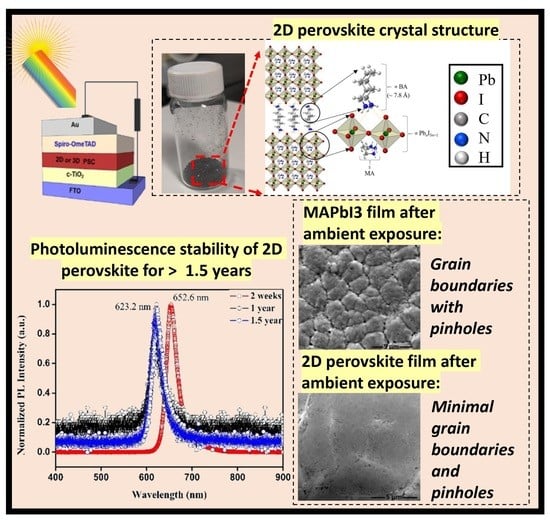
Optical Property and Stability Study of CH3(CH2)3NH3)2(CH3NH3)3Pb4I13 Ruddlesden Popper 2D Perovskites for Photoabsorbers and Solar Cells and Comparison with 3D MAPbI3
Abstract
1. Introduction
2. Results and Discussion
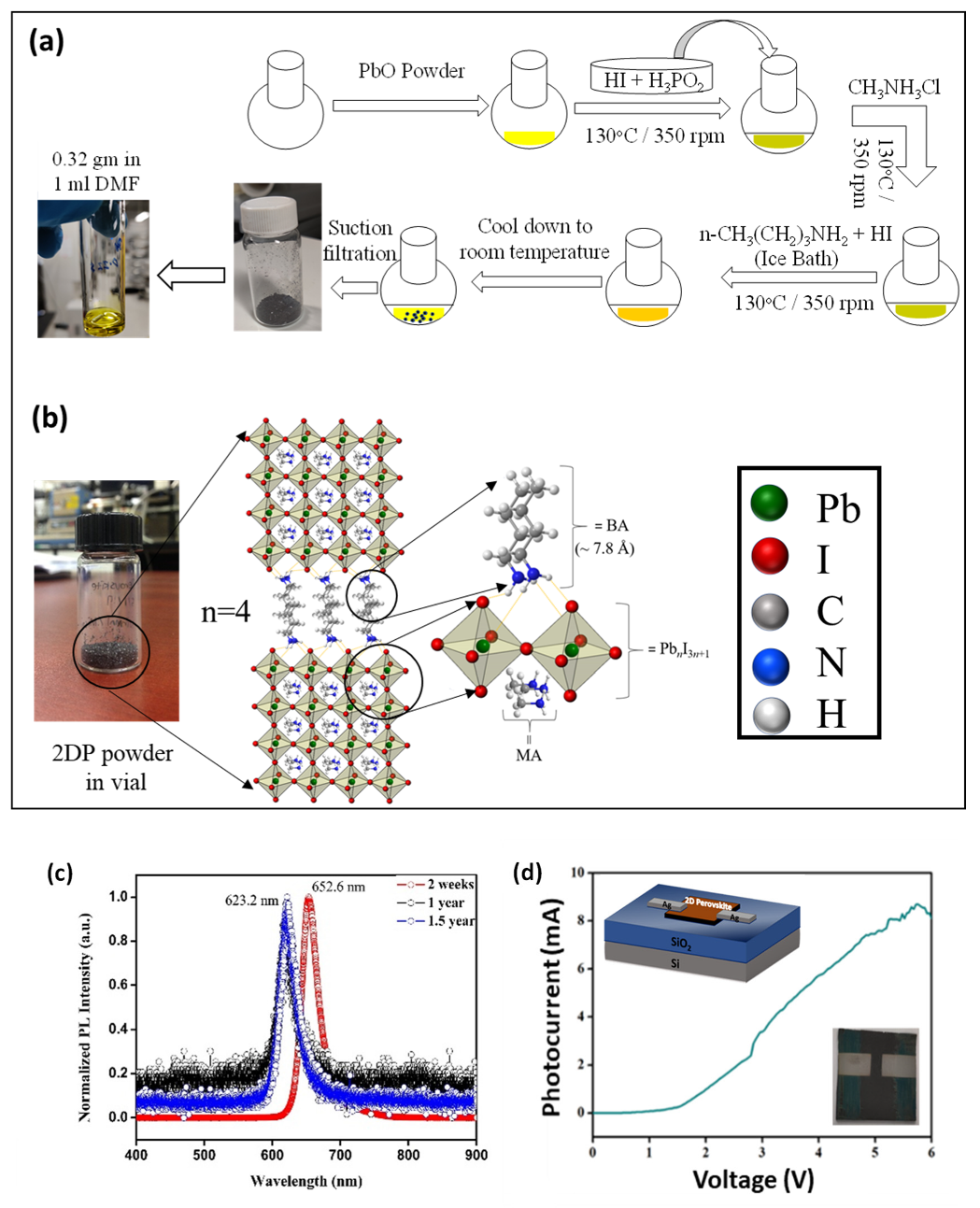
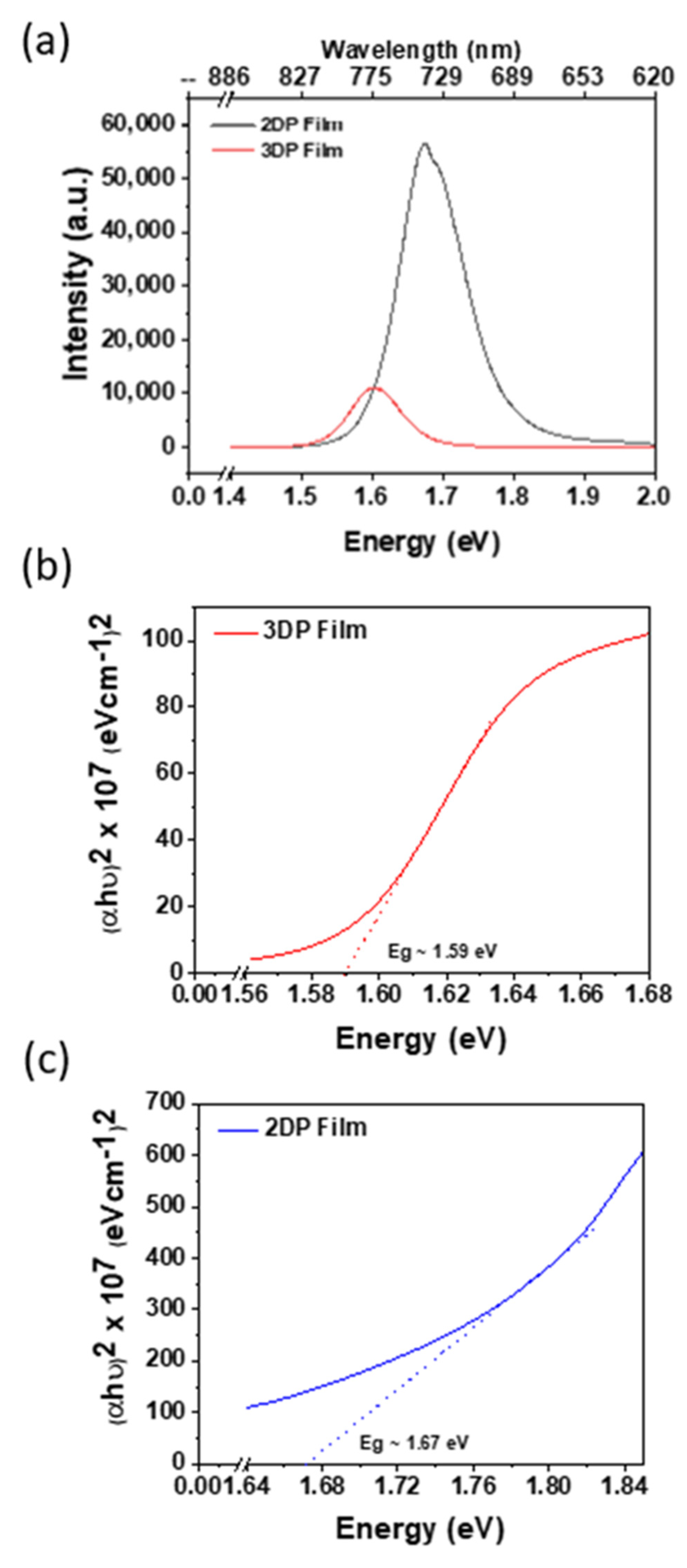
