11]. The extracts of neem, tulsi, Mexican mint, and curry leaves are reported to exhibit a wide spectrum of biological properties: anti-inflammatory, antioxidant, antimicrobial, anticarcinogenic, and anti-diabetic properties.
These plant extracts from neem, tulsi, Mexican mint, and curry leaves may act as efficient antimicrobial additives in starch-based composite film formulations. Furthermore, to improve the mechanical strength of starch-based film, carboxylmethyl cellulose (CMC) is added which possesses better mechanical strength than starchy materials. CMC is an anionic derivative of cellulose used as a thickener, stabilizer, and gelling agent prepared by the etherification of cellulose by substituting fractions of hydroxyl groups by carboxymethyl groups [12]. Therefore, the present study focused on the fabrication and characterization of biodegradable composite films by adding plant extracts from the leaves of curry tree, neem, tulsi, and Mexican mint to find their potential applicability in the food packaging industry.
2. Materials and Methods
2.1. Materials
The talipot starch was isolated from talipot flour obtained from the stem pith of talipot palm by following the procedure described in Aaliya et al. [13]. Fresh neem, tulsi, Mexican mint, and curry leaves were collected from Pondicherry University, Puducherry, India. The plants and their leaves were taxonomically identified and validated by the botanical survey of India. All the chemicals used for the study were of analytical grade.
2.2. Isolation of Plant Extracts
The plant extracts were prepared by slightly modifying the method described by Kumar et al. [14]. The leaves were collected, sorted, and cleaned with distilled water. The excess water in the leaves was wiped off and 20 g of leaves was weighed out and heated in 100 mL of distilled water for 30 min at 60 °C. The aqueous plant extract was collected in an amber-colored bottle by filtering through Whatman No. 1 filter paper and stored under refrigeration (4 ± 2 °C). The extract of the four leaves (neem, tulsi, Mexican mint, and curry leaves) was prepared using the abovementioned method.
2.3. Preparation of Talipot Starch-CMC Composite Films
The composite films were developed by the solution blending-casting technique following the procedure detailed in the work of Sudheesh et al. [15]. A total of 1 g CMC was dissolved in 10 mL distilled water by heating it on a magnetic stirrer to get a homogenous gel solution. In another beaker 10% (w/w) starch suspension was taken and gelatinized in a magnetic stirrer at 600 rpm. To obtain the gelatinized suspension, 2 mL glycerol and CMC solution were added and blended thoroughly for 10 min. Then, 5 mL plant extract was added to the composite filmnogenic solution and stirred for another 5 min. Finally, the prepared filmnogenic solution was homogenized and degassed and then poured into a Petri dish (with a 3 mm thickness) to dry in a hot air oven for 24 h at 45 °C. The dried composite film was peeled off from the Petri dish and stored in a desiccator at 25 ± 2 °C with 65% relative humidity for further analyses. The biodegradable film prepared without adding CMC and plant extracts was taken as control and regarded as native TSF, and the composite films prepared with CMC and plant extracts from curry leaves, neem, tulsi, and Mexican mint were regarded as CTSF, NTSF, TTSF, and MTSF.
2.4. Morphological Properties
The digital photographs of starch films were captured using a DSLR camera (Nikon D750, Tokyo, Japan). AFM analysis was performed using an atomic force microscope (MultiMode 8-HR, Bruker, Karlsruhe, Germany), and the roughness (Ra) of the films was determined by NanoScope analysis software. The surface of films was studied by using a scanning electron microscope (Hitachi, S-3400N, Tokyo, Japan) at 500× magnification.
2.5. Crystalline Properties
X-ray diffraction analysis of film samples was carried out using an X-ray diffractometer (BRUKER, D2 PHASER, Karlsruhe, Germany), and the data from the scans were computed from an angle of diffraction 2θ, 4–50° by following the procedure mentioned in Akhila et al. [16]. TOPAS software was applied to calculate the relative crystallinity of films. The relative crystallinity (RC) of the starch granules was estimated with the formula:
2.6. Opacity
The opacity of films (5 cm × 2 cm) was investigated on a UV Spectrophotometer (Shimadzu, UV-1800, Kyoto, Japan). The analysis was carried out at 600 nm by evaluating light transmittance. The opacity was measured using the following equation:
2.7. Barrier Properties
The composite films were kept in a desiccator with 67% relative humidity for conditioning (using a saturated solution of NaCl) and the water vapor permeability (WVP) of the films was determined using the ASTM E-96 (1993) [17] standard dry cup method. The oxygen permeability (OP) of the films was determined by evaluating the peroxide value of soybean oil covered with prepared films. The OP of the films was performed by following the method described in the previous study of Sudheesh et al. [18].
