1. Introduction
Pasteurization is a widely applied heat treatment method used to reduce the risks posed by pathogenic microorganisms in the dairy industry and other food and beverage sectors [
1]. It primarily functions to eliminate harmful/pathogenic microbes in milk such as bacteria, yeasts, and molds, by applying precise temperatures for defined durations. Studies show that pasteurization can reduce microbial loads in milk by approximately 95–99% [
1,
2], significantly enhancing both its microbial safety and shelf life. In addition to ensuring safety, pasteurization helps retain the organoleptic properties of milk, preserving freshness, flavor, and overall sensory quality [
3]. According to the U.S. Food and Drug Administration (FDA), pasteurization can be achieved via several thermal regimens, including 62.8 °C for 30 min, 71.6 °C for 15 s, 88.4 °C for 0.1 s, 95.6 °C for 0.05 s, or 100 °C for 0.01 s (flash pasteurization) [
4]. Milk is a nutrient-rich food that plays an enormous role in supporting human health, particularly in early development [
5,
6,
7]. Although it is sterile within the mammary glands, milk is highly prone to microbial contamination post-milking. The source and type of milk consumed, therefore, carry significant public health implications [
8,
9,
10]. Pathogenic contaminants in raw milk including
Escherichia coli,
Salmonella,
Listeria, and
Mycobacterium tuberculosis have been associated with gastrointestinal infections, immune suppression, and, in severe cases, chronic illnesses or death [
11,
12,
13]. Such contamination can originate from multiple sources: fecal matter, mastitis-infected udders [
14], bovine diseases such as tuberculosis [
15], skin-borne bacteria, unhygienic environments [
16], insects [
17], rodents [
18], or human contact from contaminated equipment, clothing, or footwear [
19].
In many developing African countries such as Ghana and other West African nations, milk safety is often managed through traditional methods such as fermentation or boiling over firewood [
20,
21,
22]. While these approaches reduce some risk of microbial infections, they present grave limitations. Firewood dependency contributes to deforestation, depletion of agricultural residues, and loss of soil fertility, which, in turn, compromise environmental health and food security [
23,
24]. In this regard, solar energy has emerged as a promising alternative, offering a sustainable, eco-friendly, and cost-effective method for pasteurizing milk in resource-limited settings [
25,
26]. Solar pasteurizers can be locally fabricated using readily available materials and optimized based on parameters such as temperature, time, and material conductivity [
27]. However, a major gap exists in deploying low-cost, region-specific designs that meet microbial safety standards without relying on electricity or fossil fuels.
In this study, we hypothesized that a solar-powered milk pasteurization system, constructed entirely from locally available materials, could achieve effective microbial reduction and meet public health safety standards, thereby providing a sustainable alternative to conventional and biomass-based pasteurization systems in low-resource rural settings. To test our hypothesis, this study evaluated the thermal efficiency, pasteurization capacity, and microbial inactivation potential of a solar milk pasteurization system constructed from low-cost, locally available materials. Specifically, we assessed the system’s ability to achieve and maintain target pasteurization temperatures and quantified reductions in total bacterial and coliform counts in treated milk. The goal was to determine its suitability as a viable and scalable alternative for smallholder dairy producers in off-grid or pastoral regions. This may promote clean energy innovation in food safety systems, support rural dairy development, and lay the foundation for community-scale solar-based processing technologies in West Africa and similar ecosystems.
2. Materials and Methods
2.1. Design and Fabrication of the Pasteurization System
An 8 L capacity solar pasteurization system was designed and built with locally available materials for small-scale cattle herders in northern Ghana; the pasteurization system is composed of a flat-plate solar energy collector that heats water running through copper pipes and circulates a cylindrical milk vat. Details of the technical specifications of the solar pasteurization system are described subsequently. The system works as incident solar radiation, comprising mainly visible light and infrared radiation, falls on the glass cover. Only visible light and short-wavelength infrared radiation pass through the glass. The glass is opaque to long-wavelength infrared radiation [
28,
29]. On reaching the flat plate, the metal absorbs the incident energy, heating to a relatively lower temperature. The plate then emits infrared radiation of long wavelengths that cannot pass (escape) through the glass to be lost to the surroundings. Heat is, therefore, trapped in the box where the copper tubes then absorb this heat. The hot copper tubes heat the water within the tubes, making it less dense, and it subsequently rises into the water jacket. The risen hot water is replaced by cooler water from the water vat. The hot water vat conducts heat to the milk vat, which also conducts heat to the milk, thereby raising the milk temperature. This process is known as thermosiphon circulation [
28,
29]. The process continues until the milk reaches pasteurization temperatures between 65 °C and 72 °C.
