Beneficial Effects of Ketogenic Diet on Nonalcoholic Steatohepatitis in Obese Mice Model †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anatomical Measurements and Histological Stanning
2.3. RNA Isolation, Reverse Transcription, and Real-Time Quantitative PCR
2.4. Statistical Analysis
3. Results
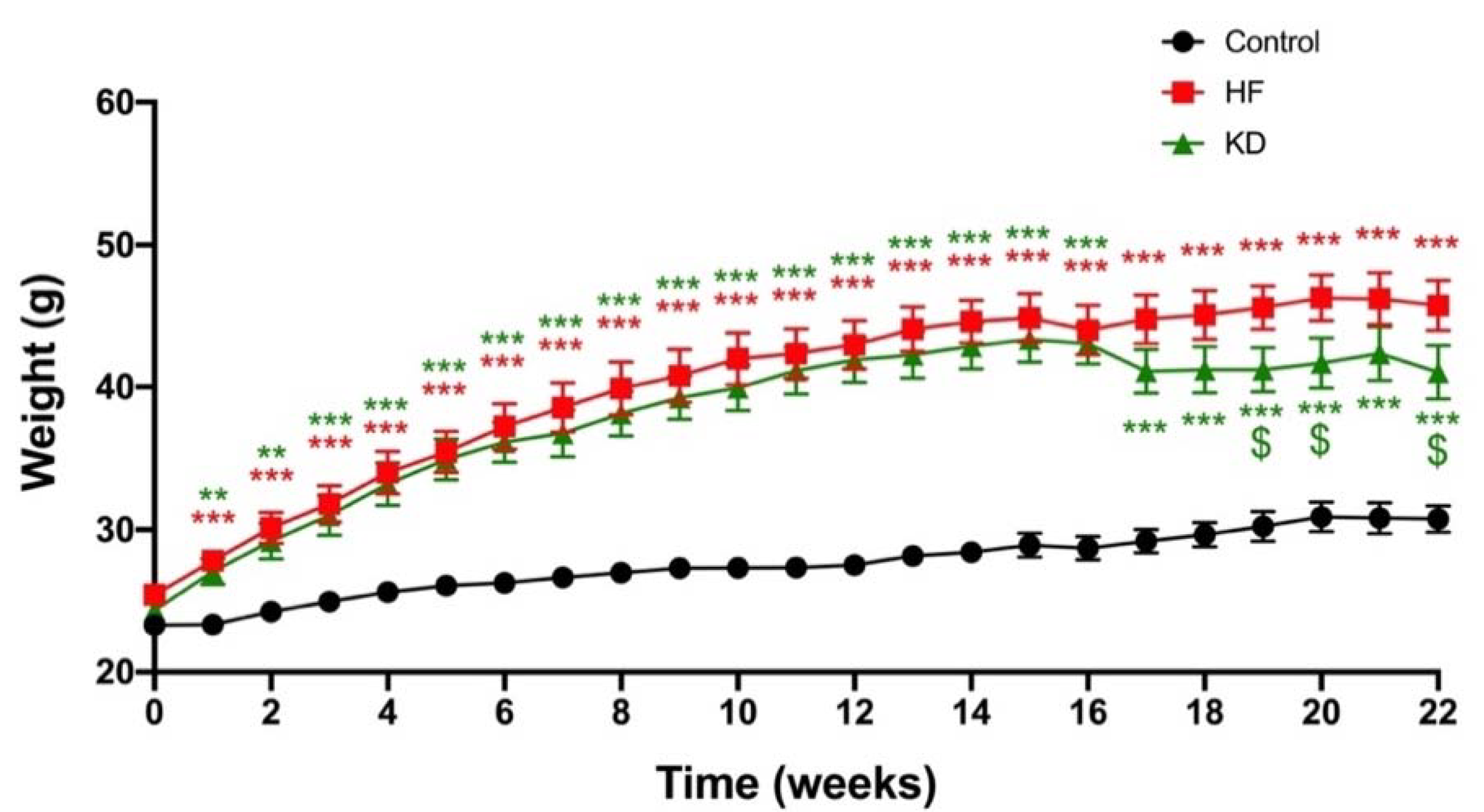
3.1. Ketogenic Diet Decreases Weight Gain
3.2. Ketogenic Diet Decreases Liver Weight Gain
3.3. Ketogenic Diet Decreases Collagen-1 and IL6 Genes Expression but Had No Effect on the Other Cytokines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 30 November 2020).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Washington, DC, USA, 2020. [Google Scholar]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a Continuum: From Obesity to Metabolic Syndrome and Diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD Development and Therapeutic Strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H. Guidelines for Obesity Management. Endocrinol. Metab. Clin. N. Am. 2016, 45, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Koutoukidis, D.A.; Astbury, N.M.; Tudor, K.E.; Morris, E.; Henry, J.A.; Noreik, M.; Jebb, S.A.; Aveyard, P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2019, 179, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, A.R.; Pissios, P.; Otu, H.; Xue, B.; Asakura, K.; Furukawa, N.; Marino, F.E.; Liu, F.-F.; Kahn, B.B.; Libermann, T.A.; et al. A High-Fat, Ketogenic Diet Induces a Unique Metabolic State in Mice. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E1724–E1739. [Google Scholar] [CrossRef] [PubMed]
- Badman, M.K.; Kennedy, A.R.; Adams, A.C.; Pissios, P.; Maratos-Flier, E. A Very Low Carbohydrate Ketogenic Diet Improves Glucose Tolerance in Ob/Ob Mice Independently of Weight Loss. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E1197–E1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, A.L.; Hallberg, S.J.; Creighton, B.C.; Volk, B.M.; Link, T.M.; Abner, M.K.; Glon, R.M.; McCarter, J.P.; Volek, J.S.; Phinney, S.D. A Novel Intervention Including Individualized Nutritional Recommendations Reduces Hemoglobin A1c Level, Medication Use, and Weight in Type 2 Diabetes. JMIR Diabetes 2017, 2, e5. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Ooi, G.J.; Burton, P.R.; Earnest, A.; Laurie, C.; Kemp, W.W.; Nottle, P.D.; McLean, C.A.; Roberts, S.K.; Brown, W.A. Visual Liver Score to Stratify Non-Alcoholic Steatohepatitis Risk and Determine Selective Intraoperative Liver Biopsy in Obesity. Obes. Surg. 2018, 28, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Spengler, E.K.; Loomba, R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of NAFLD and NASH. Mayo Clin. Proc. 2015, 90, 1233–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanches, S.C.L.; Ramalho, L.N.Z.; Augusto, M.J.; da Silva, D.M.; Ramalho, F.S. Nonalcoholic Steatohepatitis: A Search for Factual Animal Models. BioMed Res. Int. 2015, 2015, 574832. [Google Scholar] [CrossRef] [PubMed]