2.8. Antimicrobial Activity
The antimicrobial activity of films was identified via the inhibition zone method by following the method suggested in the work of Wu et al. [19]. The antimicrobial activities of the composite films with and without plant extracts against S. aureus and E. coli were determined by evaluating the disk inhibition zones’ diameters. Broth cultures of 106 CFU/mL of both pathogenic microorganisms were made and smeared on culture media. Film discs of 6 mm diameter were placed over the agar plates containing culture media and incubated at 37 °C for 24 h. The zones developed around the film discs indicated the inhibition of bacterial growth, and the inhibition zone diameter was determined by measuring with a caliper in millimeters.
2.9. Biodegradation Study
Biodegradation studies of films were determined by following the method suggested by Oluwasina et al. [20]. The film samples were cut in a square shape of 2 cm × 2 cm and dried in a hot air oven for 3 h. The samples were then weighed and it was recorded as W0. The weighed samples were buried in the soil (3.5 cm depth) in perforated plastic containers. After 24 h of burial, the films were taken back, cleaned, and dried in a hot air oven for 3 h at 105 °C. The dried films were then weighed and it was noted as W1. The film samples were buried again and 10 mL of distilled water was sprinkled on the soil. Later, all the film samples were taken out every three days and the weight reduction was recorded for the period of study (16 days). The percentage of film degradation was calculated with the formula:
The biodegradation of films was conveyed with regards to the weight loss (%) versus storage days.
2.10. Statistical Analysis
The data from each analysis were put through one-way analysis of variance (ANOVA) and Duncan’s multiple range test (DMRT) using the software IBM SPSS Statistics 23 (IBM Corporation, Chicago, IL, USA). The experimental data are denoted as mean ± standard deviation (SD), at a significant difference of p ≤ 0.05.
3. Result and Discussion
3.1. Morphological Properties
The digital photographs and SEM images of native and plant extract added films are illustrated in Figure 1a,b. The digital photograph of TSF exhibited a clear film and the films prepared with plant extracts, CTSF, NTSF, TTSF, and MTSF, also showed clear films with the characteristic color of their respective plant extracts.
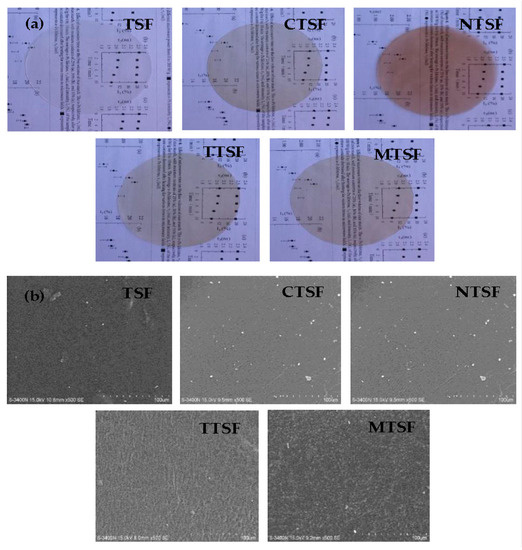
Figure 1.
(a) Digital photographs and (b) scanning electron monographs of biodegradable composite films with and without plant extracts.
SEM of native TSF showed a smooth surface with little irregularity. A few white spots on the CTSF, NTSF, TTSF, and MTSF samples showed homogeneous distribution of plant extracts throughout the film and the surfaces showed slight heterogeneity when extracts were added. This advanced into the development of a greater compact matrix, with enhanced mechanical and moisture resistance by the active compounds in extracts [21].
The roughness of native TSF, CTSF, NTSF, TTSF, and MTSF samples was 20.32 nm, 28.13 nm, 25.14 nm, 22.99 nm, and 29.13 nm, respectively (Table 1). The incorporation of plant extracts showed a significant increase (p ≤ 0.05) in the Ra values, possibly because of biopolymer–extract interactions. Thus the incorporation of curry leaves, neem, tulsi, and Mexican mint extracts retained the dense structure of the starch film, indicating that there was an impregnable interaction between the polymeric constituents and the extracts, even though it gave a rougher texture to the talipot starch films [11]. Increased surface roughness can be attributed to the improved opacity and hydrophobic properties of the film [20].

Table 1.
Roughness (Ra), relative crystallinity (RC), and opacity of biodegradable composite films with and without plant extracts.
