Bioactive Compounds from Andean Berry (Vaccinium meridionale Swartz) Juice Inhibited Cell Viability and Proliferation from SW480 and SW620 Human Colon Adenocarcinoma Cells †
Abstract
1. Introduction
2. Materials and Methods
2.1. Andean Berry Juice (ABJ) Preparation
2.2. Cell Culture
2.2.1. Cell Viability and Antiproliferative Effect Using Sulforhodamine B Assay
2.2.2. Quantification of Ki67 Protein
2.2.3. In Silico Analysis
3. Results and Discussion
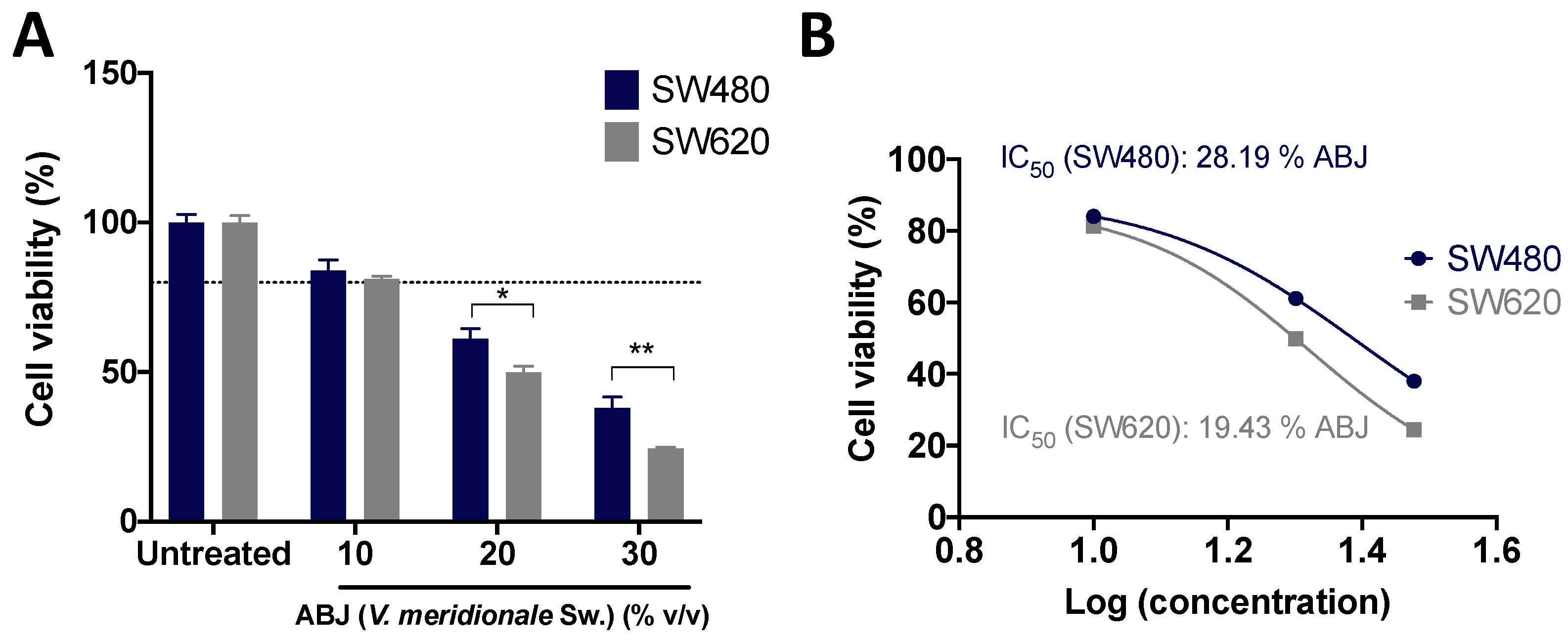
3.1. ABJ Treatments Inhibited Cell Viability and Reduced Proliferation of SW480 and SW620 Cells
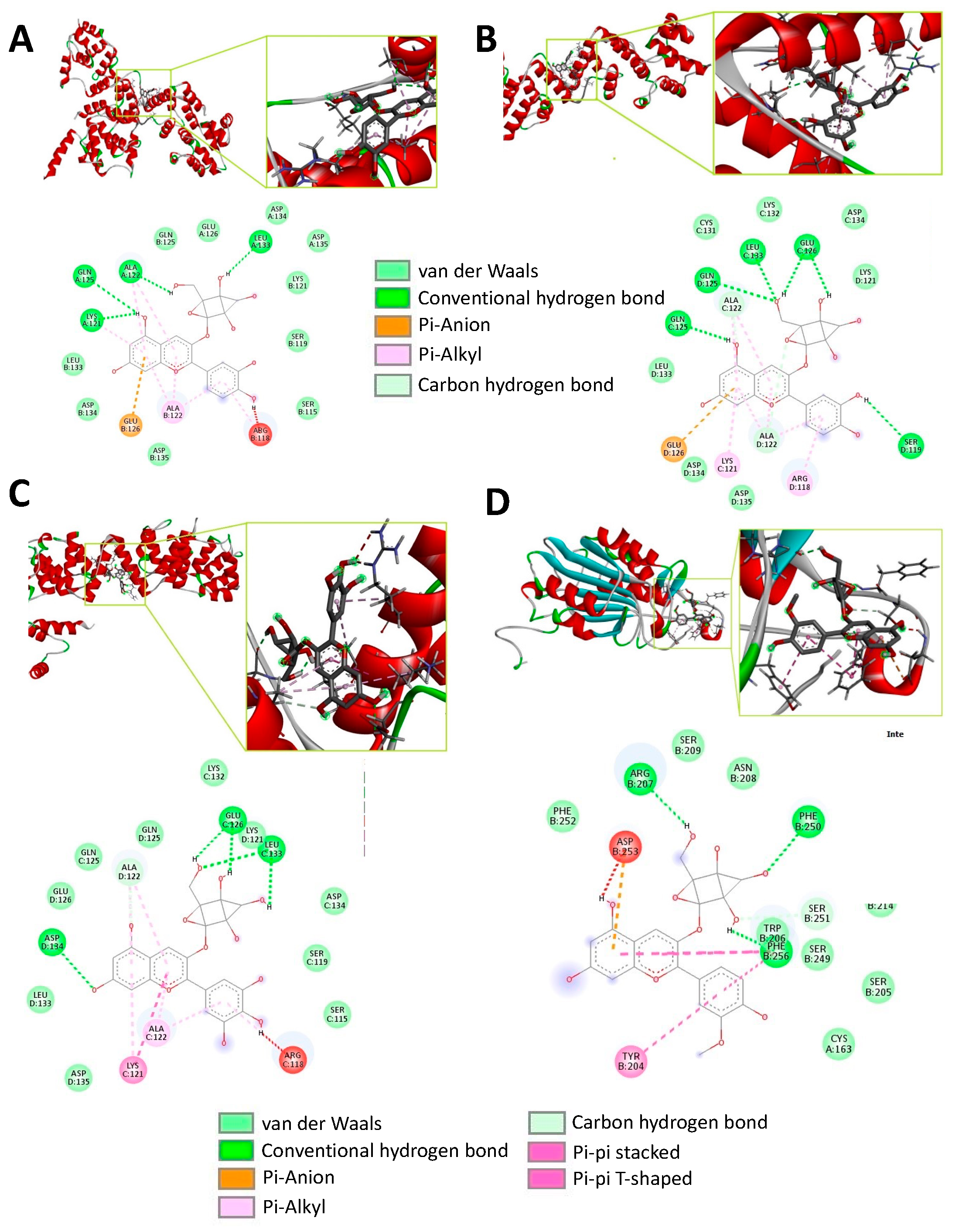
3.2. Molecular Docking Analysis between Caspase 8 and Selected ABJ Anthocyanins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abreu, O.A.; Barreto, G.; Prieto, S. Vaccinium (Ericaceae): Ethnobotany and Pharmacological Potential. Emir. J. Food Agric. 2014, 26, 577–591. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Franco-Tobón, Y.N.; Agudelo, C.; Arango, S.S.; Rojano, B. Andean Berry (Vaccinium Meridionale Swartz). In Fruit and Vegetable Phytochemicals; Yahia, E.M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 869–882. ISBN 9781119158042. [Google Scholar]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Maldonado-Celis, M.E.; Campos-Vega, R. Andean Berry (Vaccinium Meridionale Swartz) Juice in Combination with Aspirin Modulated Anti-Inflammatory Markers on LPS-Stimulated RAW 264.7 Macrophages. Food Res. Int. 2020, 137, 109541. [Google Scholar] [CrossRef] [PubMed]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Reyes-Dieck, C.; Yahia, E.M.; Maldonado-Celis, M.E. Antiproliferative Potential of Andean Berry (Vacciniu m Meridionale Swartz) Juice in Combination with Aspirin in Human SW480 Colon Adenocarcinoma Cells. J. Food Biochem. 