2.1.1. Flat-Plate Solar Collector
In this experiment, a sheet of clear transparent glass acting as a solar collector with gross and efficient areas of 1.45 m
2 and 1.12 m
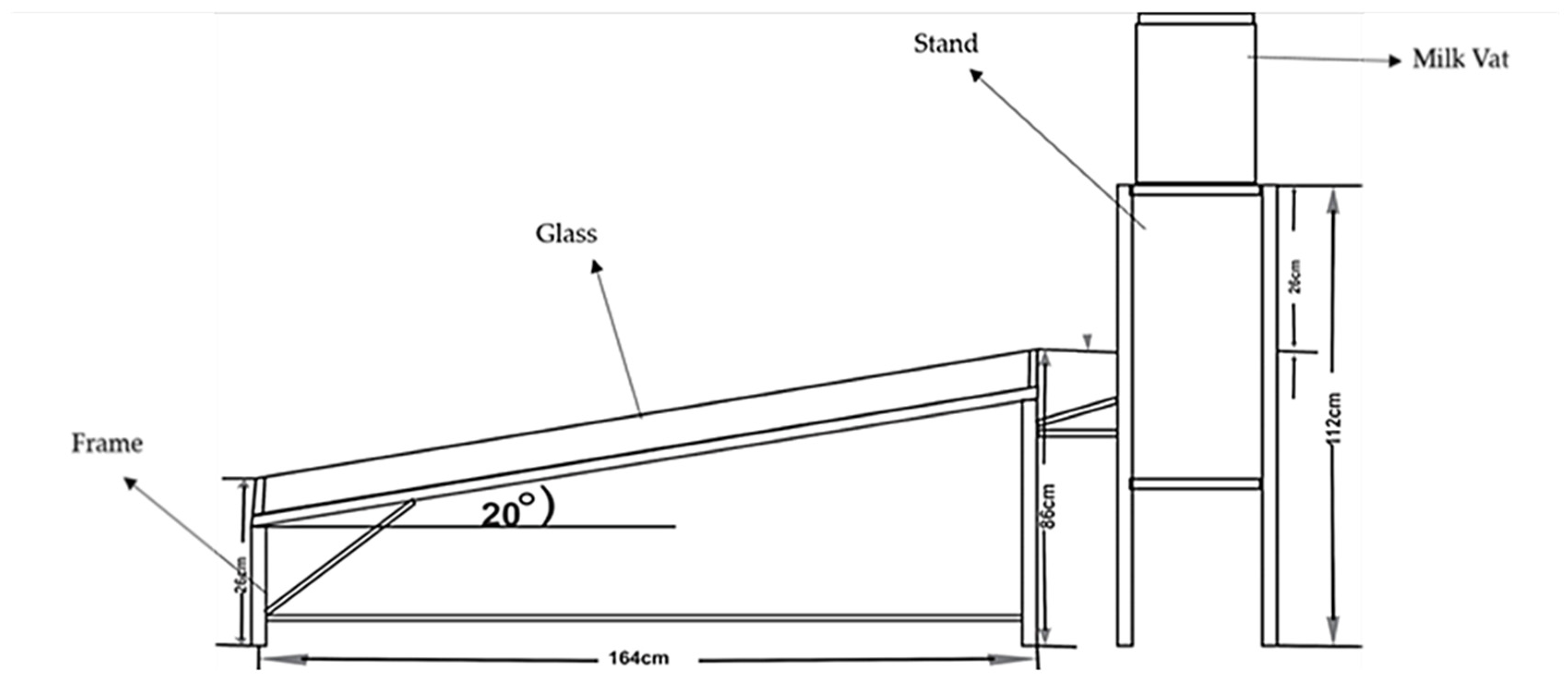
2, respectively, was used. The absorber was made of a galvanized steel sheet to which nine 15.15 mm nominal diameter copper tubes were welded to allow water circulation to and from the milk vat. The surfaces of the plate and tubes were painted black to improve the absorption of solar radiation. The glazing was ordinary normal window glass of thickness 5.0 mm. The insides of the casing (wooden frame) were lined with 5.0 mm thick aluminum foil to serve as insulation. The transparent glass (solar collector) was tilted at an angle of 17° from the horizontal and oriented southward, in alignment with optimal solar exposure in Navrongo, Upper East Region of Ghana (Northern Hemisphere). The radiation reaching the glass cover passed through, falling on the collector, transforming the solar radiation into heat energy. The pasteurizer’s frame and milk vat stand were constructed with wood (waawa boards) using the construction parameters shown in
Figure 1. The heat energy is then conducted into the water from the collector through the collector pipes, whose ends are connected to two rubber tubes to limit heat loss. One plastic tube from the collector connected to the copper tubes circulates hot water from the collector to the top of the milk vat. Another plastic tube from the bottom of the milk vat circulates cooler water into the collector. Plastic tubes are used to maintain maximum heat because they lie outside the solar box. The portions of metal tubes projecting from the collector and vat into the atmosphere were insulated with aluminum foil to minimize heat losses.