3.2. Crystalline Properties
XRD patterns of native TSF, CTSF, NTSF, TTSF, and MTSF are shown in Figure 2. Native talipot starch film exhibited crystalline peaks at 17.09°, 19.52°, and 21.99° indicating a V-type crystalline pattern that can be ascribed to the plasticization effect of starch. However, the addition of plant extracts reduced the peak intensities in the range of 17.09–21.99°. This behavior agrees with the previous results suggesting that plant extracts decreased the retrogradation tendency of starch [22].
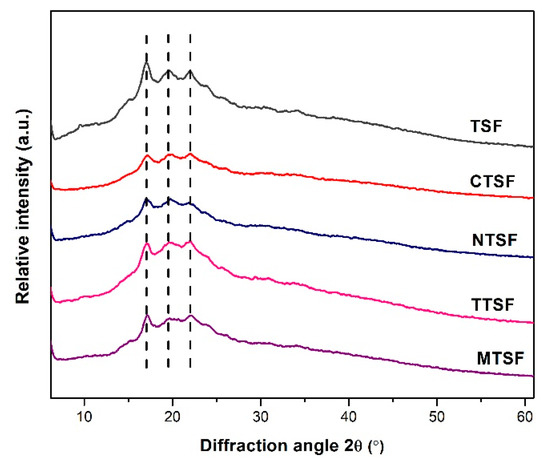
Figure 2.
XRD of biodegradable composite films with and without plant extracts.
RC values of different talipot starch films are given in Table 1, where the native TSF showed a RC of 2.03%. XRD patterns of films incorporated with plant extracts exhibited a decreased crystallinity fraction compared with native TSF. This indicated that the addition of plant extracts induced a plasticizing action on starch–CMC–glycerol films, likely because of the interference of extract that affects the interaction of starch chains. This leads to an increment in the amorphism of the film structures and implies that plant extracts had hindered the phenomenon of retrogradation [23]. This result can be due to the development of intermolecular hydrogen bonds between amylose/amylopectin chains and polyphenols that potentially averts the starch molecules’ reassociation to a more ordered structure [22].
3.3. Opacity
The opacity of films connotes the dense structure of polymeric films [18]. The opacity of native TSF was 1.55 AU/mm and significantly increased (p ≤ 0.05) to 3.63 AU/mm, 5.21 AU/mm, 4.04 AU/mm, and 4.61 AU/mm in CTSF, NTSF, TTSF, and MTSF, respectively (Table 1). In general, the inclusion of plant extracts enhanced the UV-blocking ability of the talipot starch films. Due to the natural pigmentation of the plant extracts, the films prepared by adding the extracts became darker and hence increased the opacity of the plant extract added talipot starch films [22]. The increase in opacity relates to the irreversible phase partition to polymer-rich and polymer-deficient domains, and it can be related to the observation here that plant extracts decelerated the retrogradation process. Besides, the increased roughness with the addition of CTSF, NTSF, TTSF, and MTSF scatters light, and this contributes to lower light transmittance and higher opacity. A comparable increase in film opacity was perceived in blueberry waste extract incorporated in an active starch film [24]. Kumar et al. [14] in their experiment with moth bean starch–basil leaf extract active film reported that enhancement in the opacity is due to the pigments and polyphenolic compounds of basil extracts that can greatly retard the propagation of light through the films hence increasing the opacity. The higher opacity of plant extract added films is potentially beneficial for packaging photocatalytic reaction susceptible food products [18].
3.4. Barrier Properties
The WVP of packaging material is a crucial parameter as it measures the water exchange between food products and the environment. The WVP of native TSF (3.67 g Pa−1h−1m−1 × 10−6) is greater than that of plant extract-added talipot starch films (Figure 3a). The higher WVP of native TSF ascribes a higher number of free glycerol and hydroxyl groups [15]. The inclusion of plant extract decreased the WVP compared to the native TSF, which is desirable, suggesting that plant extract-incorporated films have a more effectual moisture barrier than native TSF. The plant extracts’ compounds might have occupied the empty spaces of the polymer matrices, slowing the water vapor diffusion through the film [11]. The tortuous path hence developed reduces the rate of water molecule diffusion through the film that leads to the reduction in WVP [2]. The results of the WVP of plant extract-added talipot films were in accordance with the result reported in the moth bean starch–basil leaf extract active film [14]. They reported that basil extract in the active film improved the moisture barrier resistance by enhancing the internal interactions between the starch matrices that attenuate water molecules. A lower WVP of developed talipot starch films thus deprives or decreases the transport of moisture between the food products and packages.