2021, 45, e13760. [Google Scholar] [CrossRef] [PubMed]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Maldonado-Celis, M.E. Andean Berry (Vaccinium Meridionale Swartz) Juice, in Combination with Aspirin, Displayed Antiproliferative and pro-Apoptotic Mechanisms in Vitro While Exhibiting Protective Effects against AOM-Induced Colorectal Cancer in Vivo. Food Res. Int. 2022, 157, 111244. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K.; Sulforhodamine, B. Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.D.; Luzardo-Ocampo, I.; Campos-Vega, R.; Loarca-Piña, G.; Maldonado-Celis, M.E. Bioaccessibility during in Vitro Digestion and Antiproliferative Effect of Bioactive Compounds from Andean Berry (Vaccinium Meridionale Swartz) Juice. J. Agric. Food Chem. 2018, 66, 7358–7366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Ali Ashfaq, U.; Usman Mirza, M. Medicinal Plant Phytochemicals and Their Inhibitory Activities against Pancreatic Lipase: Molecular Docking Combined with Molecular Dynamics Simulation Approach. Nat. Prod. Res. 2018, 32, 1123–1129. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.D.; Arango, S.; Cortés-mancera, F.; Rojano, B.; Maldonado-Celis, M.E.; Maldonado, M.E.; Carlos, D.A.; Sandra, A.; Fabián, C.-M.; Benjamín, R.; et al. Antiproliferative and Pro-Apoptotic Effects of Andean Berry Juice (Vaccinium Meridionale Swartz) on Human Colon Adenocarcinoma SW480 Cells. J. Med. Plants Res. 2017, 11, 393–402. [Google Scholar] [CrossRef][Green Version]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total Cranberry Extract vs. Its Phytochemical Consituents: Antiproliferative and Synergistic Effects against Human Tumor Cell Lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Costea, T.; Hudiță, A.; Ciolac, O.-A.; Gălățeanu, B.; Ginghină, O.; Costache, M.; Ganea, C.; Mocanu, M.-M. Chemoprevention of Colorectal Cancer by Dietary Compounds. Int. J. Mol. Sci. 2018, 19, 3787. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Quintero, M.L.; Varela, S.A.; Lopera-Rodriguez, J.A.; Llano-Ramirez, M.A. Molecular Affinity between Caffeic and Chlorogenic Acid with Proteins Associated with Apoptosis Pathways Using Docking Molecular Analysis. In Proceedings of the 2021 IEEE 2nd International Congress of Biomedical Engineering and Bioengineering (CI-IB&BI), Bogota, Colombia, 13–15 October 2021; pp. 1–4. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agudelo-Quintero, M.L.; Luzardo-Ocampo, I.; Lopera-Rodríguez, J.A.; Maldonado-Celis, M.E.; Arango-Varela, S.S. Bioactive Compounds from Andean Berry (Vaccinium meridionale Swartz) Juice Inhibited Cell Viability and Proliferation from SW480 and SW620 Human Colon Adenocarcinoma Cells. Biol. Life Sci. Forum 2022, 18, 17. https://doi.org/10.3390/Foods2022-12984
Agudelo-Quintero ML, Luzardo-Ocampo I, Lopera-Rodríguez JA, Maldonado-Celis ME, Arango-Varela SS. Bioactive Compounds from Andean Berry (Vaccinium meridionale Swartz) Juice Inhibited Cell Viability and Proliferation from SW480 and SW620 Human Colon Adenocarcinoma Cells. Biology and Life Sciences Forum. 2022; 18(1):17. https://doi.org/10.3390/Foods2022-12984
Chicago/Turabian StyleAgudelo-Quintero, Myriam L., Ivan Luzardo-Ocampo, Jorge A. Lopera-Rodríguez, Maria E. Maldonado-Celis, and Sandra S. Arango-Varela. 2022. "Bioactive Compounds from Andean Berry (Vaccinium meridionale Swartz) Juice Inhibited Cell Viability and Proliferation from SW480 and SW620 Human Colon Adenocarcinoma Cells" Biology and Life Sciences Forum 18, no. 1: 17. https://doi.org/10.3390/Foods2022-12984
APA StyleAgudelo-Quintero, M. L., Luzardo-Ocampo, I., Lopera-Rodríguez, J. A., Maldonado-Celis, M. E., & Arango-Varela, S. S. (2022). Bioactive Compounds from Andean Berry (Vaccinium meridionale Swartz) Juice Inhibited Cell Viability and Proliferation from SW480 and SW620 Human Colon Adenocarcinoma Cells. Biology and Life Sciences Forum, 18(1), 17. https://doi.org/10.3390/Foods2022-12984






