Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus pluvialis Flotow: Focus on Gastrointestinal System †
Abstract
:1. Introduction
2. Astaxanthin: “The Red Gold”
2.1. Main Sources of Astaxanthin
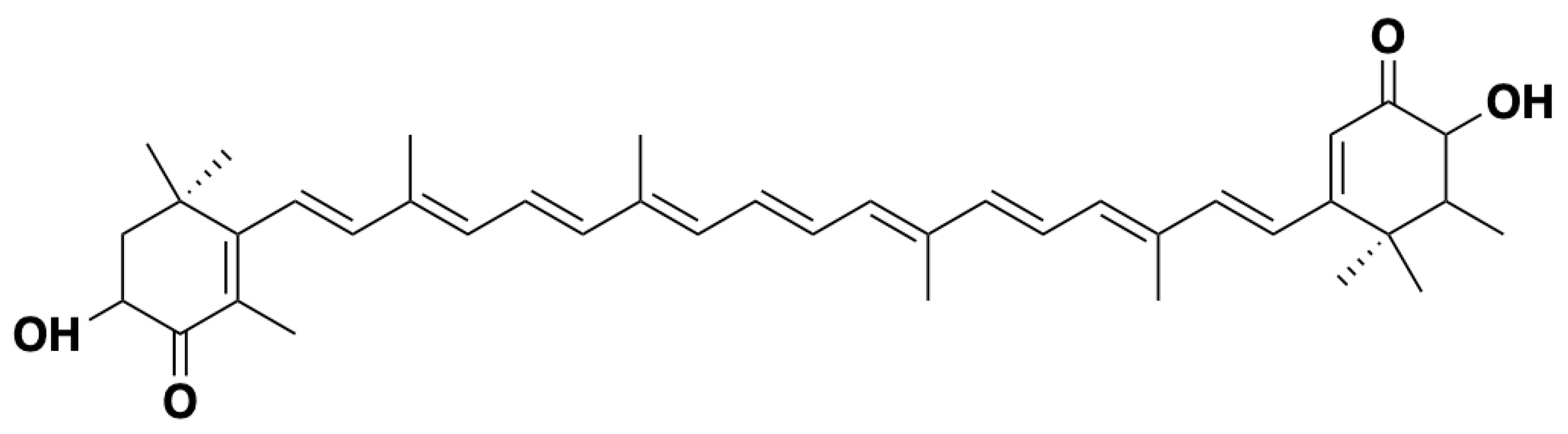
2.2. Chemical Structure of Astaxanthin
3. Astaxanthin: The “Supernutrient”
4. Areas of Action and Use of Astaxanthin
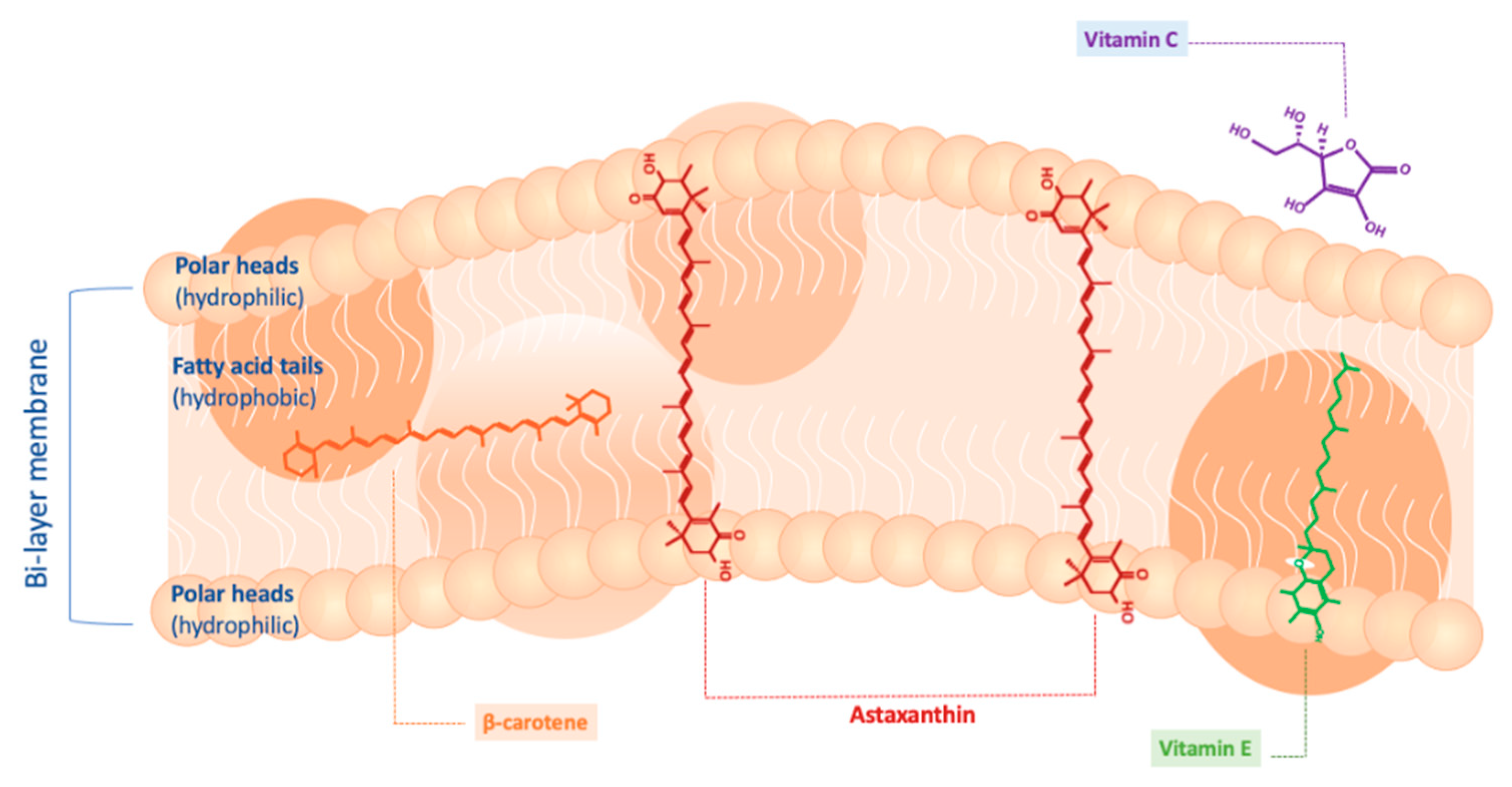
4.1. Antioxidant Action
4.2. Anti-Inflammatory and Gastrointestinal Protective Action
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.-P.; Peng, J.; Yin, K.; Wang, J.-H. Potential Health-Promoting Effects of Astaxanthin: A High-Value Carotenoid Mostly from Microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, C.; Yang, Y.; Kim, B.; Pham, T.; Bae, M.; Park, Y.-K.; Lee, J.-Y. Astaxanthin Plays Anti-Inflammatory and Antioxidant Effects by Inhibiting NFkB Nuclear Translocation and NOX2 Expression in Macrophages. FASEB J. 2015, 29, 603–608. [Google Scholar]
- Candore, G.; Scapagnini, G.; Caruso, C. Aging and Anti-Aging Strategies. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1055–1061. ISBN 978-3-540-89656-2. [Google Scholar]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive Effect of Dietary Astaxanthin on UVA-Induced Skin Photoaging in Hairless Mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.M.; Jones, H.D.T.; Han, D.; Hu, Q.; Beechem, T.E.; Timlin, J.A. Carotenoid Distribution in Living Cells of Haematococcus Pluvialis (Chlorophyceae). PLoS ONE 2011, 6, e24302. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus Pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [Green Version]
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of Astaxanthin Supplementation on Paraoxonase 1 Activities and Oxidative Stress Status in Young Soccer Players. Phytother. Res. 2013, 27, 1536–1542. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Natural Astaxanthin Hawaii’s Supernutrient by William Sears MD: VERY GOOD Paperback (2015)|Discover Books. Available online: https://www.abebooks.com/9780979235344/Natural-Astaxanthin-Hawaiis-Supernutrient-William-0979235340/plp (accessed on 1 March 2022).
