Oral Administration of Rauwolfia serpentina Plant Extract Mitigated Immobilization Stress-Induced Behavioral and Biochemic and Deficits in Rats †
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Prepararation of Plant Extract
2.3. Immobilization Procedure
2.4. Behavioral Analysis
2.4.1. Activity in a Novel Environment (Open Field)
2.4.2. Light–Dark Transition Test
2.5. Blood Sample Collection
2.6. Biochemical Estimation of Glucose, Catalase and Superoxide Dismutase in Plasma
2.6.1. Determination of Catalase (EC1.11.1.6)
2.6.2. Determination of Superoxide Dismutase (EC.1.15.1.1)
2.6.3. Estimation of Glucose in Plasma by GOD-PAP Method
2.6.4. Estimation of Leptin and Corticosterone in Plasma by ELISAKit
3. Experimental Protocol
Statistical Analysis
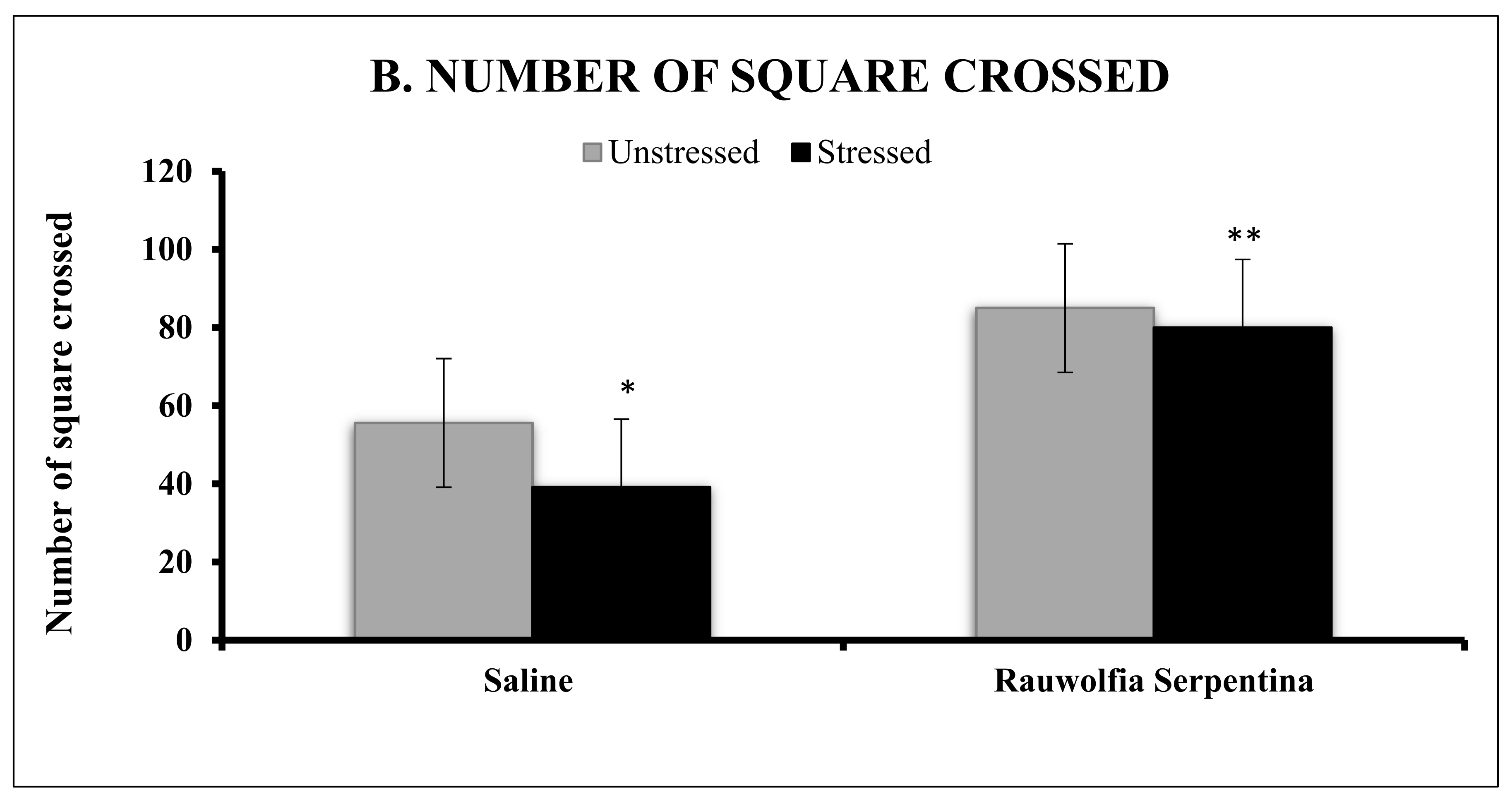
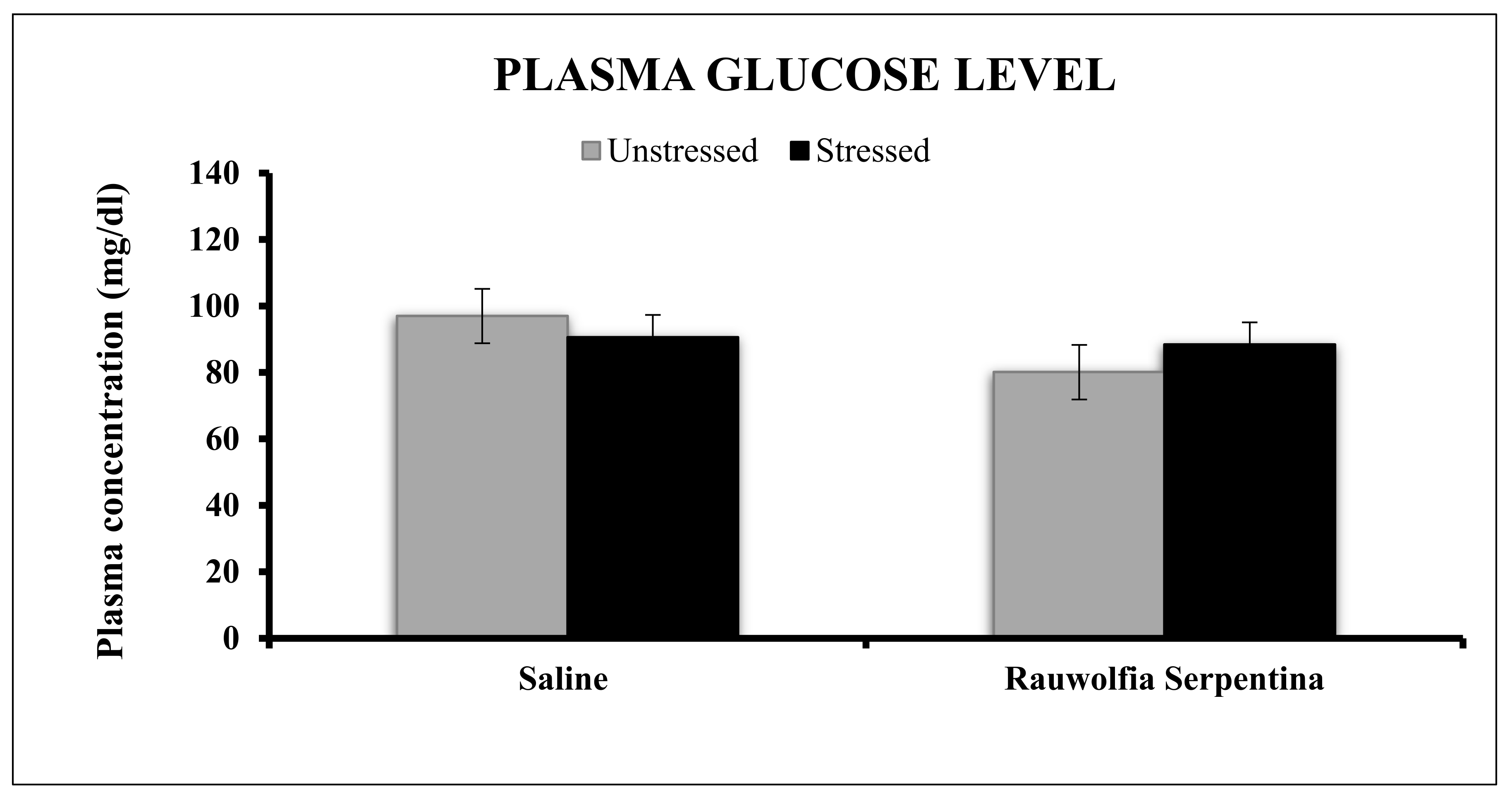
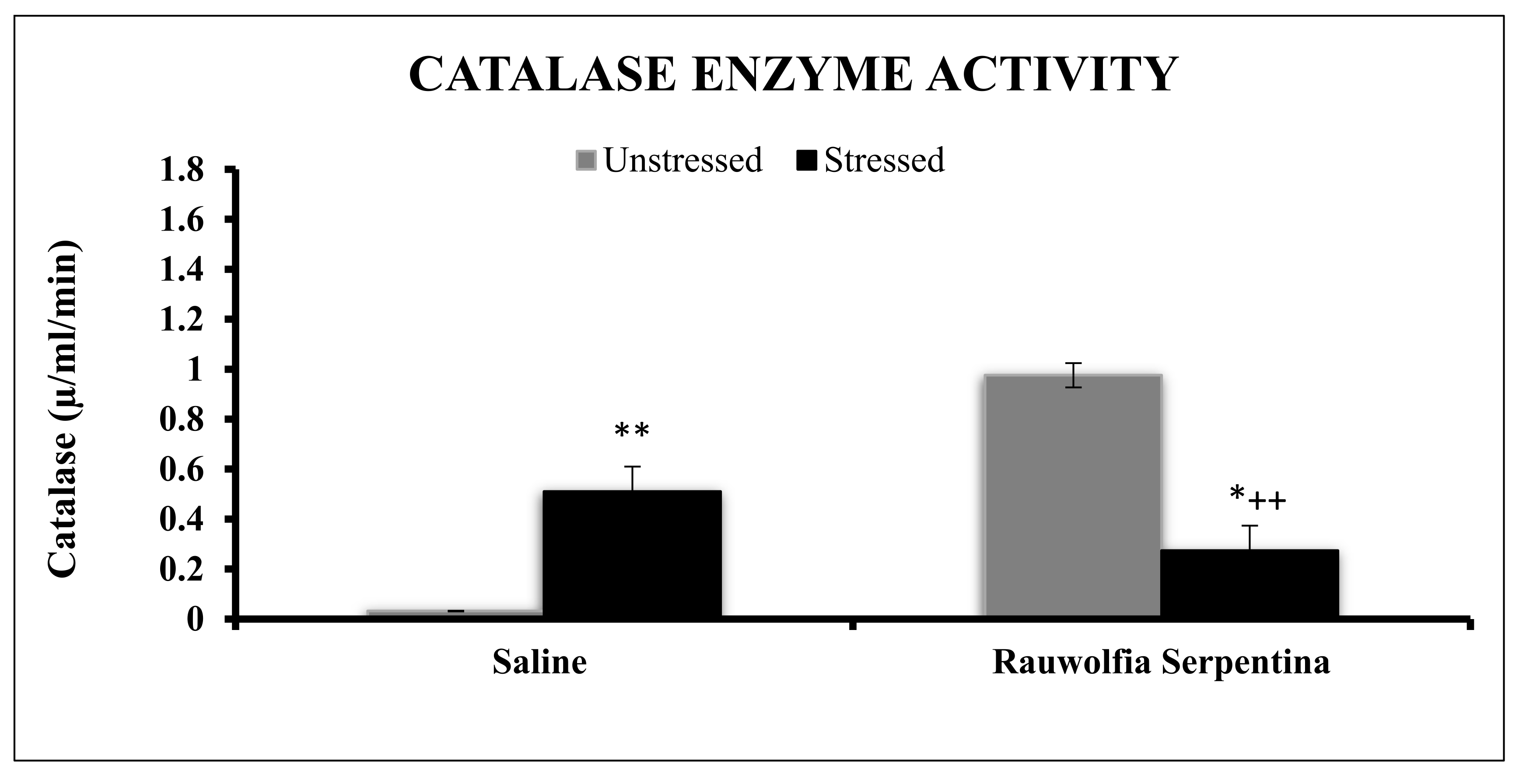
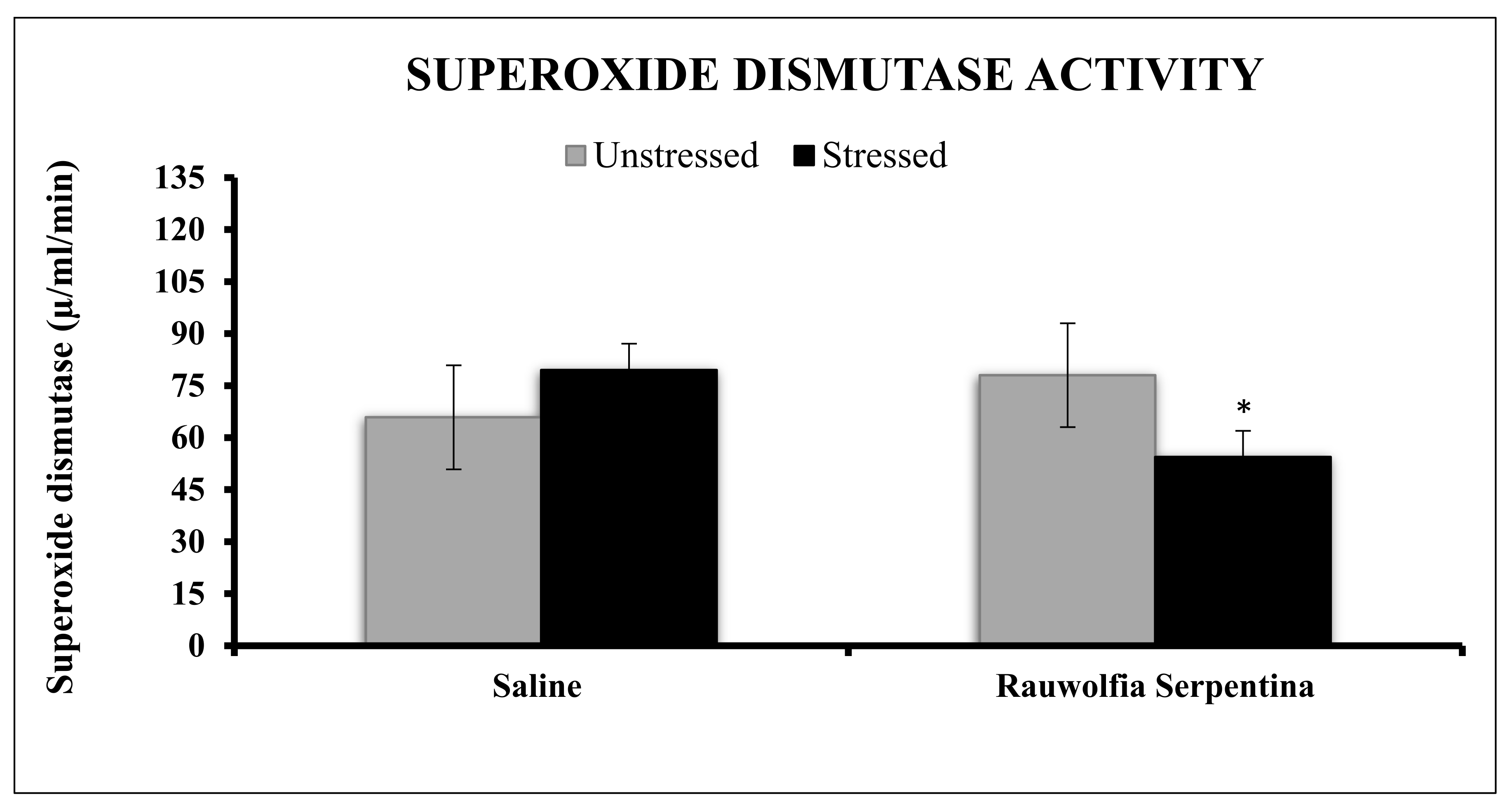
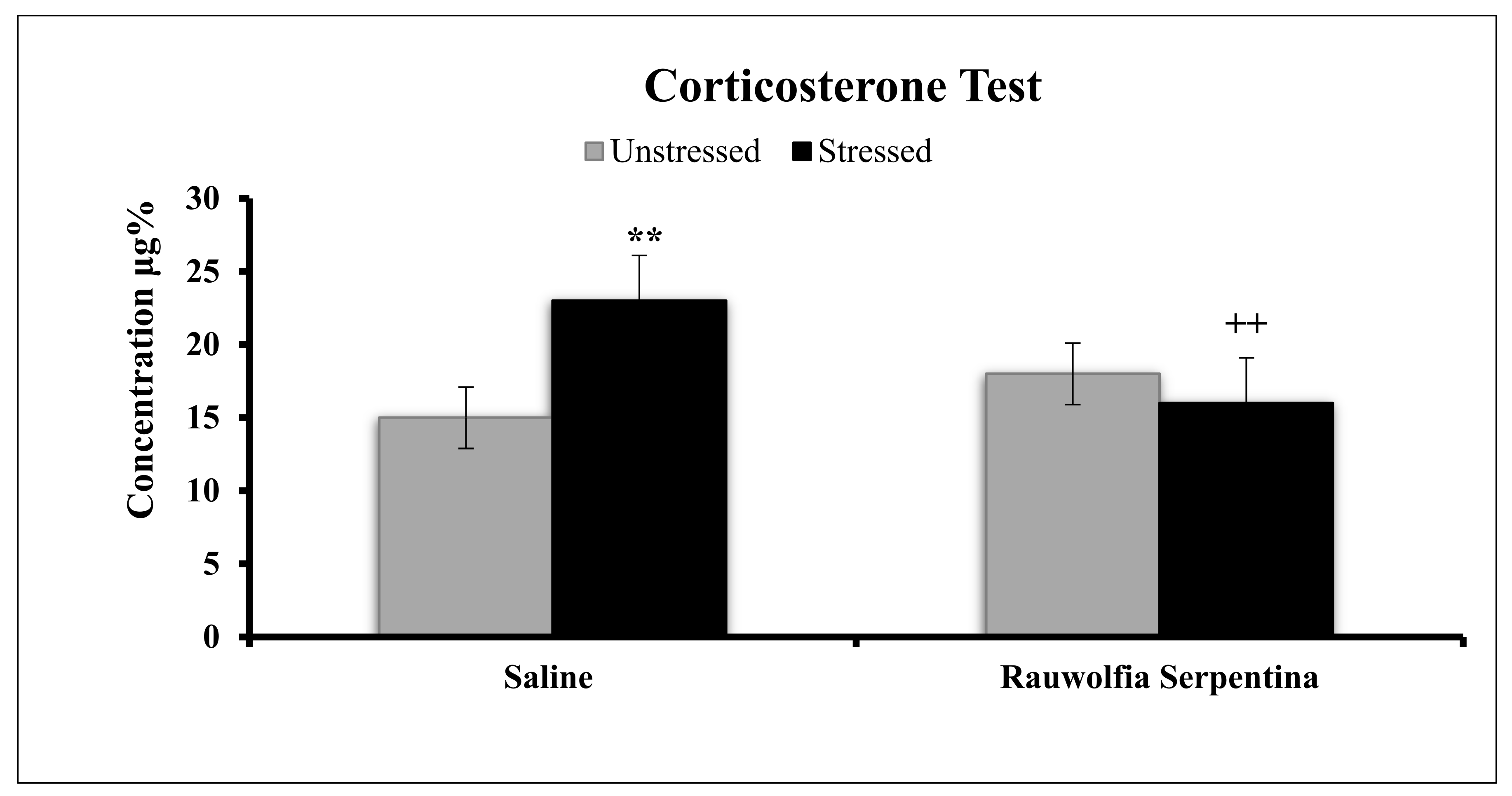
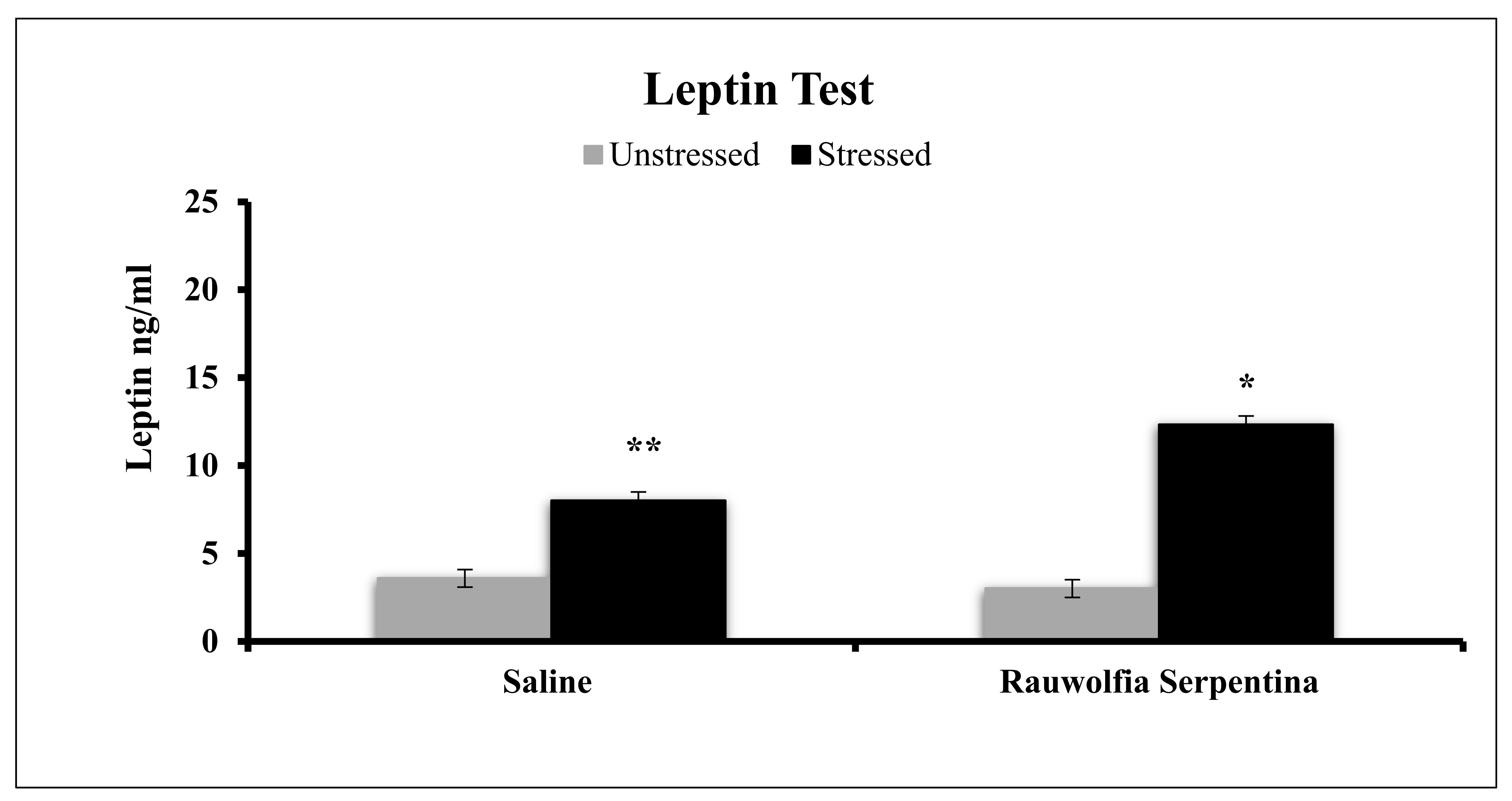
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| Catalase | CAT |
| Superoxide dismutase | SOD |
| Oxidative stress | OS |
| Reactive oxygen species | ROS |
