Phytochemical and Antioxidant Properties of Athamanta turbith (L.) Brot Collected from Serbia †
Abstract
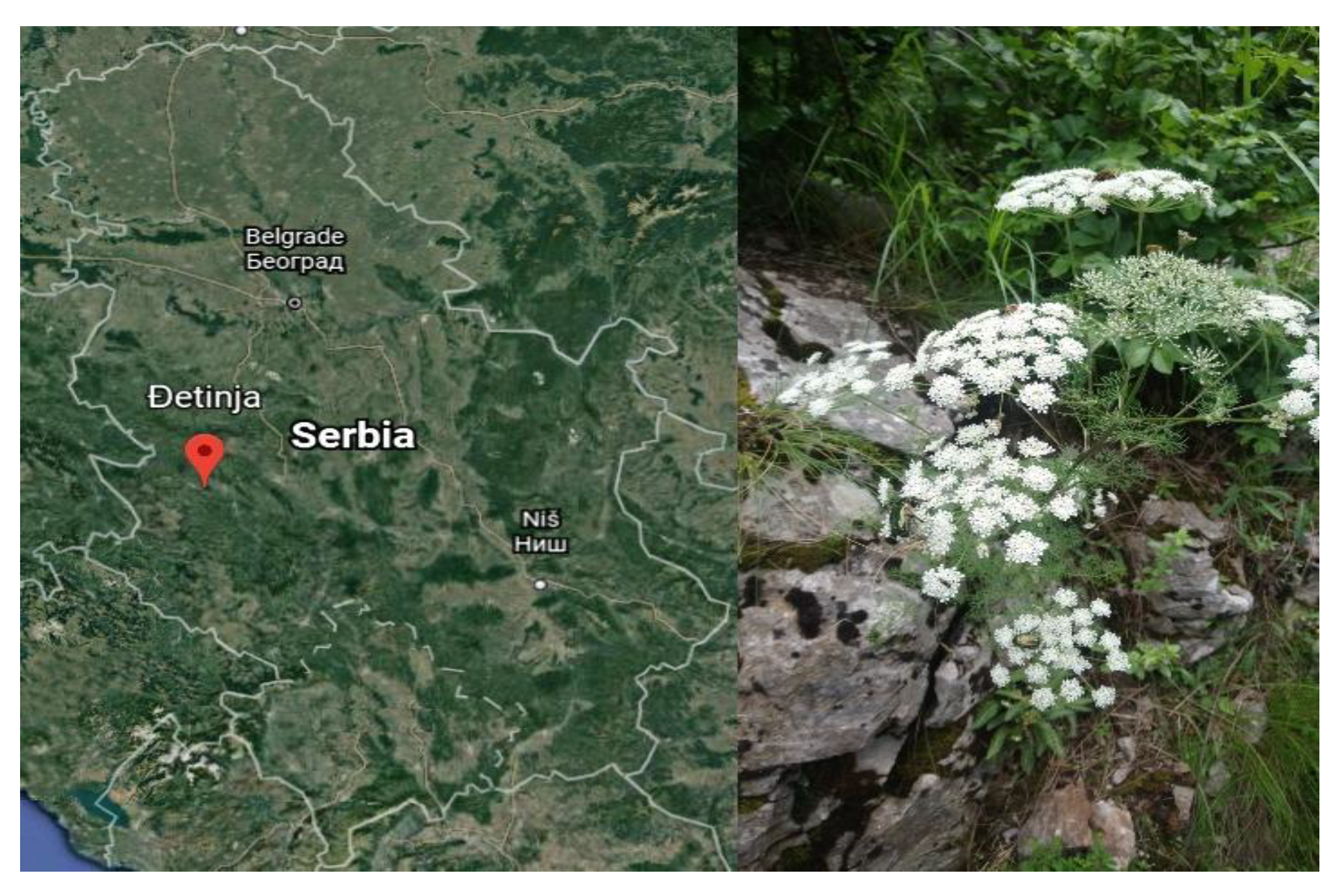
1. Introduction
2. Material and Methods
2.1. Preparation of Extracts
2.2. Determination of Bioactive Compounds
2.3. Determination of Antioxidant Activity
2.4. Statistics
3. Results and Discussion
3.1. Phytochemical Composition
3.2. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Režek Jambrak, A.; Lorenzo, J.M.; Collado, M.C.; Granato, D.; Barba, F.J. Effects of ultrasound-assisted extraction and solvent on the phenolic profile, bacterial growth, and anti-inflammatory/antioxidant activities of Mediterranean olive and fig leaves extracts. Molecules 2020, 25, 1718. [Google Scholar] [CrossRef] [PubMed]
- Berkani, F.; Serralheiro, M.L.; Dahmoune, F.; Ressaissi, A.; Kadri, N.; Remini, H. Ultrasound assisted extraction of phenolic compounds from a jujube by-product with valuable bioactivities. Processes 2020, 8, 1441. [Google Scholar] [CrossRef]
- Ng, A.; Parker, M.L.; Parr, A.J.; Saunders, P.K.; Smith, A.C.; Waldron, K.W. Physicochemical characteristics of onion (Allium cepa L.) tissues. J. Agric. Food Chem. 2000, 48, 5612–5617. [Google Scholar] [CrossRef] [PubMed]
- Santas, J.; Carbo, R.; Gordon, M.; Almajano, M. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008, 107, 1210–1216. [Google Scholar] [CrossRef]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Gawron-Gzella, A.; Królikowska, A.; Pietrzak, M. Antioxidant activity of teas obtained from leaves of Camellia sinensis (L.) Kuntze in course of various production processes available on Polish market. Herba Pol. 2018, 64, 60–67. [Google Scholar] [CrossRef][Green Version]
- Rajurkar, S.N.; Hande, M.S. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Tropic. Biomed. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedical family as source for industrial uses. Ind. Crop. Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
| Sample | Extraction Technique | TPC * (mg/g GAE) | TFC (mg/g QE) | HCAs (mg/g CGAE) |
|---|---|---|---|---|
| Inflorescence | UAE | 2.73 ± 0.13 a,** | 1.36 ± 0.02 a | 1.41 ± 0.004 a |
| SE | 1.95 ± 0.15 b | 1.56 ± 0.02 b | 1.45 ± 0.11 a | |