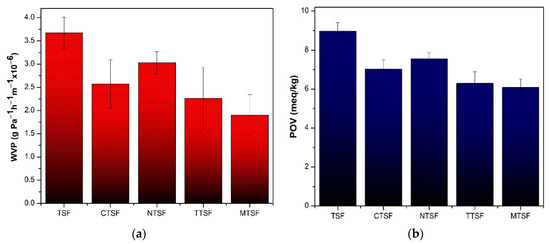
Figure 3.
(a) WVP and; (b) OP of biodegradable composite films with and without plant extracts.
The OP of talipot starch films was found by measuring the peroxide value (POV) of oil covered by the developed starch films. The POV of soybean oil covered by native TSF, CTSF, NTSF, TTSF, and MTSF is 8.97 meq/kg, 7.02 meq/kg, 7.54 meq/kg, 6.29 meq/kg, and 6.08 meq/kg, respectively (Figure 3b). The lower POV of soybean oil covered with plant extract-added films suggests the lower OP of the starch films. The extract–starch–CMC matrices improved the compactness of the film surface and resulted in higher oxygen barrier properties [15]. The reduced OP of composite films increases their applicability in packaging food products susceptible to lipid oxidation [25].
3.5. Antibacterial Activity
The bacterial inhibitory effects of talipot starch films on the growth of Gram-positive S. aureus and Gram-negative E. coli are given in Table 2. No inhibition zone was found for the native TSF against both S. aureus and E. coli and the test microorganisms were grown homogeneously in all regions of the Petri dishes. However, the addition of plant extracts from the leaves of curry tree, neem, tulsi, and Mexican mint showed a significant antibacterial effect against the bacterial pathogens. The diameters of the inhibition zones of the films with CTSF, NTSF, TTSF, and MTSF against S. aureus and E. coli were in the range of 18.14–22.13 mm and 17.16–24.83 mm, respectively. The development of the inhibition zone might be because of the leaching of antibacterial bioactive constituents from the talipot films that are incorporated into neem, tulsi, Mexican mint, and curry leaves extracts. A similar result of antibacterial activity was observed in the neem extract with an added bi-layered polyvinyl alcohol–chitosan nanofibrous mat [8]. The talipot starch films with antibacterial activities suggest that they might be advantageous in packaging foods in future practical applications.

Table 2.
Antibacterial activity of biodegradable composite films with and without plant extracts against S. aureus and E. coli.
3.6. Biodegradability Properties
The biodegradability analysis simulates the degradation of biodegradable films in the natural environment by the action of microorganisms in the soil [11]. The biodegradability of talipot starch film with and without plant extracts studied for 15 days is depicted in Figure 4. All the talipot starch films exhibited a continuous reduction in weight as the storage period increased. The films began to change their integrity after the fourth day of the experiment indicating the initiation of degradation. However, it was noticed that on the seventh day, the films with plant extracts CTSF, NTSF, TTSF, and MTSF exhibited a significant reduced degradation rate when compared to the native TSF, and after the tenth day of the experiment, the films started to degrade into smaller pieces.
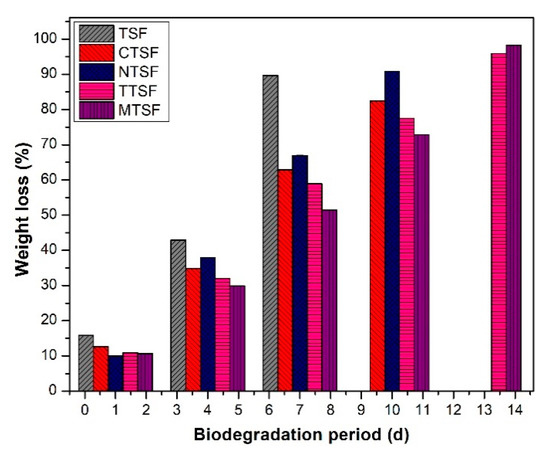
Figure 4.
Biodegradability study on soil of biodegradable composite films with and without plant extracts.
The incorporation of plant extracts to talipot starch films augmented CTSF, NTSF, TTSF, and MTSF stability against the biodegradation process compared to native TSF because of the antimicrobial agents present in the extract. The reduced WVP and antimicrobial activity of CTSF, NTSF, TTSF, and MTSF contribute to the increased biodegradation stability of talipot starch film. However, the biodegradability of starch films is affected by the type and moisture of soil, microorganisms in the soil, weather conditions, and film properties such as thickness, water absorption, and WVP [20]. The comparatively short degradation time of talipot starch films observed in the study demonstrates that the residues of these films can be potentially discarded in urban gardens unescorted by any industrial interventions [1]. Thereby it can possibly reduce the expenses of processing the packaging residues and reduce environmental pollution.