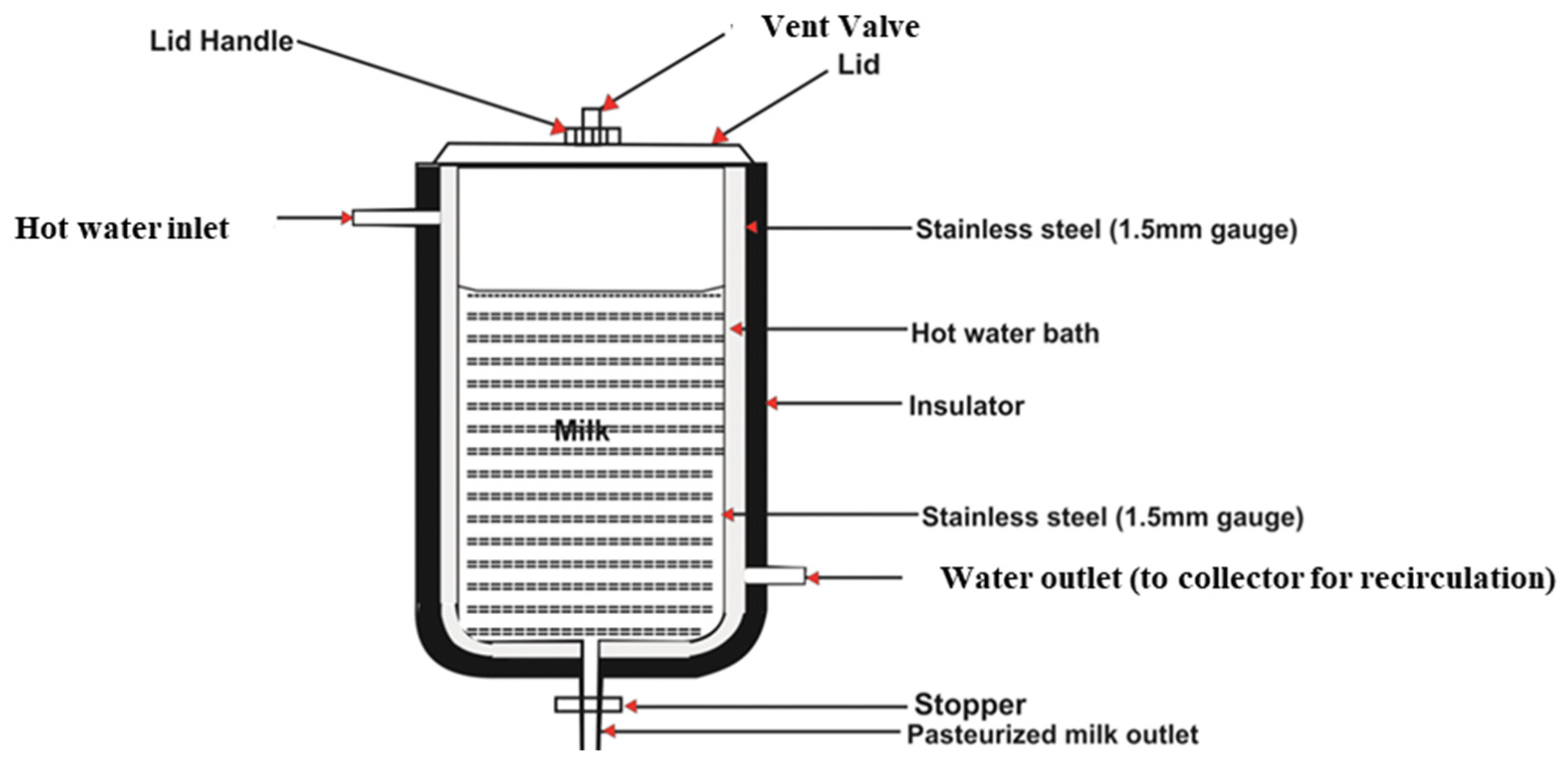
The figure shows a solar pasteurization system’s hot water bath chamber fabricated from durable 1.5 mm stainless steel. At the top is a lid handle that provides access to the vat and water jacket where the milk and water are placed to commence the pasteurization process. Heated water recirculates through a water outlet back to the solar collector, while a stopper prevents heat and fluid loss. The pasteurized milk then exits through a dedicated outlet, completing the process. This design ensures efficient heat transfer and temperature maintenance, making it particularly suitable for off-grid or resource-limited applications.
2.1.2. Milk Vat
The milk vat consists of a 0.15 cm thick stainless steel cylindrical tank, a 2.2 cm wide hot water jacket, and an outer layer (insulator) of 0.5 cm thick aluminum foil insulation. The milk vat was placed on a stand such that the bottom of the vat was 26 cm above the top of the collector (
Figure 2). This enables water to flow freely at a maximum rate by convection from the collector tubes through the milk vat and back to the collector tubes. The capacity of the water was approximately 6.0 L. The collector and plastic tubes held about 1.0 L of water. The water jacket was directly heated by the solar collector, which, in turn, heated the milk to pasteurization temperatures. The vat had a lid that was insulated with 0.5 cm aluminum foil and could be opened; it also had a 1.55 cm nominal diameter copper pipe at the bottom, which acted as an outlet for pasteurized milk, with a customized stopper to control the flow of the milk (
Figure 1).
The design highlights the angular placement of the glass and the sturdy construction of the supporting stand [
28,
29].
2.2. Experimental Set-Up and Evaluation of the Performance of Solar Milk Pasteurizer and Location of Trial
The solar milk pasteurization system was installed in an open area located between the Departments of Applied Biology and Applied Biochemistry at the University for Development Studies (UDS), Navrongo Campus as shown in
Figure 3. During the study period, the ambient temperature ranged between 32 °C and 43 °C, providing favorable conditions for solar-based heat applications. Geographically, Navrongo is positioned at 10°53′5″ N, 1°5′25″ W, and lies within the Sahelian ecological zone, characterized by flat terrain, arid grasslands, and scattered shrubbery. These environmental and climatic features make the location ideal for testing and implementing solar energy-based technologies, particularly in the context of sustainable food processing solutions [
30].
