1. Introduction
Neural interfaces are bioelectronic devices that aim to communicate and control the nervous tissue by stimulating or inhibiting a group of neurons according to different modalities, in addition to recording electrical signals in a specific area [
1]. The types of neural interfaces differ in design between microelectrode arrays and probes that contain parts for electrical recording and sometimes for stimulation purposes [
2]. Despite the technological development in the field of neural interface fabrication in terms of materials engineering and biocompatibility with neural tissue, the barrier of biological and electrical stability, which plays an important role in the functional performance of the neural interface, remains a problem that is not completely or ideally solved. Some of the factors that influence the functionality of neural interfaces come from interactions between brain tissue and the implanted device, such as neuronal degeneration and glial scarring. Moreover, the response of the nervous tissue against the foreign body leads to a corrosive environment that accelerates the structural deterioration of the electrodes [
3].
Electrocorticogram (ECoG) signals recorded directly from the cerebral cortex have a much higher signal-to-noise ratio than electroencephalogram (EEG). Despite its close contact with the cerebral cortex, ECoG signals are still prone to noise. This noise can be categorized by origin into two different types: (1) noise that is common in all channels (for example, noise generated by a signal reference and surrounding instruments) and (2) noise that is unique to a particular channel (for example, electrode and surrounding tissue response). Therefore, some or all channels are prone to effects by common or different types of noise. Therefore, in the case of neural interfaces that contain a large number of recording sites and possibly other functions that generate noise, it is not reliable to use a filter with the same parameters on all channels [
4].
Filters or noise-removal approaches can be categorized into [
5] (1) well-known digital filters, such as bandpass filters, bandstop filters, low-bandwidth and high-bandwidth filters, where parameters are empirically determined; (2) adaptive filters that optimize parameters using a raw signal, a reference signal and an optimization algorithm with feedback process [
6]; (3) hybrid methods that depend on the integration of several methods to achieve optimal noise cancellation [
5].
In this paper, we propose an approach to determine the noisiest ranges of frequencies in all channels of the neural interface based on the probability of their occurrence in all channels. The proposed approach starts with calculating the short-time Fourier transform (STFT) of 3 s of each channel, then estimating the most periodic frequencies that have more rates of occurrence compared with active and dominant bands and, finally, filtering those frequencies out using the Kaiser-Window-based band-pass filter. The proposed approach is tested using ECoG recording of anesthetized rats with penicillin-induced epilepsy.