- Yang, Y.; Kim, B.; Lee, J.Y. Astaxanthin Structure, Metabolism, and Health Benefits. J. Hum. Nutr. Food Sci. 2013, 1, 1–1003. [Google Scholar]
- Seabra, L.M.J.; Pedrosa, L.F.C. Astaxanthin: Structural and Functional Aspects. Rev. Nutr. 2010, 23, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of Its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of Astaxanthin in Haematococcus Algal Extract: The Effects of Timing of Diet and Smoking Habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef] [Green Version]
- Ranga Rao, A.; Raghunath Reddy, R.L.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. Characterization of Microalgal Carotenoids by Mass Spectrometry and Their Bioavailability and Antioxidant Properties Elucidated in Rat Model. J. Agric. Food Chem. 2010, 58, 8553–8559. [Google Scholar] [CrossRef]
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching Activities of Common Hydrophilic and Lipophilic Antioxidants against Singlet Oxygen Using Chemiluminescence Detection System. Carotenoid Sci. 2007, 11, 16–20. [Google Scholar] [CrossRef]
- Paterson, E.; Gordon, M.H.; Niwat, C.; George, T.W.; Parr, L.; Waroonphan, S.; Lovegrove, J.A. Supplementation with Fruit and Vegetable Soups and Beverages Increases Plasma Carotenoid Concentrations but Does Not Alter Markers of Oxidative Stress or Cardiovascular Risk Factors. J. Nutr. 2006, 136, 2849–2855. [Google Scholar] [CrossRef] [Green Version]
- Kidd, P. Astaxanthin, Cell Membrane Nutrient with Diverse Clinical Benefits and Anti-Aging Potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, Canthaxanthin and Beta-Carotene Differently Affect UVA-Induced Oxidative Damage and Expression of Oxidative Stress-Responsive Enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Hernández Blanco, I.; Hoffmann, T.; Martin, H.-D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative Assessment of Antioxidant Properties of Natural Colorants and Phytochemicals: Carotenoids, Flavonoids, Phenols and Indigoids. The Role of β-Carotene in Antioxidant Functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Yun, Y.-S.; Park, J.M.; Yang, J.-W. Determination of the Time Transferring Cells for Astaxanthin Production Considering Two-Stage Process of Haematococcus Pluvialis Cultivation. Bioresour. Technol. 2011, 102, 11249–11253. [Google Scholar] [CrossRef]
- Han, H.; Lim, J.W.; Kim, H. Astaxanthin Inhibits Helicobacter Pylori-Induced Inflammatory and Oncogenic Responses in Gastric Mucosal Tissues of Mice. J. Cancer Prev. 2020, 25, 244–251. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Wang, X.; Willén, R.; Wadström, T. Astaxanthin-Rich Algal Meal and Vitamin C Inhibit Helicobacter Pylori Infection in BALB/cA Mice. Antimicrob. Agents Chemother. 2000, 44, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.P.; Holck, S.; Kupcinskas, L.; Kiudelis, G.; Jonaitis, L.; Janciauskas, D.; Permin, H.; Wadström, T. Gastric Inflammatory Markers and Interleukins in Patients with Functional Dyspepsia Treated with Astaxanthin. FEMS Immunol. Med. Microbiol. 2007, 50, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Sakai, S.; Nishida, A.; Ohno, M.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; Andoh, A. Astaxanthin, a Xanthophyll Carotenoid, Prevents Development of Dextran Sulphate Sodium-Induced Murine Colitis. J. Clin. Biochem. Nutr. 2019, 64, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Kochi, T.; Shimizu, M.; Sumi, T.; Kubota, M.; Shirakami, Y.; Tanaka, T.; Moriwaki, H. Inhibitory Effects of Astaxanthin on Azoxymethane-Induced Colonic Preneoplastic Lesions in C57/BL/KsJ-Db/Db Mice. BMC Gastroenterol. 2014, 14, 212. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Lim, J.W.; Kim, H. Astaxanthin Inhibits Mitochondrial Dysfunction and Interleukin-8 Expression in Helicobacter Pylori-Infected Gastric Epithelial Cells. Nutrients 2018, 10, 1320. [Google Scholar] [CrossRef] [Green Version]