The figure illustrates a solar-powered milk pasteurization system with interconnected components. At the center, an insulated water vat receives heated water through a hot water inlet, while cooled water returns to the solar collector via a cold water downpipe. The milk vat, supported by a dedicated stand, connects to the system through a milk outlet for processed product removal. Below, the solar collector assembly features copper tubes enclosed in a collector box, which absorbs thermal energy to heat the circulating water. This closed-loop design ensures efficient heat transfer for continuous pasteurization.
2.3. The Climate of Navrongo
Navrongo is located in the Upper East Region of Ghana, West Africa, and lies within the tropical savanna climatic zone, classified as “Aw” under the Köppen climate classification, where “A” denotes a tropical climate with consistently high temperatures throughout the year, and “w” indicates a distinct dry season occurring in winter. This region experiences two distinct seasons, a rainy season and a dry season, both of which contribute to marked variations in temperature, humidity, and precipitation throughout the year.
Temperatures in Navrongo are consistently warm, ranging from approximately 19.6 °C to 41 °C, with the hottest months occurring between March and May. During this period, daytime temperatures can exceed 40 °C, making it one of the hottest regions in the country. In contrast, the coolest months fall between November and January, where temperatures average around 19.6 °C, largely due to the influence of the Harmattan, a dry, dusty wind originating from the Sahara (Nomad Season Weather Atlas).
Rainfall patterns are highly seasonal. The rainy season spans from May to September, with August typically recording the highest rainfall, averaging around 130 mm. Conversely, the dry season extends from November to March, during which rainfall is minimal—often as low as 2 mm in December and January (WeatherAPI). This seasonal disparity results in significant fluctuations in relative humidity, which peaks at approximately 78% in August, aligning with the height of the rainy season.
Navrongo also enjoys high solar irradiance, making sunshine duration a critical climatic feature. From December through May, the region receives between 7.7 and 11.9 h of sunshine per day on average, making it ideal for solar energy applications (World Weather & Climate Information). Monthly sunshine days’ range from 8.6 to 30.8 days, depending on the time of year [
30].
These climatic conditions, characterized by high temperatures, pronounced wet and dry seasons, and abundant sunshine, make Navrongo a suitable location for studies involving solar energy, heat-dependent technologies, and tropical agricultural practices.
2.4. Source of Milk Samples
Fresh cow milk samples were collected immediately on harvesting from small-scale farmers in Navrongo in the Upper East Region of Ghana. The milk samples were aseptically collected from farmers into 10 L sterile plastic containers (for sterility purposes, we cleaned all plastic containers thoroughly with 80% ethanol before using them to collect milk samples). Collected samples were placed on ice to retain freshness while under transportation to the study site. The samples were put into two groups, with the first group (1 L) stored in a 5 °C refrigerator for further microbial analysis on the raw milk, and the second group was pasteurized using the fabricated system.
2.5. Milk Pasteurization
The outer water jacket, including the copper tubing, was filled with water, while the inner milk vat was charged with raw milk. Pasteurization occurred via thermosiphon circulation, a passive heat transfer process where heated water from the solar collector naturally flowed into the water jacket and then returned to the collector. This established a continuous circulation loop as solar energy heated the collector throughout the day. As the system operated, water circulated from the bottom of the water jacket to the collector tubes and back, progressively raising the temperature of the water jacket. The heated water in turn transferred thermal energy to the milk, gently elevating its temperature to pasteurization levels. The milk was stirred at 10 min intervals using a stainless steel stirrer to ensure uniform heat distribution. Once the target pasteurization temperature was reached, the circulation of water was halted, and the milk was maintained at that temperature for 30 min (the holding time). During the pasteurization process, the following parameters were monitored at hourly intervals using a liquid-in-glass thermometer: milk temperature (Tm, °C), hot water jacket temperature (Tw, °C), ambient temperature (Ta, °C) and solar collector temperature (Tc, °C). These measurements were essential for evaluating the thermal performance of the solar pasteurizer and ensuring effective microbial reduction in the milk. The complete and actual constructed system is shown in
Figure 4 below.
2.6. Microbial Analysis of Pasteurized and Unpasteurized Milk
For microbiological safety, we adopted international standards such as the ISO 4833-1:2013 [
31] for total viable count, ISO 4832:2006 [
32] for coliform enumeration, and ISO 6888-1:1999 [
33] for
Staphylococcus aureus. Using stomacher bags, milk samples were aseptically collected immediately before and after pasteurization and stored in industrial ice packs and processed within 1 h of collection to prevent microbial growth post-collection.
All experimental procedures were carried out under a biosafety cabinet to reduce potential contaminations. We performed total bacteria counts using Plate Count Agar (PCA) (Oxoid, UK). Samples were serially diluted 10-fold in 0.1% PBS, and 1 mL aliquots from appropriate dilutions were spread-plated in triplicates. The plates were incubated at 37 °C for 48 h, and colony-forming units (CFU/mL) were enumerated from plates with at visible colonies ranging from 30 to 300. For coliform enumeration, we used Violet Red Bile Glucose (VRBG) agar adhering strictly to ISO 4832:2006 [
32] guidelines. After plating and incubation at 37 °C for 24 h, colonies with characteristic red coloration and bile precipitate zones were considered for enumeration as presumptive colonies.