| Glutathione peroxidase | GPx |
| Nigella sativa | NS |
| Olea europaea | OE |
| Methanolic root extract | MREt |
| Paraventricular nucleus | PVN |
| Corticotropin-releasing factor | CRF |
| Adrenocorticotropin hormone | ACTH |
References
- Raio, C.M.; Phelps, E.A. The influence of acute stress on the regulation of conditioned fear. Neurobiol. Stress 2015, 1, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.R. Coping with Stress: Commonsense Strategies; McFarland: Jefferson, NC, USA, 2007; ISBN 978-0-7864-2875-5. [Google Scholar]
- Dhir, A.; Padi, S.S.V.; Naidu, P.S.; Kulkarni, S.K. Protective effect of naproxen (non-selective COX-inhibitor) or rofecoxib (selective COX-2 inhibitor) on immobilization stress-induced behavioral and biochemical alterations in mice. Eur. J. Pharmacol. 2006, 535, 192–198. [Google Scholar] [CrossRef]
- Imbe, H.; Iwai-Liao, Y.; Senba, E. Stress-induced hyperalgesia: Animal models and putative mechanisms. Front. Biosci. 2006, 11, 2179–2192. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. The Ever-Changing Brain: Cellular and Molecular Mechanisms for the Effects of Stressful Experiences. Dev. Neurobiol. 2012, 72, 878–890. [Google Scholar] [CrossRef]