| Vegetative shoot | UAE | 1.06 ± 0.02 c | 0.70 ± 0.002 c | 1.07 ± 0.009 b |
| SE | 0.87 ± 0.01 c | 0.53 ± 0.05 d | 0.85 ± 0.008 c | |
| Rhizome | UAE | 0.37 ± 0.03 d | n.d. | 0.71 ± 0.00 d |
| SE | 0.40 ± 0.01 d | n.d. | 0.66 ± 0.00 d |
| Sample | Extraction Technique | ABTS+ * (% inh.) | DPPH∙ (% inh.) | TAC (mg/g AAE) | CUPRAC (mg/g AAE) | FRP (mg/g AAE) |
|---|---|---|---|---|---|---|
| Inflorescence | UAE | 51.43 ± 0.06 a,** | 77.68 ± 0.55 a | 3.60 ± 0.07 a | 39.15 ± 3.03 a | 11.06 ± 0.52 a |
| SE | 92.11 ± 0.48 b | 77.77 ± 0.57 a | 3.53 ± 0.29 a | 41.83 ± 1.29 a | 18.37 ± 1.70 b | |
| Vegetative shoot | UAE | 23.67 ± 0.00 c | 33.86 ± 0.14 b | 1.75 ± 0.00 b | 12.52 ± 1.10 b | 1.59 ± 0.09 c,d |
| SE | 34.00 ± 0.06 d | 50.34 ± 0.41 c | 1.54 ± 0.01 b | 8.42 ± 1.03 b | 3.33 ± 0.30 c | |
| Rhizome | UAE | 13.91 ± 0.13 e | 10.00 ± 0.17 d | 1.72 ± 0.14 b | n.d. | 0.46 ± 0.06 d |
| SE | 9.14 ± 0.66 f | 5.67 ± 0.31 e | 1.78 ± 0.007 b | n.d | 0.46 ± 0.05 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilibarda, S.N.; Vuković, S.Z.; Milinčić, D.D.; Mačukanović-Jocić, M.P.; Jarić, S.; Kostić, A.Ž. Phytochemical and Antioxidant Properties of Athamanta turbith (L.) Brot Collected from Serbia. Biol. Life Sci. Forum 2022, 11, 30. https://doi.org/10.3390/IECPS2021-11947
Kilibarda SN, Vuković SZ, Milinčić DD, Mačukanović-Jocić MP, Jarić S, Kostić AŽ. Phytochemical and Antioxidant Properties of Athamanta turbith (L.) Brot Collected from Serbia. Biology and Life Sciences Forum. 2022; 11(1):30. https://doi.org/10.3390/IECPS2021-11947
Chicago/Turabian StyleKilibarda, Sofija N., Sandra Z. Vuković, Danijel D. Milinčić, Marina P. Mačukanović-Jocić, Snežana Jarić, and Aleksandar Ž. Kostić. 2022. "Phytochemical and Antioxidant Properties of Athamanta turbith (L.) Brot Collected from Serbia" Biology and Life Sciences Forum 11, no. 1: 30. https://doi.org/10.3390/IECPS2021-11947
APA StyleKilibarda, S. N., Vuković, S. Z., Milinčić, D. D., Mačukanović-Jocić, M. P., Jarić, S., & Kostić, A. Ž. (2022). Phytochemical and Antioxidant Properties of Athamanta turbith (L.) Brot Collected from Serbia. Biology and Life Sciences Forum, 11(1), 30. https://doi.org/10.3390/IECPS2021-11947







