1. Introduction
A meta-analysis study by Gonzales-Barron et al. [
1] revealed
S. aureus as the pathogen of highest incidence in goat raw milk (35.2%; 95% CI: 23.2–49.3%), and the overall occurrence of
S. aureus in goat’s milk cheeses to be noticeably high (16.0%; CI: 7.92–29.8%). Moreover, cheeses made of raw milk, regardless of their origin, presented an even higher prevalence of
S. aureus (38.7%; 95% CI: 9.28–79.6%). These concerning prevalence values underscore the importance of improving cheese manufacture to control
S. aureus development. For this, biopreservative agents such as plant extracts can be used [
2].
Previous investigation from our research group has demonstrated the antimicrobial capacity of several plant extracts against
S. aureus in vitro by the determination of their minimum inhibitory concentration (MIC) [
3]. Lemon balm extract resulting from hydroethanolic (70%
v/
v) solid–liquid extraction presented a MIC of 2.5 mg/mL against
S. aureus, whereas the equivalent extract obtained from spearmint showed an MIC of 1.25 mg/mL against this pathogen [
3]. Such outcomes suggest the potential of lemon balm and spearmint extracts to be incorporated into foods as preservatives against microbial spoilage.
In this sense, the objective of this work was to assess the antimicrobial effect against S. aureus of lemon balm and spearmint extracts in goat’s raw milk cheeses, when directly incorporated into curd, and to characterise the survival kinetic parameters of this pathogen by means of an extended Bigelow model. With this approach, values of decimal reduction time (D) can be described as a function of pH and incorporation of plant extract, and the inactivation parameters of S. aureus may help on the optimisation of the manufacturing process to ensure the microbial safety of cheeses.
2. Materials and Methods
2.1. Plant Material and Extraction Procedure
Dried lemon balm and spearmint aerial parts were provided by Pragmático Aroma Lda. (“Mais Ervas”, Trás-os-Montes, Portugal) and mechanically ground. Extracts were obtained using ethanol 70% (v/v) as solvent in a shaking water bath (150 rpm) at 60 °C for 90 min. The sample/solvent ratio was 1:20. The mixtures were filtrated (7–10 μm), and the ethanolic fraction was evaporated. The remaining aqueous fraction was frozen and lyophilised.
2.2. Inoculation of S. aureus in Milk and Cheese Production
Staphylococcus aureus ATCC 6538 kept on a fresh slant was cultivated twice at 37 °C, 200 rpm, for 16 h, first on tryptic soy broth (TSB) and then on tryptic soy broth with pH adjusted to 6.34, to mimic goat’s milk pH. On the day of inoculation, the second subculture was centrifuged at 10,640× g at 4 °C for 10 min, for removing debris and residual culture media. After centrifugation, the supernatant was discarded, and pellets were washed with sterile 0.9% physiological solution. Centrifugation and washing procedures were repeated twice and cells were re-suspended in sterile 0.9% physiological solution to reach ~7 log CFU/mL.
Lab-scale cheeses were prepared by adding the rennet (0.75 mL/L milk) and inoculum (5 mL/L milk) to milk at ~34 °C. Through this procedure, each cheese reached a S. aureus target concentration of 4 to 5 log CFU/g, depending on the milk’s initial contamination. After 30 min at 34 °C, curdled milk was cut and drained, and 1% (w/w) of lyophilised spearmint or lemon balm extract was added to the curd and mixed, while an inoculated control without extract was kept. The curd was then placed in 50 mL tubes and centrifuged at 6000 rpm at 20 °C for 3.5 min. The supernatant (whey) was removed, and cheeses of ~5 g were cut from the compacted curd and placed in a 15% brine solution for 10 min for salting. Finally, the weight in g of each cheese was annotated and cheeses were kept in a climate-controlled chamber (10 °C, 98% RH) for fermentation and maturation to take place for 15 days.
2.3. Microbiological and Physicochemical Analyses
The analyses were conducted on days 0 (day of cheese production), 2, 4, 7, 10, 13, and 15. For the microbiological determinations, for every test unit, appropriate serial dilutions were prepared by homogenising the cheese in 50 mL of buffered peptone water for 60 s. In order to determine the concentration of
S. aureus, 0.1 mL of aliquot was plated on Baird-Parker agar, supplemented with Egg Yolk Tellurite, following ISO norms [
4]. Typical colonies were counted after 48 h following incubation at 37 °C.