Confirmation was performed with brilliant green bile broth and indole production tests where necessary.
Mannitol Salt Agar (
MSA) was used to isolate
Staphylococcus aureus. After incubation at 37 °C for 24 h, yellow colonies surrounded by yellow zones were recorded as presumptive
S. aureus. Confirmation was performed via coagulase testing. To verify successful pasteurization, the alkaline phosphatase test was performed using the Fluorophos Test System (Advanced Instruments), following IDF Standard 155:2007 [
34]. A negative phosphatase result indicated sufficient heat treatment [
35].
2.7. Statistical Analysis
All data were subjected to ANOVA (one-way analysis of variance), and the means were separated by Tukey’s family error rate multiple comparison test (<0.05) using Microsoft excel software package 2013. All microbial tests were performed in triplicate (n = 3) for each sample point (before and after pasteurization), and results were reported as mean ± standard deviation (SD). Microbial reduction was calculated using log10 CFU/mL transformations. Paired t-tests were conducted to assess statistical significance of microbial reduction, with p < 0.05 considered significant. Confidence intervals (95%) were also computed.
4. Discussion
Solar pasteurization has been used widely as a sustainable alternative for liquid pasteurization and treatment [
36,
37,
38,
39]. Nevertheless, ambient temperature conditions and thermal bottleneck remain major impediments, as highlighted in recent solar pasteurization works by Ray and Jain (2011) and Wang (2013) [
40,
41]. The present study aimed to address that challenge by designing and evaluating a solar milk pasteurizer fabricated from locally available materials. The solar milk pasteurizer developed in this study operates based on the principle of thermosiphon circulation, utilizing solar radiation to generate heat that is indirectly transferred to milk through a water jacket system. The core component of the device is a flat-plate solar collector, covered with a 5 mm thick transparent glass panel. This glass allows the transmission of short-wavelength solar radiation including visible light and shortwave infrared, while blocking longwave infrared radiation. As sunlight strikes the collector, a black-painted galvanized steel plate absorbs the energy and becomes heated. The heat is then conducted into copper tubes welded to the absorber plate. These tubes contain water, which heats up, becomes less dense, and rises naturally into the surrounding water jacket that encases the stainless steel milk vat. This movement of heated water initiates a natural convection loop, where cooler water from the bottom of the water jacket flows back into the collector tubes to be reheated. This cycle continues uninterrupted as long as solar radiation is available, allowing for continuous and passive heating of the milk vat. The stainless steel milk vat, insulated with aluminum foil, is heated indirectly by the surrounding hot water. As the heat is transferred through the jacket to the milk, the temperature of the milk gradually rises.
To ensure uniform heat distribution and prevent stratification, the milk is manually stirred at 10 min intervals using a stainless steel stirrer. Once the milk reaches the desired pasteurization temperature, typically between 65 °C and 72 °C, the system’s circulation is halted. The milk is then held at this temperature for 30 min to complete the pasteurization process and ensure effective microbial inactivation. The system was designed to process up to 8 L of milk per cycle and is constructed entirely from locally available materials, including stainless steel, copper tubing, wood (for structural framing), and aluminum insulation. The solar collector is tilted at an angle of 17 degrees facing the equator to optimize solar absorption, making it highly effective in sun-rich regions such as Navrongo, Ghana, where the average ambient temperatures range from 32 °C to 43 °C during the dry season.