- Weiss, J.M.; Goodman, P.A.; Losito, B.G.; Corrigan, S.; Charry, J.M.; Bailey, W.H. Behavioral depression produced by an uncontrollable stressor: Relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res. Rev. 1981, 3, 167–205. [Google Scholar] [CrossRef]
- Parveen, T.; Haider, S.; Mumtaz, W.; Razi, F.; Tabassum, S.; Haleem, D.J. Attenuation of stress-induced behavioral deficits by lithium administration via serotonin metabolism. Pharmacol. Rep. 2013, 65, 336–342. [Google Scholar] [CrossRef]
- Day, P. Killers in the Brain: Essays in Science and Technology from the Royal Institution; Oxford University Press: Oxford, UK, 1999; ISBN 978-0-19-850540-2. [Google Scholar]
- Doble, A. The role of excitotoxicity in neurodegenerative disease: Implications for therapy. Pharmacol. Ther. 1999, 81, 163–221. [Google Scholar] [CrossRef]
- Beal, M.F.; Hyman, B.T.; Koroshetz, W. Do defecs in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci. 1993, 16, 125–131. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondrial dysfunction in neurodegenerative diseases. Biochim. Biophys. Acta (BBA)-Bioenerg. 1998, 1366, 211–223. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Ji, L.L. Exercise and oxidative stress: Role of the cellular antioxidant systems. Exerc. Sport Sci. Rev. 1995, 23, 135–166. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.-H. Severe Life Stress and Oxidative Stress in the Brain: From Animal Models to Human Pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.E.; Specht, E.; Broedbaek, K.; Henriksen, T.; Ellervik, C.; Mandrup-Poulsen, T.; Tonnesen, M.; Nielsen, P.E.; Andersen, H.U.; Weimann, A. RNA modifications by oxidation: A novel disease mechanism? Free Radic. Biol. Med. 2012, 52, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Tiwari, S. Plants: A rich source of herbal medicine. J. Nat. Prod. 2008, 1, 27–35. [Google Scholar]
- Kumari, R.; Rathi, B.; Rani, A.; Bhatnagar, S. Rauvolfia serpentina L. Benth. ex Kurz.: Phytochemical, Pharmacological and Therapeutic Aspects. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 348–355. [Google Scholar]
- Ghani, A. Medicinal plants of Bangladesh: Chemical constituents and uses. In Medicinal Plants of Bangladesh: Chemical Constituents and Uses; Asiatic Society of Bangladesh: Nimtali, Bangladesh, 1998. [Google Scholar]