Physicochemical analyses comprised the measurement of pH, which was carried out using a pH meter (Hanna Instruments, model HI5522, Woonsocket, RI, USA) equipped with a HI1131 glass penetration probe.
2.4. Modelling
For every treatment, a log–decay function with tail in differential form as primary model, with varying
D-value, coupled to a secondary model Bigelow equation of
D-value as a function of
pH (with parameters log
Dref at
pH 7.0 and
zpH) was adjusted, as follows:
Herein, N is the population density (CFU/g), k is the inactivation rate (1/day), is the physiological state of the cells, is the residual population density (CFU/g), D (days) is the decimal reduction time at the constant temperature T (10 °C) and at the pH of the cheese, pHref is the reference pH (set to 7), zpH is the distance of pH from pHref which leads to a ten-fold change in decimal reduction time, and Dref is the decimal reduction time at pHref (days).
3. Results and Discussions
Bigelow-type secondary models were used to describe the inactivation of
S. aureus in goat’s raw milk cheeses during maturation as affected by spearmint and lemon balm extracts. The survival curves of
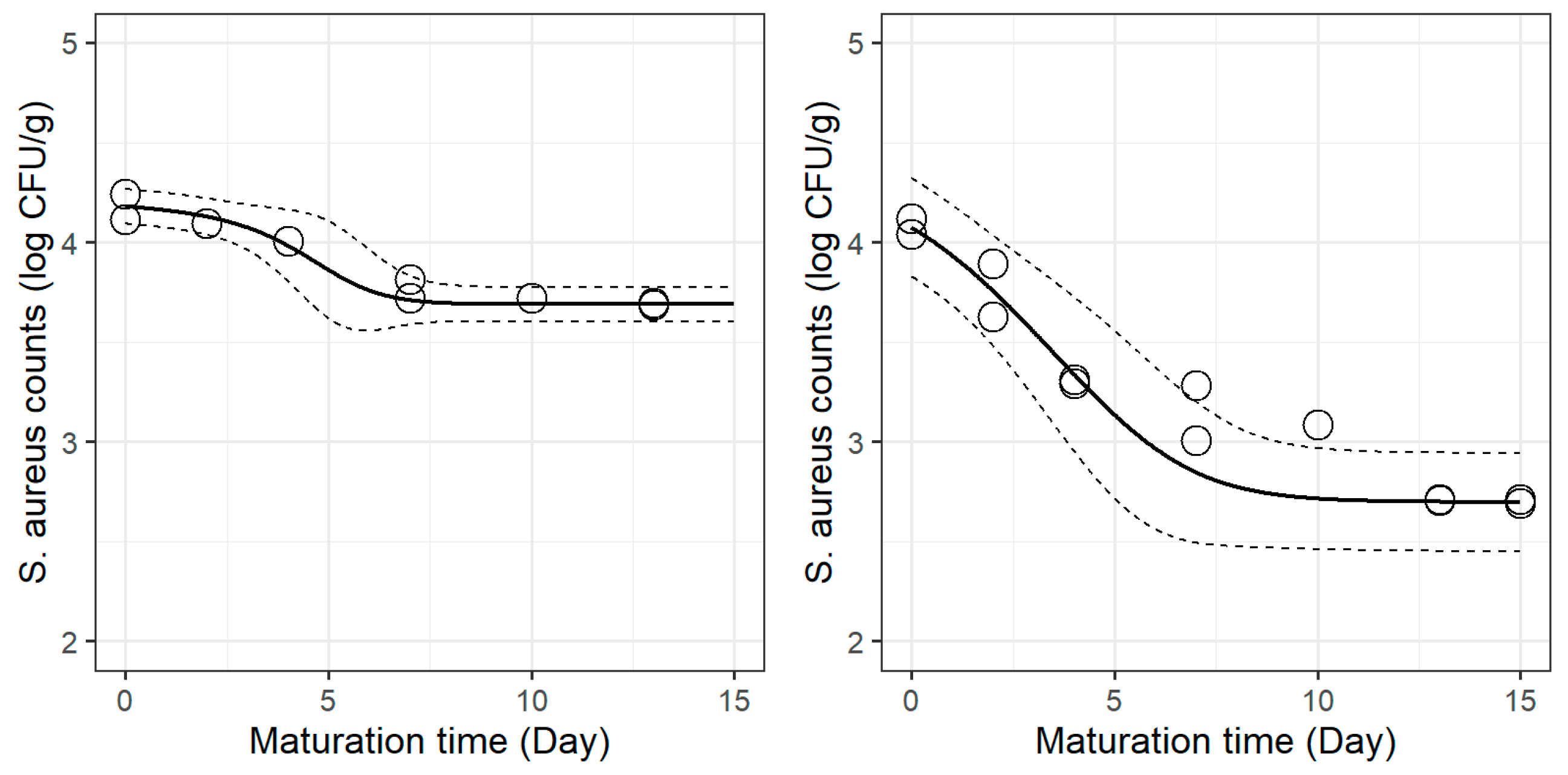
S. aureus in cheese without and with plant extracts, as depicted by the dynamic models, are presented in
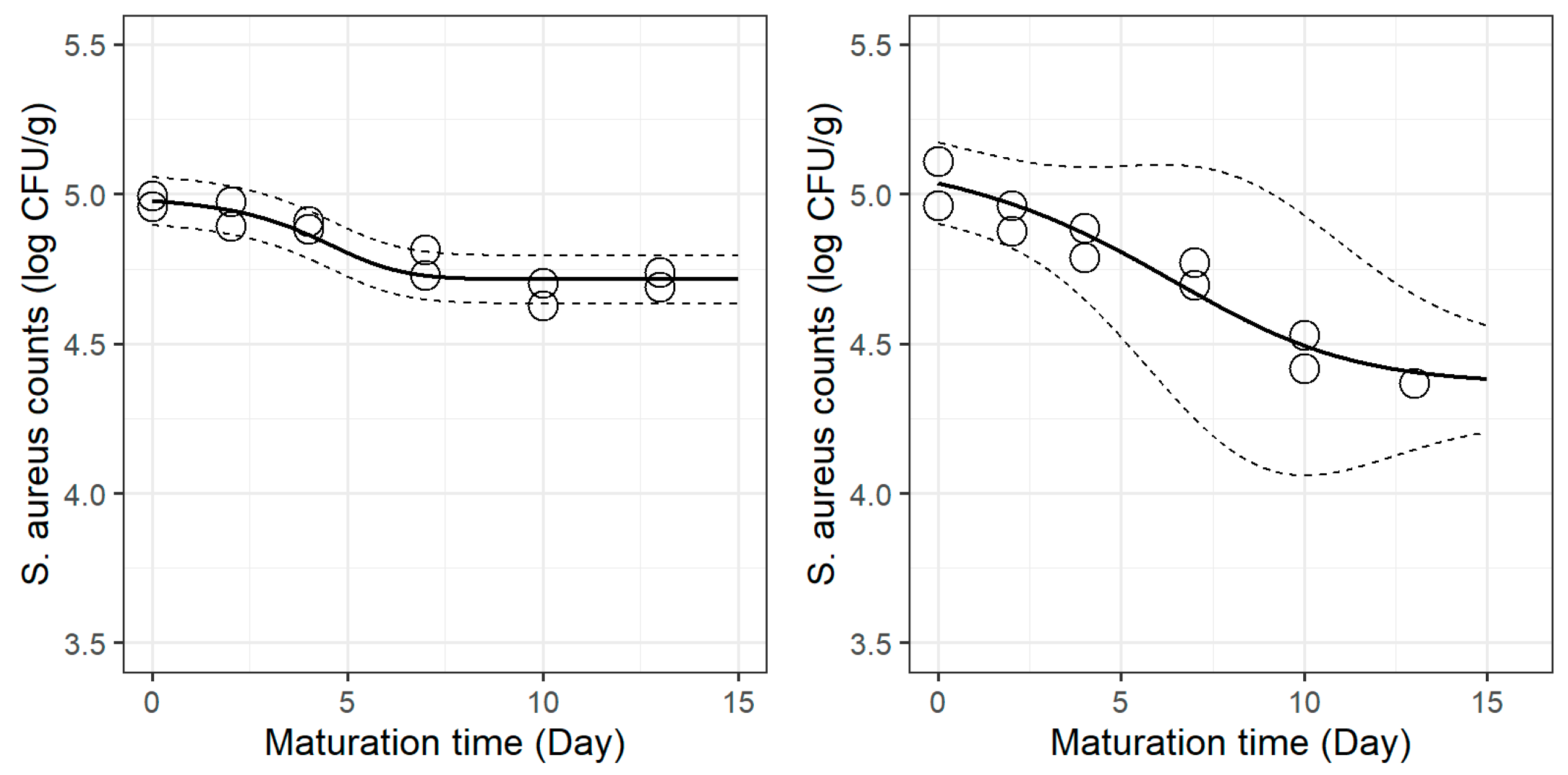
Figure 1 (spearmint) and
Figure 2 (lemon balm). The results of the Bigelow parameters for each treatment are shown in
Table 1.