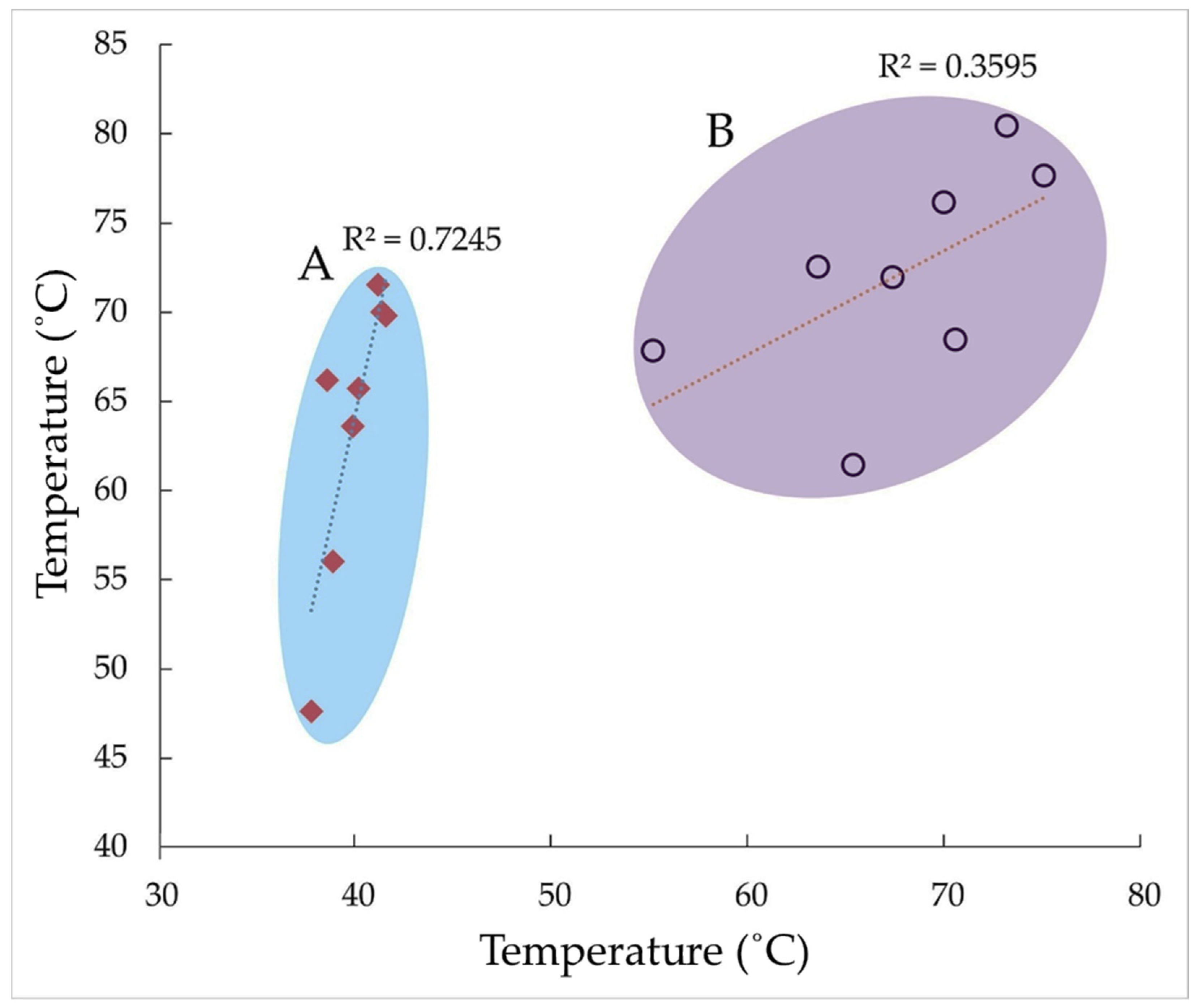
To understand the system’s performance, thermal efficiency was analyzed through correlations among component temperatures and environmental conditions. The thermal performance analysis of the pasteurization system reveals key essential relationships and operational qualities that collectively determine its efficiency. Starting with the effectiveness of the ambient temperature, we observe in this study a strong impact on milk temperature, with R
2 = 0.7245, as shown in
Figure 6A, and a moderate impact on water temperature, with R
2 = 0.6458, as indicated in
Figure 7A, while the collector temperature remains largely unaffected (R
2 = 0.0695) (
Figure 5A). This pattern suggests that while ambient conditions significantly affected the milk and heat transfer medium (water), the collector operates relatively independently of environmental fluctuations. The pasteurization system shows excellent thermal coupling between water and milk with R
2 = 0.9141 (
Figure 5B), indicating the water bath serves as an effective heat transfer medium for pasteurization. However, this efficiency contrasts sharply with the poor thermal transfer from collector to milk (R
2 = 0.128) (
Figure 7B) and the weak water-to-collector relationship (R
2 = 0.3595), as shown in
Figure 6B. These findings point to a significant thermal bottleneck at the collector stage, where much of the generated heat fails to effectively reach the product. This thermal profile suggests the current system configuration has both strengths and weaknesses. On the positive side, the water bath reliably regulates milk temperature regardless of collector performance, and the collector maintains stable operation independent of ambient conditions. However, the substantial heat loss between collector and milk represents a significant inefficiency, compounded by the system’s sensitivity to ambient temperature fluctuations that affect process stability. To improve system performance, priority should be given to enhancing the collector–milk heat exchange, potentially through redesign of heat transfer surfaces or optimization of flow patterns. Additional insulation for the water bath could reduce its ambient temperature sensitivity, while verification of milk flow rates might reveal opportunities to increase contact time and heat transfer efficiency. Regular monitoring of ambient conditions combined with thermal imaging could help identify specific heat loss points for targeted improvements. The observed “thermal bottleneck” at the collector stage suggests a mismatch between the heat generation and utilization components. While the water bath currently compensates for this deficiency by providing reliable temperature control, the system as a whole operates below its potential efficiency. Future research should focus on quantifying the specific energy losses across components, testing alternative collector designs, and evaluating advanced insulation materials. The consistent temperature measurements (in °C) and calculated R
2 values provide a solid foundation for developing more sophisticated predictive thermal models to guide these optimizations. In 2022, Khan et al. performed a comparative analysis of solar collectors for pasteurization and reported similar findings. They stipulated in their study that evacuated tube collectors retain stable temperatures despite ambient temperature fluctuations, with flat-plate collectors showing higher sensitivity. This implies that collector design and positioning plays a crucial role in thermal independence [
42].