- Qureshi, S.A.; Udani, S.K. Hypolipidaemic activity of Rauwolfia serpentina benth. Pak. J. Nutr. 2009, 8, 1103–1106. [Google Scholar] [CrossRef]
- Itoh, A.; Kumashiro, T.; Yamaguchi, M.; Nagakura, N.; Mizushina, Y.; Nishi, T.; Tanahashi, T. Indole Alkaloids and Other Constituents of Rauwolfia serpentina. J. Nat. Prod. 2005, 68, 848–852. [Google Scholar] [CrossRef]
- Dey, A.; De, J.N. Ethnobotanical aspects of Rauvolfia serpentina (L). Benth. ex Kurz. in India, Nepal and Bangladesh. JMPR 2011, 5, 144–150. [Google Scholar]
- Tyler, V.E.; Robbers, J.E.; Brady, L.R. Pharmacognosy, 9th ed.; Lee & Febiger: Philadelphia, PA, USA, 1988; ISBN 978-0-8121-1071-5. [Google Scholar]
- Mehdi, B.J.; Tabassum, S.; Haider, S.; Perveen, T.; Nawaz, A.; Haleem, D.J. Nootropic and anti-stress effects of rice bran oil in male rats. J. Food Sci. Technol. 2015, 52, 4544–4550. [Google Scholar] [CrossRef] [PubMed]
- Perveen, T.; Hashmi, B.M.; Haider, S.; Tabassum, S.; Saleem, S.; Siddiqui, M.A. Role of Monoaminergic System in the Etiology of Olive Oil Induced Antidepressant and Anxiolytic Effects in Rats. Available online: https://www.hindawi.com/journals/isrn/2013/615685/ (accessed on 27 July 2018).