The dynamic models adequately fitted the survival curves with root mean square errors (RMSEs) of 0.1172 and 0.0633 for spearmint and lemon balm, respectively (
Table 1), producing significant parameter estimates (
p < 0.05).
From
Table 1, the parameter log
Dref was affected by the addition of the extracts (0.621 (SE = 0.061) for spearmint; 1.189 (SE = 0.200) for lemon balm) in comparison to the controls (0.932 (SE = 0.166) and 0.996 (SE = 0.056)).
In the case of cheeses with spearmint extract, log
Dref was lower than that of the control, implying a greater inactivation rate of the pathogen. The survival curves presented in
Figure 1 show that the incorporation of this extract reduced the initial lag phase (the “shoulder”) and promoted
S. aureus inactivation earlier in maturation.
Oppositely, when adding lemon balm extract to the cheese, the estimated log
Dref was higher than that of the control, thus suggesting a slower inactivation rate. However,
S. aureus’ inactivation was steadier and more prolonged throughout maturation, compared to the control cheeses, in which
S. aureus’ inactivation phase was rather short and the stationary phase (“tail”) was reached sooner (
Figure 2).
In both cases, the addition of plant extracts significantly decreased the time to achieve one log reduction, which in practical terms corresponded to up to 1.36 log CFU/g reduction by the end of maturation. Such outcomes support the usefulness of incorporating spearmint and lemon balm extracts to reduce S. aureus burden in this dairy product.
The fermentation process was affected by the presence of extracts in the sense that the pH drop in the beginning of the maturation (until day 4) was slower, and the pH value reached after 14 days was slightly higher, compared to the corresponding control group (data not shown). This is reflected by the higher
zpH values of the cheeses with spearmint and lemon balm extracts in
Table 1 (3.172 (SE = 0.660) and 2.339 (SE = 0.835), respectively) and means that a greater difference between pH and
pHref is necessary to lead to a ten-fold change in
D when incorporating plant extracts in cheese, than the one needed for the same variation in
D in the controls. This outcome is likely a result of plant extracts affecting, to some extent, the production of organic acids by bacteriocinogenic lactic acid bacteria that drop the pH during fermentation. Nonetheless, after day 4, the pH trend for both treatments and controls were similar.
The results of this work indicate that the main effect of adding 1% lemon balm extract in curd is on S. aureus’ lag phase and the zpH; while 1% spearmint extract affects lag phase, zpH, and log Dref. Therefore, spearmint extract is more efficient in controlling S. aureus in goat’s raw milk cheese.
4. Conclusions
Using Bigelow-type secondary models, this work characterised S. aureus’ survival parameters in goat’s raw milk cheese. The results indicate that both parameters, log Dref and zpH, were affected by the addition of extracts. The zpH values are increased by the addition of extracts due to their interaction with the ongoing fermentation.
The dynamic models also demonstrated that the addition of lemon balm and spearmint extracts reduced the time needed to achieve one log reduction in S. aureus, thus showing their ability to act as biopreservatives against this pathogen during cheese maturation.