2. Materials and Methods
2.1. Animal Preparation, Electrode Implantation and Data Analysis
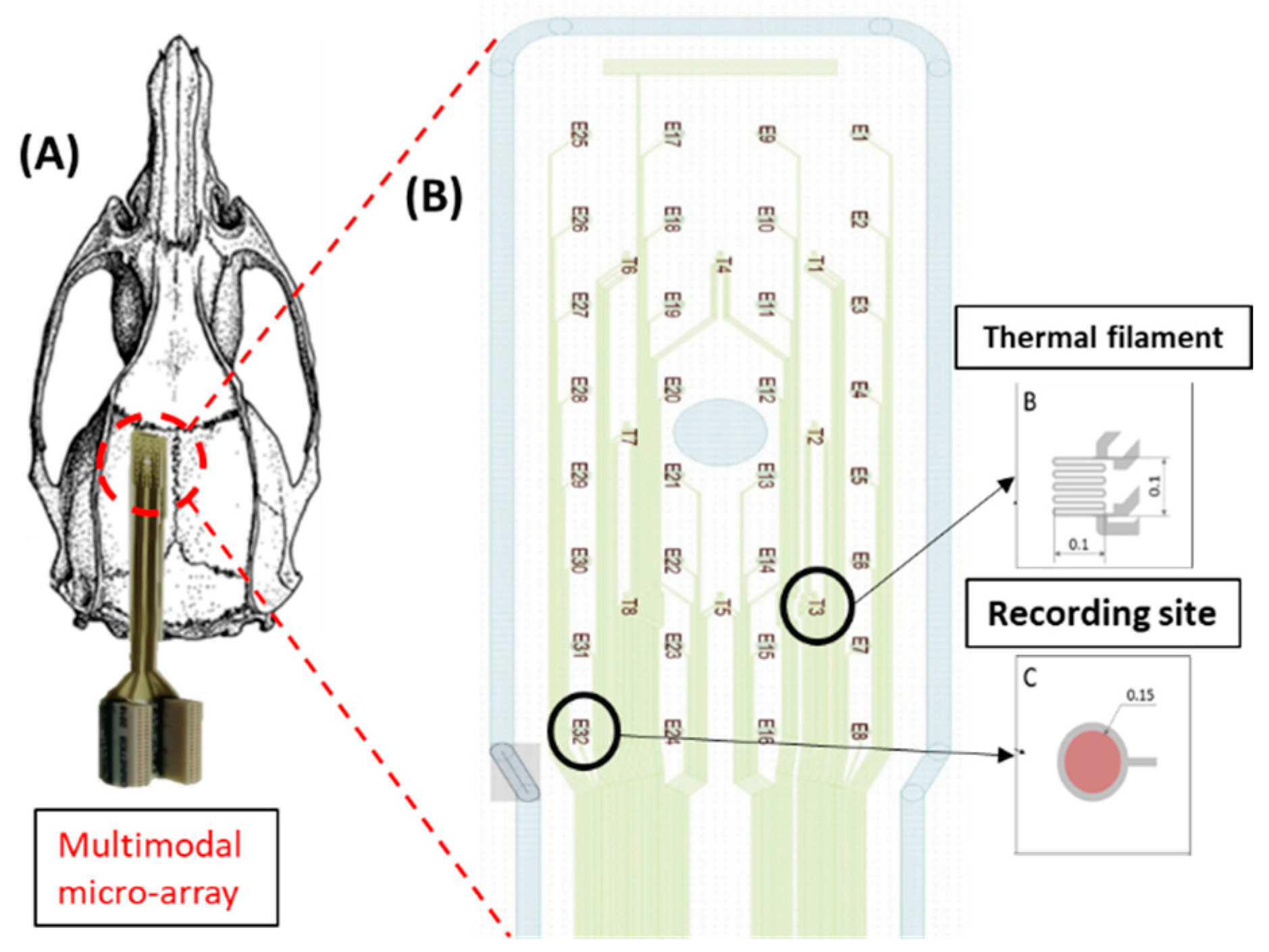
Recording experiments were carried out on four adult male rats weighing 240–250 g in the Research Centre for Natural Sciences, Eötvös Lóránd Research Network. All experiments were performed according to the EC Council Directive of 22 September 2010 (2010/63/EU) and all procedures were reviewed and approved by the Animal Care Committee of the Research Centre for Natural Sciences and by the National Food Chain Safety Office of Hungary (license number: PE/EA/775-7/2020). Rats were kept in a stable environment with a controlled temperature (22 ± 1 °C). The ECoG signals are recorded using flexible polymer-based multimodal microarray of 32 recording sites (
Figure 1B) [
7]. The multimodal microarray was implanted on the left somatosensory cortex at the same site where the injection was performed (
Figure 1A). The common reference electrode was fixed on the skull. The rats were injected with penicillin (1 μL, injected over 10 min, 500 IU, inserted into a 1.5 mm depth 34G needle with a rate of 10 μm/s). After penicillin administration, the needle is pulled out. The ECoG signals are recorded using 32 channels (Intan, 4/SP, AD Instruments, Sydney, Australia) with 20 kHz sampling, 16-bit resolution, 0.1–7500 Hz. ECoG signals are filtered and pre-processed offline using Matlab (MathWorks, Natick, MA, USA). Notch filter (50 Hz) is used to remove electrical supply artefacts.
2.2. Time-Frequency Analysis
The STFT of a signal is implemented by calculating the discrete Fourier transform of the M-length of data using a sliding window over the signal then repeating these steps using an interval of the R signal element. The result of implementing STFT is a matrix where the row order (m) expresses the frequencies of the spectrum power, the column order expresses the time window (
k) of the signal and the
STFT(
m,
k) is density of the spectrum power at the time frequency in dB [
8]:
where:
k = 0, 1, …, N − 1
S(m,k): indicates the m-index time-frequency spectrogram.
N: window segment length.
N′: the shifting step of the time window.
w(n) = window method of an N-point sequence.
2.3. Proposed Filtering Approach
The classical principle of the adaptive filter consists of using a primary signal with a reference signal through feedback error to modify the transfer function or parameters of the filter. In this work, the principle of updating the filter parameters depends on re-estimating the noisy frequencies by detecting the non-repeating frequencies in all channels. The proposed approach consists of the following steps as shown in (
Figure 2):
- (1)
Implementing the STFT on 3 s length of all active channels using (Equation (1)).