4. Conclusions
The current research confirmed that a few white spots on the SEM images of CTSF, NTSF, TTSF, and MTSF showed the homogeneous distribution of plant extracts throughout the film and the surfaces showed slight heterogeneity when extracts were added. The increased surface roughness of CTSF, NTSF, TTSF, and MTSF attributes to the improved opacity and hydrophobic properties of the film. The reduced crystallinity of plant extract-added films suggests that plant extracts reduce the retrogradation tendency of starch. The higher opacity of plant extract-added films is potentially beneficial for packaging photocatalytic reaction-susceptible food products. The lower WVP and OP of developed talipot starch films thus decrease or deprive the transfer of moisture and oxygen between the food products and packages. Antimicrobial activity as assessed by the inhibition zone method showed that composite films showed good antimicrobial activity against S. aureus and E. coli. The incorporation of plant extracts into talipot starch composite films augmented CTSF, NTSF, TTSF, and MTSF stability against the biodegradation process compared to native TSF because of the antimicrobial agents present in the extract. The comparatively short degradation time of talipot starch films observed in the study demonstrates that the residues of these films can be potentially eliminated easily, and are unescorted by any industrial interventions. Thereby it can possibly reduce the expenses of processing the packaging residues and reduce environmental pollution. Thus, the amalgamation of biodegradable polymers with natural plant extracts is helpful in producing environmental friendly films and coatings for the food packaging industries.
Supplementary Materials
The presentation material of this work is available online at https://www.mdpi.com/article/10.3390/IECBM2022-13393/s1.
Author Contributions
Conceptualization, B.A.; methodology, B.A. and K.V.S.; software, B.A.; formal analysis, P.K. and B.A.; data curation, P.K. and B.A.; writing—original draft preparation, B.A.; writing—review and editing, K.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
We thank A. Shijin, Veterinary Surgeon, Government of Kerala.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boeira, C.P.; Flores, D.C.B.; Alves, J.D.S.; de Moura, M.R.; Melo, P.T.S.; Rolim, C.M.B.; Nogueira-Librelotto, D.R.; da Rosa, C.S. Effect of Corn Stigma Extract on Physical and Antioxidant Properties of Biodegradable and Edible Gelatin and Corn Starch Films. Int. J. Biol. Macromol. 2022, 208, 698–706. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of Yerba Mate Extract on the Performance of Starch Films Obtained by Extrusion and Compression Molding as Active and Smart Packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, G.; Singh, D. Synthesis and Characterization of Starch Nanocellulosic Films Incorporated with Eucalyptus Globulus Leaf Extract. Int. J. Food Microbiol. 2020, 332, 108765. [Google Scholar] [CrossRef]
- Baek, S.K.; Kim, S.; Song, K. Bin Cowpea Starch Films Containing Maqui Berry Extract and Their Application in Salmon Packaging. Food Packag. Shelf Life 2019, 22, 100394. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; John, N.E.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Sabu, S.; Sasidharan, A.; Mir, S.A.; George, J. Impact of Microwave Irradiation on Chemically Modified Talipot Starches: A Characterization Study on Heterogeneous Dual Modifications. Int. J. Biol. Macromol. 2022, 209, 1943–1955. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Sabu, S.; Sasidharan, A.; Mir, S.A.; George, J.; Khaneghah, A.M. Effect of Low Dose γ-Irradiation on the Structural and Functional Properties, and in Vitro Digestibility of Ultrasonicated Stem Starch from Corypha umbraculifera L. Appl. Food Res. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Dasgupta, T.; Rao, A.R.; Yadava, P.K. Chemomodulatory Action of Curry Leaf (Murraya Koenigii) Extract on Hepatic and Extrahepatic Xenobiotic Metabolising Enzymes, Antioxidant Levels, Lipid Peroxidation, Skin and Forestomach Papillomagenesis. Nutr. Res. 2003, 23, 1427–1446. [Google Scholar] [CrossRef]
- Ali, A.; Shahid, M.A.; Hossain, M.D.; Islam, M.N. Antibacterial Bi-Layered Polyvinyl Alcohol (PVA)-Chitosan Blend Nanofibrous Mat Loaded with Azadirachta Indica (Neem) Extract. Int. J. Biol. Macromol. 2019, 138, 13–20. [Google Scholar] [CrossRef]