The pasteurizer successfully reached and maintained pasteurization temperatures, demonstrating its potential for microbial reduction and improved milk safety in savanna ecological zones. The pasteurization system achieved an average milk temperature of 66.2 °C within one hour, aligning with the low-temperature, long-time (LTLT) pasteurization protocol [
1,
43]. This finding is consistent with other solar pasteurization studies from Franco et al. [
44], who reported a 1.25 h come-up time for goat milk using a solar concentrator. Although this heating duration is slightly longer than conventional electric pasteurizers, it remains acceptable for resource-limited environments where affordability and energy sustainability are priorities. Additionally, optimization strategies such as reducing milk volume or incorporating multiple collectors in series could significantly shorten heating times and improve system efficiency.
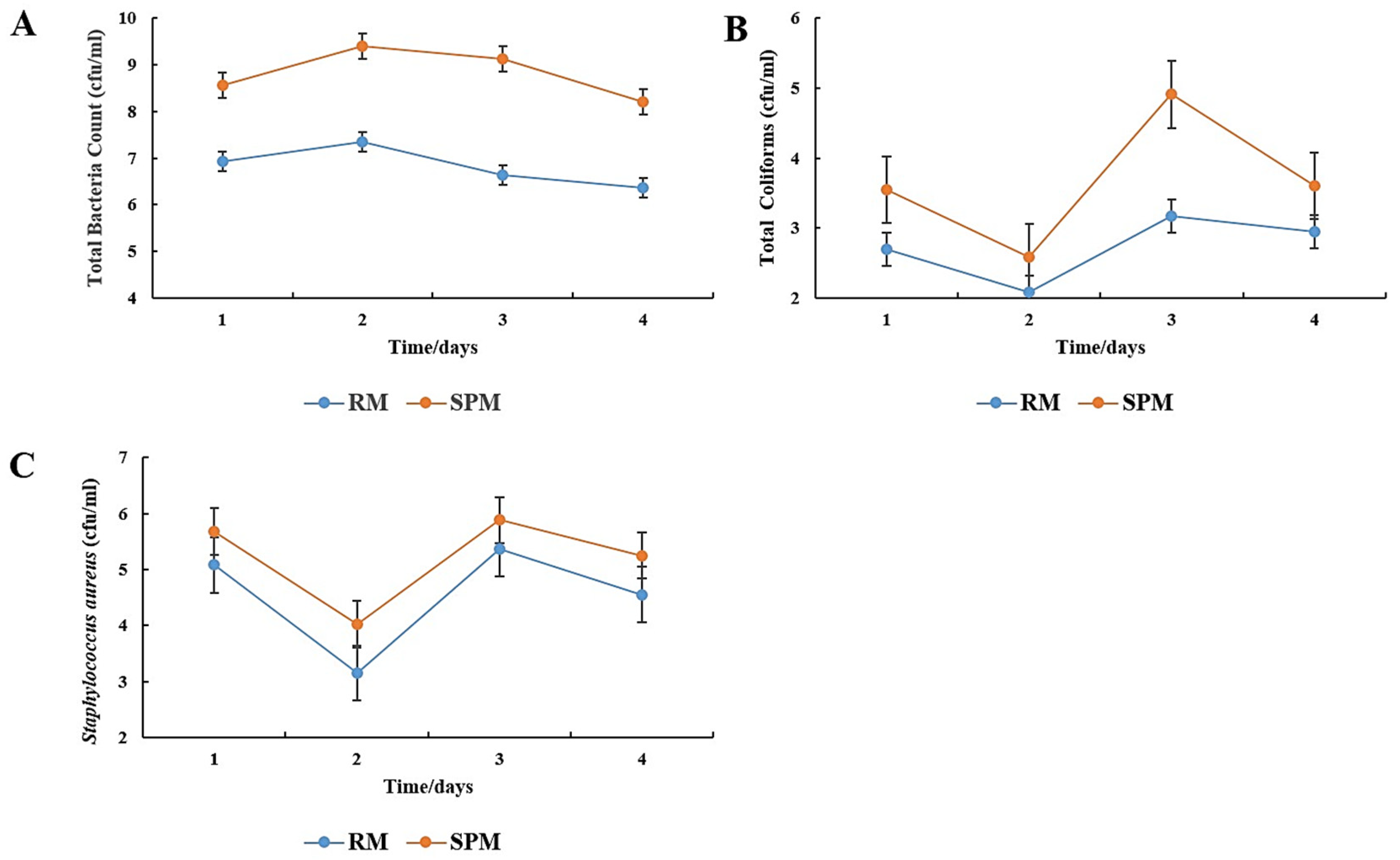
Beyond thermal metrics, microbial safety is a critical factor in determining pasteurization efficacy and consumer health protection. To evaluate the performance of the system, microbial assessments were conducted before and after pasteurization. These included total bacterial counts (TBC), coliforms, and
Staphylococcus aureus to confirm the adequacy of thermal treatment, as shown in
Figure 8A–C. Microbial evaluation of the raw versus solar-pasteurized milk revealed significant reductions in total bacterial counts (
Figure 8A), total coliforms (
Figure 8B), and
Staphylococcus aureus (
Figure 8C), with all values falling within safe thresholds for human consumption. These reductions are comparable to the findings by Mulwa [
45] and Wayua et al. [
46], who similarly observed microbial load reduction in camel and cow milk following solar pasteurization. The use of alkaline phosphatase testing further validated the efficacy of thermal inactivation, although this study did not assess lipase or spore-forming bacteria, which are known to survive some pasteurization protocols. Future studies should incorporate these elements to comprehensively evaluate enzymatic and thermal resilience. A detailed analysis of temperature correlations during pasteurization revealed insightful dynamics. A strong positive correlation between water and milk temperatures (
Figure 5B) highlights the importance of efficient thermal conduction through the water jacket. Conversely, collector temperature showed a weak correlation with milk temperature (
Figure 7B), suggesting that system efficiency depends more on water circulation dynamics and thermal insulation than direct solar input. Additionally, ambient temperature showed a positive correlation with both milk and water temperatures, implying that external environmental conditions can indirectly influence pasteurization efficiency, especially during peak solar periods. The pasteurization system’s design features, including the thermosiphon circulation and water jacket heating, provided relatively stable and consistent heating patterns. However, the fluctuations in microbial counts on certain days may reflect variations in the initial microbial load of the raw milk and environmental factors such as solar intensity. Notably, coliform counts, while significantly reduced, approached levels that warrant attention on certain days, possibly due to contamination during collection or initial milk quality. These issues underscore the need for improved sanitary practices and better container handling, especially under field conditions.