- Batool, F.; Shah, A.H.; Ahmed, S.D.; Saify, Z.S.; Haleem, D.J. Protective effects of aqueous fruit extract from Sea Buckthorn (Hippophae rhamnoides L. Spp. Turkestanica) on haloperidol-induced orofacial dyskinesia and neuronal alterations in the striatum. Med. Sci. Monit. 2010, 16, BR285–BR292. [Google Scholar] [PubMed]
- Naz, F.; Shireen, E. Suppression and treatment of Haloperidol induced extra-pyramidal side effects and anxiety syndrome by the coadministeration of red rice bran oil in rats. Int. J. Endors Health Sci. Res. 2014, 2, 82–92. [Google Scholar] [CrossRef]
- Cheema, M.A.R.; Nawaz, S.; Gul, S.; Salman, T.; Naqvi, S.; Dar, A.; Haleem, D.J. Neurochemical and behavioral effects of Nigella sativa and Olea europaea oil in rats. Nutr. Neurosci. 2018, 21, 185–194. [Google Scholar] [CrossRef]
- Azmi, M.B.; Qureshi, S.A. Methanolic Root Extract of Rauwolfia serpentina Benth Improves the Glycemic, Antiatherogenic, and Cardioprotective Indices in Alloxan-Induced Diabetic Mice. Available online: https://www.hindawi.com/journals/aps/2012/376429/ref/ (accessed on 5 July 2017).
- Haider, S.; Khaliq, S.; Ahmed, S.P.; Haleem, D.J. Long-term tryptophan administration enhances cognitive performance and increases 5HT metabolism in the hippocampus of female rats. Amino Acids 2006, 31, 421–425. [Google Scholar] [CrossRef]
- Haleem, D.J.; Parveen, T. Brain regional serotonin synthesis following adaptation to repeated restraint. Neuroreport 1994, 5, 1785–1788. [Google Scholar] [CrossRef]
- Haleem, D.J.; Kennett, G.; Curzon, G. Adaptation of female rats to stress: Shift to male pattern by inhibition of corticosterone synthesis. Brain Res. 1988, 458, 339–347. [Google Scholar] [CrossRef]
- Kennett, G.A.; Dickinson, S.L.; Curzon, G. Enhancement of some 5-HT-dependent behavioural responses following repeated immobilization in rats. Brain Res. 1985, 330, 253–263. [Google Scholar] [CrossRef]
- Haleem, D.J.; Naz, H.; Parveen, T.; Haider, S.; Ahmed, S.P.; Khan, N.H.; Haleem, M.A. Serotonin and serotonin 1-A receptors in the failure of ethanol-treated rats to adapt to a repeated stress schedule. J. Stud. Alcohol. 2002, 63, 389–396. [Google Scholar] [CrossRef]
- Haleem, D.J. Behavioral deficits and exaggerated feedback control over raphe-hippocampal serotonin neurotransmission in restrained rats. Pharmacol. Rep. 2011, 63, 888–897. [Google Scholar] [CrossRef]
- Patterson, B.D.; Payne, L.A.; Chen, Y.-Z.; Graham, D. An Inhibitor of Catalase Induced by Cold in Chilling-Sensitive Plants. Plant. Physiol. 1984, 76, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.S. Effects of double stress on antioxidant enzyme activity in Vigna radiata (L.) Wilczek. Acta Bot. Croat. 2013, 72, 145–156. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Gross, J.J.; Thompson, R.A. Emotion Regulation: Conceptual Foundations. In Handbook of Emotion Regulation; Guilford Press: New York, NY, USA, 2007; pp. 3–24. ISBN 978-1-59385-148-4. [Google Scholar]
- Esch, T.; Stefano, G.B.; Fricchione, G.L.; Benson, H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol. Lett. 2002, 23, 199–208. [Google Scholar]
- McEwen, B.S. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004, 1032, 1–7. [Google Scholar] [CrossRef]
- Poleszak, E.; Wlaź, P.; Kedzierska, E.; Nieoczym, D.; Wyska, E.; Szymura-Oleksiak, J.; Fidecka, S.; Radziwoń-Zaleska, M.; Nowak, G. Immobility stress induces depression-like behavior in the forced swim test in mice: Effect of magnesium and imipramine. Pharmacol. Rep. 2006, 58, 746–752. [Google Scholar]
- Belzung, C.; Misslin, R.; Vogel, E.; Dodd, R.H.; Chapouthier, G. Anxiogenic effects of methyl-β-carboline-3-carboxylate in a light/dark choice situation. Pharmacol. Biochem. Behav. 1987, 28, 29–33. [Google Scholar] [CrossRef]
- Ardayfio, P.; Kim, K.-S. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav. Neurosci. 2006, 120, 249–256. [Google Scholar] [CrossRef]
- Sourabh, P. Ethnomedicinal uses and cultivation of Rauvolfia serpentina Benth.: A minireview. Recent Adv. Med. Plants Cultiv. 2012, 40, 153–159. [Google Scholar]
- Chaudhary, R.; Singh, B.; Chhillar, A.K. Ethanomedicinal Importances of Rauvolfia serpentine L. Benth. Ex Kurz in the Prevention and Treatment of Diseases. Available online: https://www.researchgate.net/publication/306358162_Ethanomedicinal_Importances_of_Rauvolfia_serpentine_L_Benth_Ex_Kurz_in_the_Prevention_and_Treatment_of_Diseases_PDF_created_with_pdfFactory_Pro_trial_version_wwwpdffactorycom (accessed on 1 March 2022).