- Mittal, R.; Kumar, R.; Chahal, H. Antimicrobial Activity of Ocimum Sanctum Leaves Extracts and Oil. J. Drug Deliv. Ther. 2018, 8, 201–204. [Google Scholar] [CrossRef]
- Annu; Ali, A.; Ahmed, S. Eco-Friendly Natural Extract Loaded Antioxidative Chitosan/Polyvinyl Alcohol Based Active Films for Food Packaging. Heliyon 2021, 7, e06550. [Google Scholar] [CrossRef]
- Silva, V.D.M.; Macedo, M.C.C.; Rodrigues, C.G.; dos Santos, A.N.; Loyola, A.C.D.F.E.; Fante, C.A. Biodegradable Edible Films of Ripe Banana Peel and Starch Enriched with Extract of Eriobotrya Japonica Leaves. Food Biosci. 2020, 38, 100750. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Preparation of Multifunctional Carboxymethyl Cellulose-Based Films Incorporated with Chitin Nanocrystal and Grapefruit Seed Extract. Int. J. Biol. Macromol. 2020, 152, 1038–1046. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Sabu, S.; Sasidharan, A.; Sinha, S.K.; George, J. Influence of Plasma-Activated Water on the Morphological, Functional, and Digestibility Characteristics of Hydrothermally Modified Non-Conventional Talipot Starch. Food Hydrocoll. 2022, 130, 107709. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Effect of Basil Leaves Extract on Modified Moth Bean Starch Active Film for Eggplant Surface Coating. LWT—Food Sci. Technol. 2021, 145, 111380. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Sasidharan, A.; Sabu, S.; Basheer, A.; Navaf, M.; Raghavender, C.; Sinha, S.K.; George, J. Energetic Neutral N2 Atoms Treatment on the Kithul (Caryota Urens) Starch Biodegradable Film: Physico-Chemical Characterization. Food Hydrocoll. 2020, 103, 105650. [Google Scholar] [CrossRef]
- Akhila, P.P.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Sudheesh, C.; Yadav, D.N.; Khan, M.A.; Mir, S.A.; George, J. Morphological, Physicochemical, Functional, Pasting, Thermal Properties and Digestibility of Hausa Potato (Plectranthus Rotundifolius) Flour and Starch. Appl. Food Res. 2022, 2, 100193. [Google Scholar] [CrossRef]
- ASTM. E96-93 Standard Test Methods for Water Vapor Transmission of Materials. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 1993. [Google Scholar]
- Sudheesh, C.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Akhila, P.P.; Mir, S.A.; Sabu, S.; Sasidharan, A.; Sudheer, K.P.; Sinha, S.K.; et al. Effect of Energetic Neutrals on the Kithul Starch Retrogradation; Potential Utilization for Improving Mechanical and Barrier Properties of Films. Food Chem. 2023, 398, 133881. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of Citric Acid Induced Crosslinking on the Structure and Properties of Potato Starch/Chitosan Composite Films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Olaleye, F.K.; Olusegun, S.J.; Oluwasina, O.O.; Mohallem, N.D.S. Influence of Oxidized Starch on Physicomechanical, Thermal Properties, and Atomic Force Micrographs of Cassava Starch Bioplastic Film. Int. J. Biol. Macromol. 2019, 135, 282–293. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant Edible Films Based on Chitosan and Starch Containing Polyphenols from Thyme Extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Materska, M.; Łupina, K. Corn Starch and Methylcellulose Edible Films Incorporated with Fireweed (Chamaenerion angustifolium L.) Extract: Comparison of Physicochemical and Antioxidant Properties. Int. J. Biol. Macromol. 2021, 190, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Medina Jaramillo, C.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and Plasticizing Effect of Yerba Mate Extract on Cassava Starch Edible Films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Uranga, J.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Valorisation of Blueberry Waste and Use of Compression to Manufacture Sustainable Starch Films with Enhanced Properties. Int. J. Biol. Macromol. 2018, 115, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, C.; Sunooj, K.V.; Jamsheer, V.; Sabu, S.; Sasidharan, A.; Aaliya, B.; Navaf, M.; Akhila, P.P.; George, J. Development of Bioplastic Films from Γ−Irradiated Kithul (Caryota urens) Starch; Morphological, Crystalline, Barrier, and Mechanical Characterization. Starch/Staerke 2021, 73, 2000135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).