This study did not include a conventional pasteurizer control, which is a limitation. However, the observed microbial reductions align with established pasteurization targets [
47,
48,
49]. Additionally, physicochemical properties such as milk composition and shelf life were not assessed but remain critical for consumer acceptability and marketability. Future work should focus on these parameters and compare the effects of solar and conventional pasteurization on nutritional and sensory attributes.
The environmental and economic implications of this solar pasteurization system were also assessed to determine feasibility in low-resource settings. A comprehensive cost analysis was performed and is presented in
Supplementary Table S1 to assess the economic feasibility of the pasteurization system. The cost analysis accounted for the materials used in the construction such as wood, copper piping, transparent glass cover, insulation materials, sealants, and basic fabrication tools, with a total of approximately USD 150–USD 280. This cost includes the workmanship fee, making the device not only affordable but also reproducible in low-resource environments. In comparison, traditional firewood fuel methods incur recurring costs and labor-intensive maintenance. Afrane and Ntiamoah (2012) provided a comprehensive analysis of the life-cycle costs and environment al impacts of cooking fuels used in Ghana. They stated that firewood alone accounted for an annual environmental cost of over USD 36,000 per household [
50]. From an environmental safety perspective, solar pasteurizers provide a zero-emission, renewable alternative to firewood combustion. Relying on solar thermal technology instead of firewood in milk pasteurization eliminates the release of carbon monoxide, particulate matter (PM
2.5), and greenhouse gases (GHGs) such as CO
2, which are commonly produced during biomass burning [
51]. Using conservative estimates, a single solar unit can offset 0.8 to 1.2 tons of CO
2 annually, contributing to climate mitigation efforts and aligning with sustainable development goals (SDGs 7 and 13) [
52]. Additionally, the use of this solar device reduces deforestation pressure in rural communities where wood remains the primary source of energy. By adopting this technology, users are empowered to achieve energy self-sufficiency while improving the hygiene and quality of dairy products.
To ensure the long-term sustainability and hygiene of the system, material durability and cleaning protocols were also evaluated. The sustainability of the solar pasteurizer under long-term use hinges on the durability of its construction materials and the system’s resilience to repeated thermal cycling. Prolonged exposure to sunlight and fluctuating temperatures can induce material stress, especially at welded joints and interface regions, which may lead to gradual performance loss. While the use of stainless steel for the milk vat and aluminum foil for insulation provides a good balance of thermal conductivity and corrosion resistance, routine evaluation is recommended to detect early signs of wear or degradation. Nevertheless, even corrosion-resistant materials like aluminum and stainless steel may become prone to surface oxidation or hygiene issues, especially when exposed to residual milk, moisture, and cleaning agents in high-humidity environments [
53,
54]. These issues can pose significant microbial safety concerns if not addressed through regular and thorough cleaning [
54]. To enhance food safety, future designs could incorporate food-grade silicone seals, smoother weld finishes, and corrosion-resistant coatings that are compatible with dairy sanitation standards. In field settings such as Navrongo, Ghana, where access to high-end cleaning supplies and tools is limited, the design should favor ease of disassembly and manual cleaning. Current results suggest that the pasteurizer’s simple construction and component accessibility support this goal. However, adding features such as removable jackets, drainage ports for rinse water, and hinged or clamp-based openings could further streamline maintenance. It is also advisable to test the system over extended periods to assess material fatigue and to include UV-protective treatments for any exposed polymer or rubber parts to avoid brittleness. Community training on daily maintenance routines and simple diagnostics for leakage or heat loss could also improve the device’s lifespan and ensure safe milk handling.
Overall, this study demonstrates that a low-cost, solar-powered milk pasteurizer can serve as a viable alternative to conventional pasteurization technologies in rural and underserved regions. With minor design enhancements and broader microbiological assessments, the system holds promise for large-scale deployment to improve public health, reduce spoilage, and empower local dairy producers. These findings support our initial hypothesis that a low-cost solar milk pasteurization system, designed with locally available materials, can effectively reduce microbial contamination and meet dairy safety standards under natural sunlight conditions.