- (2)
Calculating the probability of occurrence of all major frequencies. The probability of occurrence is calculated based on the histogram of clustered frequencies with a step of 5 Hz (histogram expresses the occurrence count of 0–5 Hz, 6–10 Hz, etc.).
- (3)
Extracting the frequencies with the sudden occurrence that are not considered event-related frequencies should be previously determined by the expert. This extracting step is implemented with a threshold of 10 dB (high power density in dB according to the specialist’s decision).
- (4)
Implementing Kaiser-Window-based filter with a
β of 1.509 on extracted ranges of noisy frequencies [
9].
- (5)
Repeat the steps (1 to 4) with fixed time intervals.
3. Results
The proposed approach is implemented using Matlab (MathWorks, Natick, MA, United States) where the ECoG signals are filtered with a 50 Hz notch filter to remove power-supply artefacts. The channels in the ECoG microarray are ordered based on the distance from penicillin injection. In this work, the filtering process cares about the interictal spikes that have a main power spectrum of 0.5–90 Hz, as shown in
Figure 3A. By running instruments during in vivo experiments, many periodic frequencies of noise (for example, 98–102 Hz, 198–202 Hz, etc.) appear in the power spectrum of ECoG with different densities in all channels, as shown in
Figure 3B.
After keeping the frequencies between 0.5 and 90 Hz as an important spectrum for epileptiform discharges, the ranges of frequency in the histogram are estimated programmatically (
Figure 3B) and considered as noise, then filtered out using a finite-impulse-response (FIR) filter with a Kaiser Window. The designed filter of the proposed approach is responsible for filtering out the estimated frequencies from the probability of occurrence (
Figure 3B). The frequency response of the FIR filter is shown in
Figure 4A, in addition to the frequency response of the 2–300 Hz bandpass Butterworth filter (
Figure 4B) for comparison purposes.
After designing the FIR filter with a Kaiser Window and considering the noisy frequencies, the difference between raw and filtered ECoG signals using the proposed approach is shown in
Figure 5, where it is clear that some channels have different noises, such as ‘E20’ and ‘E7’, compared with the other. The proposed approach is able to give the same efficiency in filtering out based on a pre-known range of constructive and influencing frequencies that should be kept in all channels. The proposed approach has more efficient performance compared with Butterworth, where the signals of ‘E1’ and ‘E16’ channels are converted into smoother ECoG signals (
Figure 5).
4. Discussion
The neural interface that contains many recording sites may suffer from many factors that may decrease its functionality during long-term use [
10]. Different approaches for adaptive and hybrid filtering were proposed to handle the periodic and transient types of noise and artefacts [
5,
11]. This paper focuses more on the different types of noise that may affect specific channels of neural microarray or probe; at the same time, it is not reliable to filter out different frequencies of noise from specific channels and keep the other because it leads to wrong and inefficient information for analysis purposes. The proposed method depends on calculating the occurrence of frequencies based on STFT and considering the sudden and periodic frequencies of STFT (that are not related to the neurological event) as contamination. The proposed approach can be considered a self-adaptive filter because it is able to consider unwanted frequencies as noise and fragment the precise ranges of those frequencies based on the thresholding of the histogram (
Figure 3). The implementation of the approach shows a clear enhancement in ECoG signals (
Figure 5) by keeping the most important features and components, such as the interictal spikes, and removing precisely the periodic noise that comes from the surrounding instruments and the electrical and mechanical properties of electrodes. Some adaptive filters are developed to enhance EEG signals during the movement of patients and filter transient noise out using accelerometer-based referential signals [
12]. Our work can overcome the issue of various and transient noises [
12] by tracking the sudden noise spectrum with low probability among channels, in addition to designing one Kaiser-Window-based FIR that involves all frequencies of noise without the need for adaptive filters in cascade [
13].
This paper highlights the different noise and artefacts in specific channels of a neural interface and shows that by considering the occurrence of noise spectrum among all channels, we can design and implement an adaptive filter that can process all signals of channels with unified parameters without losing any important information.
5. Conclusions
In this paper, we propose an approach to determine the ranges of frequencies in all channels of the neural interface that cause a distortion in ECoG signals based on the probability of their occurrence in all channels. The accurate selection of frequencies in any filter, taking into account the common information among all channels, increases the reliability of analyzed signals and enhances the extracted features for evaluation stages. On the other hand, the occurrence probability of the most influencing power bands in all channels helps in keeping the most desirable information in terms of neurological events. The proposed method can be developed using a machine learning approach for a more precise selection of frequencies.