- Kakino, S.; Ohki, T.; Nakayama, H.; Yuan, X.; Otabe, S.; Hashinaga, T.; Wada, N.; Kurita, Y.; Tanaka, K.; Hara, K.; et al. Pivotal Role of TNF-α in the Development and Progression of Nonalcoholic Fatty Liver Disease in a Murine Model. Horm. Metab. Res. 2018, 50, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; McGeough, M.D.; Peña, C.A.; Schlattjan, M.; Li, H.; Inzaugarat, M.E.; Messer, K.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. NLRP3 Inflammasome Activation Is Required for Fibrosis Development in NAFLD. J. Mol. Med. 2014, 92, 1069–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Jou, J.H. Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin. Liver Dis. 2021, 17, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-M.; Xiao, Y.; Wu, X.; Zou, H.; Yang, S.; Shen, Y.; Xu, J.; Workman, H.C.; Usborne, A.L.; Hua, H. Progression and Regression of Hepatic Lesions in a Mouse Model of NASH Induced by Dietary Intervention and Its Implications in Pharmacotherapy. Front. Pharmacol. 2018, 9, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in NASH. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.A.; Mohamed, E.E.; Ahmed, D.M.; Sayed, M.A.; Hussien, A.R. A Study of Interleukin 6 as a Predictive Biomarker for Development of Nonalcholic Steatohepatitis in Patients with Nonalcholic Fatty Liver Disease. QJM Int. J. Med. 2020, 113, hcaa052-048. [Google Scholar] [CrossRef]


| Forward Primer | Reverse Primer |
|---|---|
| GTTGGATACAGGCCAGACTTTGTTG | GATTCAACTTGCGCTCATCTTAGGC |
| TGGTACTCCAGAAGACCAGAG | AACGATGATGCACTTGCAGA |
| AGTTGACGGACCCCAAAAG | AGCTGGATGCTCTCATCAGG |
| ATGGCCCAGACCCTCACA | TTGCTACGACGTGGGCTACA |
| GGCCCAGAAATCAAGGAGCA | AGACACCTTGGTCTTGGAGCTTAT |
| ACGTGGAAATCAACGGGATCA | GTTGGTATCCAGGGCTCTCC |
| TCATCGTGGCTTCTCTGGTC | GACCGTTGAGTCCGTCTTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charlot, A.; Charles, A.-L.; Georg, I.; Goupilleau, F.; Debrut, L.; Pizzimenti, M.; Mallard, J.; Pagano, A.F.; Geny, B.; Zoll, J. Beneficial Effects of Ketogenic Diet on Nonalcoholic Steatohepatitis in Obese Mice Model. Biol. Life Sci. Forum 2022, 12, 23. https://doi.org/10.3390/IECN2022-12368
Charlot A, Charles A-L, Georg I, Goupilleau F, Debrut L, Pizzimenti M, Mallard J, Pagano AF, Geny B, Zoll J. Beneficial Effects of Ketogenic Diet on Nonalcoholic Steatohepatitis in Obese Mice Model. Biology and Life Sciences Forum. 2022; 12(1):23. https://doi.org/10.3390/IECN2022-12368
Chicago/Turabian StyleCharlot, Anouk, Anne-Laure Charles, Isabelle Georg, Fabienne Goupilleau, Léa Debrut, Mégane Pizzimenti, Joris Mallard, Allan F. Pagano, Bernard Geny, and Joffrey Zoll. 2022. "Beneficial Effects of Ketogenic Diet on Nonalcoholic Steatohepatitis in Obese Mice Model" Biology and Life Sciences Forum 12, no. 1: 23. https://doi.org/10.3390/IECN2022-12368
APA StyleCharlot, A., Charles, A.-L., Georg, I., Goupilleau, F., Debrut, L., Pizzimenti, M., Mallard, J., Pagano, A. F., Geny, B., & Zoll, J. (2022). Beneficial Effects of Ketogenic Diet on Nonalcoholic Steatohepatitis in Obese Mice Model. Biology and Life Sciences Forum, 12(1), 23. https://doi.org/10.3390/IECN2022-12368