- Kupcinskas, L.; Lafolie, P.; Lignell, A.; Kiudelis, G.; Jonaitis, L.; Adamonis, K.; Andersen, L.P.; Wadström, T. Efficacy of the Natural Antioxidant Astaxanthin in the Treatment of Functional Dyspepsia in Patients with or without Helicobacter Pylori Infection: A Prospective, Randomized, Double Blind, and Placebo-Controlled Study. Phytomedicine 2008, 15, 391–399. [Google Scholar] [CrossRef]
- Liu, F.; Smith, A.D.; Solano-Aguilar, G.; Wang, T.T.Y.; Pham, Q.; Beshah, E.; Tang, Q.; Urban, J.F.; Xue, C.; Li, R.W. Mechanistic Insights into the Attenuation of Intestinal Inflammation and Modulation of the Gut Microbiome by Krill Oil Using in Vitro and in Vivo Models. Microbiome 2020, 8, 83. [Google Scholar] [CrossRef]
- Wu, L.; Lyu, Y.; Srinivasagan, R.; Wu, J.; Ojo, B.; Tang, M.; El-Rassi, G.D.; Metzinger, K.; Smith, B.J.; Lucas, E.A.; et al. Astaxanthin-Shifted Gut Microbiota Is Associated with Inflammation and Metabolic Homeostasis in Mice. J. Nutr. 2020, 150, 2687–2698. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Tie, S.; Li, J.; Su, W.; Tan, M. A Smart Cauliflower-like Carrier for Astaxanthin Delivery to Relieve Colon Inflammation. J. Control Release 2022, 342, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, L.; Qiao, X.; Xue, C.; Xu, J. Dietary Astaxanthin: An Excellent Carotenoid with Multiple Health Benefits. Crit. Rev. Food Sci. Nutr. 2021, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Wang, H.; Xiao, S.; Li, C.; Li, Y.; Liu, B. Xanthophyllomyces Dendrorhous-Derived Astaxanthin Regulates Lipid Metabolism and Gut Microbiota in Obese Mice Induced by A High-Fat Diet. Mar. Drugs 2019, 17, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, M.; Fu, X.; Zhang, Z.; Zhu, L.; Zheng, X.; Liu, J. Astaxanthin Prevents Alcoholic Fatty Liver Disease by Modulating Mouse Gut Microbiota. Nutrients 2018, 10, 1298. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ma, H.; Guan, S.; Luo, T.; Zhao, C.; Cai, G.; Zheng, Y.; Jia, X.; Di, J.; Li, R.; et al. Astaxanthin from Haematococcus Pluvialis Alleviates Obesity by Modulating Lipid Metabolism and Gut Microbiota in Mice Fed a High-Fat Diet. Food Funct. 2021, 12, 9719–9738. [Google Scholar] [CrossRef]
- Akduman, H.; Tayman, C.; Korkmaz, V.; Akduman, F.; Fettah, N.D.; Gürsoy, B.K.; Turkmenoglu, T.T.; Çağlayan, M. Astaxanthin Reduces the Severity of Intestinal Damage in a Neonatal Rat Model of Necrotizing Enterocolitis. Am. J. Perinatol. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budriesi, R.; Micucci, M.; Daglia, M.; Corazza, I.; Biotti, G.; Mattioli, L.B. Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus pluvialis Flotow: Focus on Gastrointestinal System. Biol. Life Sci. Forum 2022, 12, 31. https://doi.org/10.3390/IECN2022-12376
Budriesi R, Micucci M, Daglia M, Corazza I, Biotti G, Mattioli LB. Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus pluvialis Flotow: Focus on Gastrointestinal System. Biology and Life Sciences Forum. 2022; 12(1):31. https://doi.org/10.3390/IECN2022-12376
Chicago/Turabian StyleBudriesi, Roberta, Matteo Micucci, Maria Daglia, Ivan Corazza, Giulia Biotti, and Laura Beatrice Mattioli. 2022. "Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus pluvialis Flotow: Focus on Gastrointestinal System" Biology and Life Sciences Forum 12, no. 1: 31. https://doi.org/10.3390/IECN2022-12376
APA StyleBudriesi, R., Micucci, M., Daglia, M., Corazza, I., Biotti, G., & Mattioli, L. B. (2022). Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus pluvialis Flotow: Focus on Gastrointestinal System. Biology and Life Sciences Forum, 12(1), 31. https://doi.org/10.3390/IECN2022-12376