- Bhardwaj, N.; Yadav, M. Evaluation of the chemical composition of Rauwolfia serpentina and Leucas aspera—A comparative study. World J. Pharm. Pharm. Sci. 2016, 5, 914–920. [Google Scholar]
- Rasheed, N.; Ahmad, A.; Al-Sheeha, M.; Alghasham, A.; Palit, G. Neuroprotective and anti-stress effect of A68930 in acute and chronic unpredictable stress model in rats. Neurosci. Lett. 2011, 504, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Sorce, S.; Krause, K.-H. NOX enzymes in the central nervous system: From signaling to disease. Antioxid. Redox Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Masood, A.; Masood, N.; Gilani, R.A.; Shah, Z.A. Immobilization stress causes extra-cellular oxidant-antioxidant imbalance in rats: Restoration by L-NAME and vitamin E. Eur. Neuropsychopharmacol. 2006, 16, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kamper, E.F.; Chatzigeorgiou, A.; Tsimpoukidi, O.; Kamper, M.; Dalla, C.; Pitychoutis, P.Μ.; Papadopoulou-Daifoti, Z. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol. Behav. 2009, 98, 215–222. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef]
- Sumazian, Y.; Syahida, A.; Hakiman, M.; Maziah, M. Antioxidant activities, flavonoids, ascorbic acid and phenolic contents of Malaysian vegetables. J. Med. Plants Res. 2010, 4, 881–890. [Google Scholar]
- Badami, S.; Channabasavaraj, K.P. In Vitro. Antioxidant Activity of Thirteen Medicinal Plants of India’s Western Ghats. Pharm. Biol. 2007, 45, 392–396. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Harisaranraj, R.; Suresh, K.; Saravanababu, S. Evaluation of the Chemical Composition Rauwolfia serpentina and Ephedra vulgaris. Adv. Biol. Res. 2009, 3, 174–178. [Google Scholar]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Anusha, C.; Sarumathi, A.; Shanmugapriya, S.; Anbu, S.; Ahmad, R.S.; Saravanan, N. The effects of aqueous leaf extract of Aegle marmelos on immobilization-induced stress in male albino Wistar rats. Int. J. Nutr. Pharmacol. Neurol. Dis. 2013, 3, 11. [Google Scholar] [CrossRef]
- Dean, O.; Giorlando, F.; Berk, M. N-acetylcysteine in psychiatry: Current therapeutic evidence and potential mechanisms of action. J. Psychiatry Neurosci. 2011, 36, 78–86. [Google Scholar] [CrossRef]
- Seo, J.-S.; Park, J.-Y.; Choi, J.; Kim, T.-K.; Shin, J.-H.; Lee, J.-K.; Han, P.-L. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J. Neurosci. 2012, 32, 9690–9699. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Priftis, K.N. Regulation of the Hypothalamic-Pituitary-Adrenal Axis. Neuroimmunomodulation 2009, 16, 265–271. [Google Scholar] [CrossRef]
- Kino, T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: Implications to mood disorders. Front. Physiol. 2015, 6, 230. [Google Scholar] [CrossRef]
- Siswanto, H.; Hau, J.; Carlsson, H.-E.; Goldkuhl, R.; Abelson, K.S.P. Corticosterone concentrations in blood and excretion in faeces after ACTH administration in male Sprague-Dawley rats. In Vivo 2008, 22, 435–440. [Google Scholar]
- Haleem, D.J. Repeated corticosterone treatment attenuates behavioural and neuroendocrine responses to 8-hydroxy-2-(di-n-propylamino) tetralin in rats. Life Sci. 1992, 51, PL225–PL230. [Google Scholar] [CrossRef]
- Haleem, D.J.; Kennett, G.A.; Whitton, P.S.; Curzon, G. 8-OH-DPAT increases corticosterone but not other 5-HT1A receptor-dependent responses more in females. Eur. J. Pharmacol. 1989, 164, 435–443. [Google Scholar] [CrossRef]
- Hidalgo, J.; Armario, A.; Flos, R.; Dingman, A.; Garvey, J.S. The influence of restraint stress in rats on metallothionein production and corticosterone and glucagon secretion. Life Sci. 1986, 39, 611–616. [Google Scholar] [CrossRef]
- Ahn, T.; Bae, C.-S.; Yun, C.-H. Acute stress-induced changes in hormone and lipid levels in mouse plasma. Vet. Med. 2016, 61, 57–64. [Google Scholar] [CrossRef]
- Urhausen, A.; Gabriel, H.; Kindermann, W. Blood hormones as markers of training stress and overtraining. Sports Med. 1995, 20, 251–276. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Bauer, M.E.; Perks, P.; Lightman, S.L.; Shanks, N. Restraint stress is associated with changes in glucocorticoid immunoregulation. Physiol. Behav. 2001, 73, 525–532. [Google Scholar] [CrossRef]
- Ainsah, O.; Nabishah, B.; Osman, C.; Khalid, B. Naloxone and vitamin E block stress-induced reduction of locomotor activity and elevation of plasma corticosterone. Exp. Clin. Endocrinol. Diabetes 2009, 107, 462–467. [Google Scholar] [CrossRef]
- Bratusch-Marrain, P.R. Insulin-counteracting hormones: Their impact on glucose metabolism. Diabetologia 1983, 24, 74–79. [Google Scholar] [CrossRef][Green Version]
- Yamada, F.; Inoue, S.; Saitoh, T.; Tanaka, K.; Satoh, S.; Takamura, Y. Glucoregulatory hormones in the immobilization stress-induced increase of plasma glucose in fasted and fed rats. Endocrinology 1993, 132, 2199–2205. [Google Scholar] [CrossRef]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef]