Author Contributions
Conceptualization, E.I.; methodology and investigation, E.I., Z.F.; formal analysis, E.I.; software, E.I.; writing—original draft preparation, E.I. and Z.F.; writing—review and editing, Z.F.; supervision, Z.F. All authors have read and agreed to the published version of the manuscript.
Funding
We are thankful to the National Brain Research Program (grant no. 2017_1.2.1-NKP-2017-00002) and the Thematic Excellence Program (grant no. TKP2020-NKA-11, TKP2021-EGA-42) funded by National Research, Development and Innovation Office, Hungary. This work was partially sup-ported by the National Research, Development, and Innovation Fund (grant no. NKFIH FK 134403).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge the support of the clean room technical staff at the Insitut für Mikrosystemtechnik, Germany, and the Centre for Energy Research, Hungary. The authors are grateful to Richárd Fiáth and Ágoston Csaba Horváth for their efforts in conducting the in vivo experiments.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Cho, Y.; Shin, H.; Park, J.; Lee, S. Advanced Neural Interface toward Bioelectronic Medicine Enabled by Micro-Patterned Shape Memory Polymer. Micromachines 2021, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, R.; Zhang, H.; Cao, P.; Liu, Z.; Li, Y. Research Progress on the Flexibility of an Implantable Neural Microelectrode. Micromachines 2022, 13, 386. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Liu, Y.; Xiao, L.; Zhang, C. Advanced Metallic and Polymeric Coatings for Neural Interfacing: Structures, Properties and Tissue Responses. Polymers 2021, 13, 2834. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Coon, W.G.; De Pesters, A.; Brunner, P.; Schalk, G. The effects of spatial filtering and artifacts on electrocorticographic signals. J. Neural Eng. 2015, 12, 056008. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bian, G.-B.; Tian, Z. Removal of Artifacts from EEG Signals: A Review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Delisle-Rodriguez, D.; Villa-Parra, A.C.; Bastos-Filho, T.; López-Delis, A.; Frizera-Neto, A.; Krishnan, S.; Rocon, E. Adaptive Spatial Filter Based on Similarity Indices to Preserve the Neural Information on EEG Signals during On-Line Processing. Sensors 2017, 17, 2725. [Google Scholar] [CrossRef] [PubMed]
- Csernyus, B.; Szabó, Á.; Fiáth, R.; Zátonyi, A.; Lázár, C.; Pongrácz, A.; Fekete, Z. A multimodal, implantable sensor array and measurement system to investigate the suppression of focal epileptic seizure using hypothermia. J. Neural. Eng. 2021, 18, 0460c3. [Google Scholar] [CrossRef] [PubMed]
- Zabidi, A.; Mansor, W.; Lee, Y.K.; Fadzal, C.C.W. Short-time Fourier Transform analysis of EEG signal generated during imagined writing. In Proceedings of the 2012 International Conference on System Engineering and Technology (ICSET), Bandung, Indonesia, 1–12 September 2012; pp. 1–4. [Google Scholar] [CrossRef]
- Navid, M.S.; Niazi, I.K.; Lelic, D.; Drewes, A.M.; Haavik, H. The Effects of Filter’s Class, Cutoff Frequencies, and Independent Component Analysis on the Amplitude of Somatosensory Evoked Potentials Recorded from Healthy Volunteers. Sensors 2019, 19, 2610. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Z. Recent advances in silicon-based neural microelectrodes and microsystems: A review. Sens. Actuators B Chem. 2015, 215, 300–315. [Google Scholar] [CrossRef]
- Ranjan, R.; Sahana, B.C.; Bhandari, A.K. Motion Artifacts Suppression from EEG Signals Using an Adaptive Signal Denoising Method. IEEE Trans. Instrum. Meas. 2022, 71, 1–10. [Google Scholar] [CrossRef]
- Rosanne, O.; Albuquerque, I.; Cassani, R.; Gagnon, J.-F.; Tremblay, S.; Falk, T.H. Adaptive Filtering for Improved EEG-Based Mental Workload Assessment of Ambulant Users. Front. Neurosci. 2021, 15, 611962. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.G.; Laciar, E.; Patiño, H.D.; Valentinuzzi, M.E. Artifact removal from EEG signals using adaptive filters in cascade. In Proceedings of the 16th Argentine Bioengineering Congress and the 5th Conference of Clinical Engineering, San Juan, Argentina, 26–28 September 2007; p. 012081. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).