- Leung, K.; Munck, A. Peripheral actions of glucocorticoids. Annu. Rev. Physiol. 1975, 37, 245–272. [Google Scholar] [CrossRef] [PubMed]
- Droste, S.K.; de Groote, L.; Atkinson, H.C.; Lightman, S.L.; Reul, J.M.H.M.; Linthorst, A.C.E. Corticosterone Levels in the Brain Show a Distinct Ultradian Rhythm but a Delayed Response to Forced Swim Stress. Endocrinology 2008, 149, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- Donald, W.; Kufe, M.D.; Raphael, E.; Pollock, M.D.; Ralph, R.; Weichselbaum, M.D.; Robert, C. Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Hamilton, ON, Canada, 2003; ISBN 978-1-55009-213-4. [Google Scholar]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish. Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Flodmark, L.E.W.; Urke, H.A.; Halleraker, J.H.; Arnekleiv, J.V.; Vøllestad, L.A.; Poléo, A.B.S. Cortisol and glucose responses in juvenile brown trout subjected to a fluctuating flow regime in an artificial stream. J. Fish. Biol. 2002, 60, 238–248. [Google Scholar] [CrossRef]
- Martínez, M.; Martínez, L.R. Cortisol and Glucose: Reliable indicators of fish stress? Pan-Am. J. Aquat. Sci. 2009, 14, 158–178. [Google Scholar]
- Slieker, L.J.; Sloop, K.W.; Surface, P.L.; Kriauciunas, A.; LaQuier, F.; Manetta, J.; Bue-Valleskey, J.; Stephens, T.W. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J. Biol. Chem. 1996, 271, 5301–5304. [Google Scholar] [CrossRef]
- Armario, A.; Castellanos, J.M.; Balasch, J. Effect of acute and chronic psychogenic stress on corticoadrenal and pituitary-thyroid hormones in male rats. Horm. Res. 1984, 20, 241–245. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Qu, D.; Frederich, R.C.; Susulic, V.S.; Lowell, B.B.; Maratos-Flier, E.; Flier, J.S. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes 1996, 45, 909–914. [Google Scholar] [CrossRef]
- Patterson-Buckendahl, P.; Pohorecky, L.A.; Kvetnansky, R. Differing effects of acute and chronic stressors on plasma osteocalcin and leptin in rats. Stress 2007, 10, 163–172. [Google Scholar] [CrossRef]
- Zareian, P.; Karimi, M.V.; Dorneyani, G. The comparison of the effects of acute swimming stress on plasma corticosterone and leptin concentration in male and female rats. Acta Med. Iran. 2011, 49, 284–287. [Google Scholar]
- Chandralekha, G.; Jeganathan, R.; Viswanathan; Charan, J.C. Serum Leptin and Corticosterone Levels after Exposure to Noise Stress in Rats. Malays. J. Med. Sci. 2005, 12, 51–56. [Google Scholar] [PubMed]
- Haleem, D.J. Investigations into the involvement of leptin in responses to stress. Behav. Pharmacol. 2014, 25, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Heiman, M.L.; Ahima, R.S.; Craft, L.S.; Schoner, B.; Stephens, T.W.; Flier, J.S. Leptin Inhibition of the Hypothalamic-Pituitary-Adrenal Axis in Response to Stress. Endocrinology 1997, 138, 3859–3863. [Google Scholar] [CrossRef] [PubMed]
- Kehne, J.H.; Cain, C.K. Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: Evidence from animal models. Pharmacol. Ther. 2010, 128, 460–487. [Google Scholar] [CrossRef]
- Saiyudthong, S.; Marsden, C.A. Acute effects of bergamot oil on anxiety-related behaviour and corticosterone level in rats. Phytother. Res. 2011, 25, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Haleem, D.J.; Jabeen, B.; Parveen, T. Inhibition of restraint-induced anorexia by injected tryptophan. Life Sci. 1998, 63, PL205–PL212. [Google Scholar] [CrossRef]
- Samad, N.; Perveen, T.; Haider, S.; Haleem, M.A.; Haleem, D.J. Inhibition of restraint-induced neuroendocrine and serotonergic responses by buspirone in rats. Pharmacol. Rep. 2006, 58, 636–642. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, W.B.; Shireen, E.; Masroor, M.; Kiran, S.; Memon, N.; Junaid, N.; Haleem, D.J. Oral Administration of Rauwolfia serpentina Plant Extract Mitigated Immobilization Stress-Induced Behavioral and Biochemic and Deficits in Rats. Biol. Life Sci. Forum 2022, 12, 32. https://doi.org/10.3390/IECN2022-12393
Ali WB, Shireen E, Masroor M, Kiran S, Memon N, Junaid N, Haleem DJ. Oral Administration of Rauwolfia serpentina Plant Extract Mitigated Immobilization Stress-Induced Behavioral and Biochemic and Deficits in Rats. Biology and Life Sciences Forum. 2022; 12(1):32. https://doi.org/10.3390/IECN2022-12393
Chicago/Turabian StyleAli, Wafa Binte, Erum Shireen, Maria Masroor, Sehrish Kiran, Nida Memon, Nashran Junaid, and Darakhshan J. Haleem. 2022. "Oral Administration of Rauwolfia serpentina Plant Extract Mitigated Immobilization Stress-Induced Behavioral and Biochemic and Deficits in Rats" Biology and Life Sciences Forum 12, no. 1: 32. https://doi.org/10.3390/IECN2022-12393
APA StyleAli, W. B., Shireen, E., Masroor, M., Kiran, S., Memon, N., Junaid, N., & Haleem, D. J. (2022). Oral Administration of Rauwolfia serpentina Plant Extract Mitigated Immobilization Stress-Induced Behavioral and Biochemic and Deficits in Rats. Biology and Life Sciences Forum, 12(1), 32. https://doi.org/10.3390/IECN2022-12393