2.1. Synthesis and Characterization of 2DRP
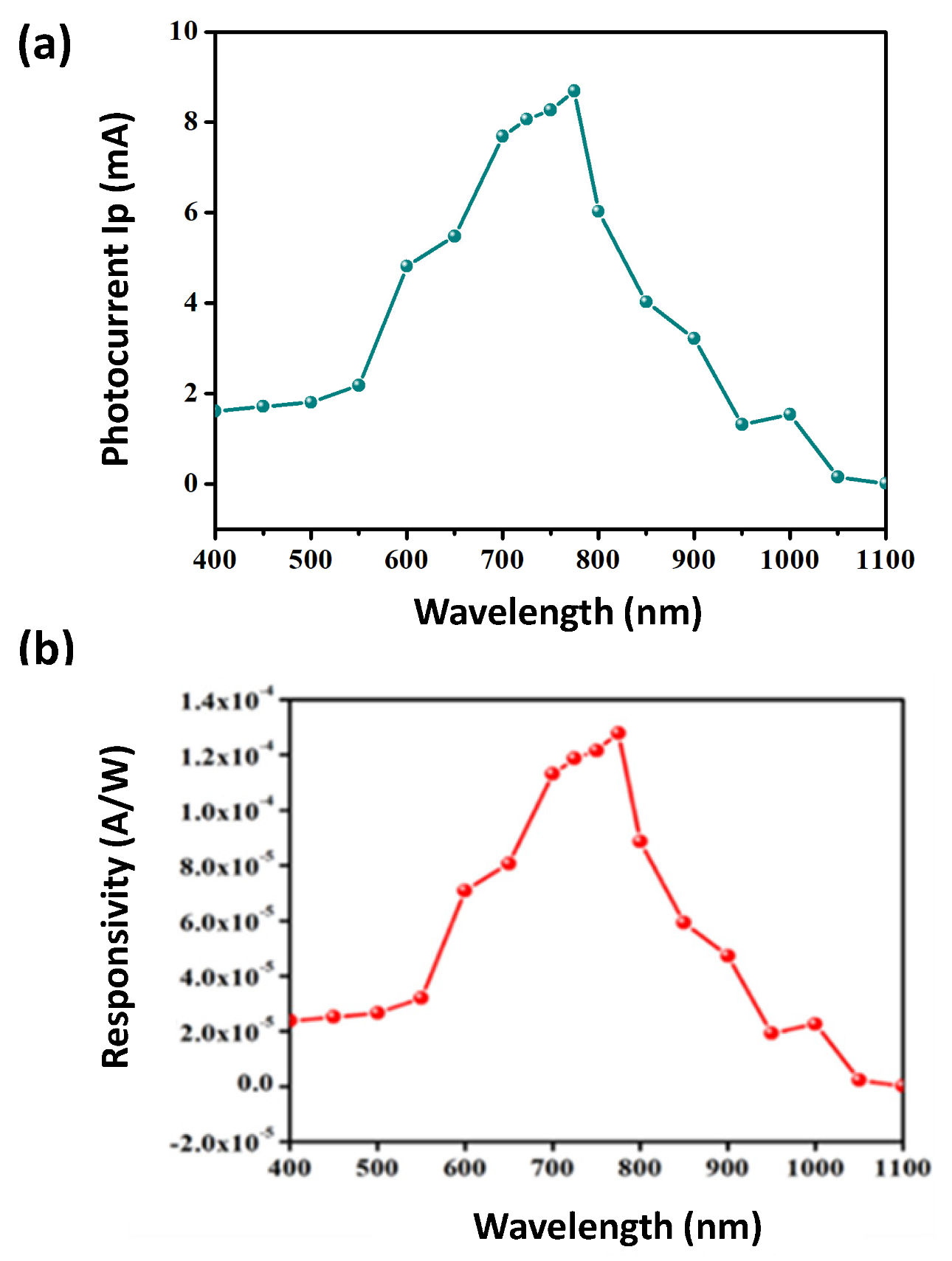
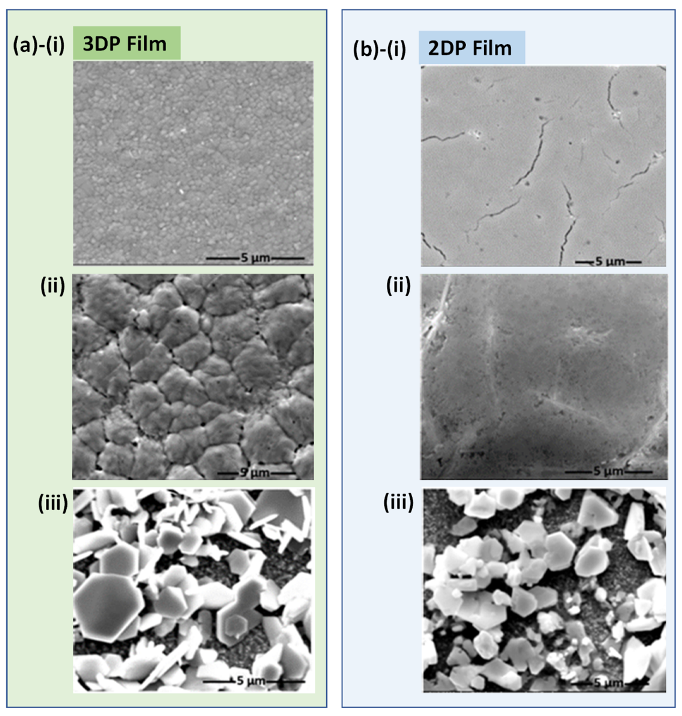
2.2. Comparative Characterization of 3D and 2D PSCs
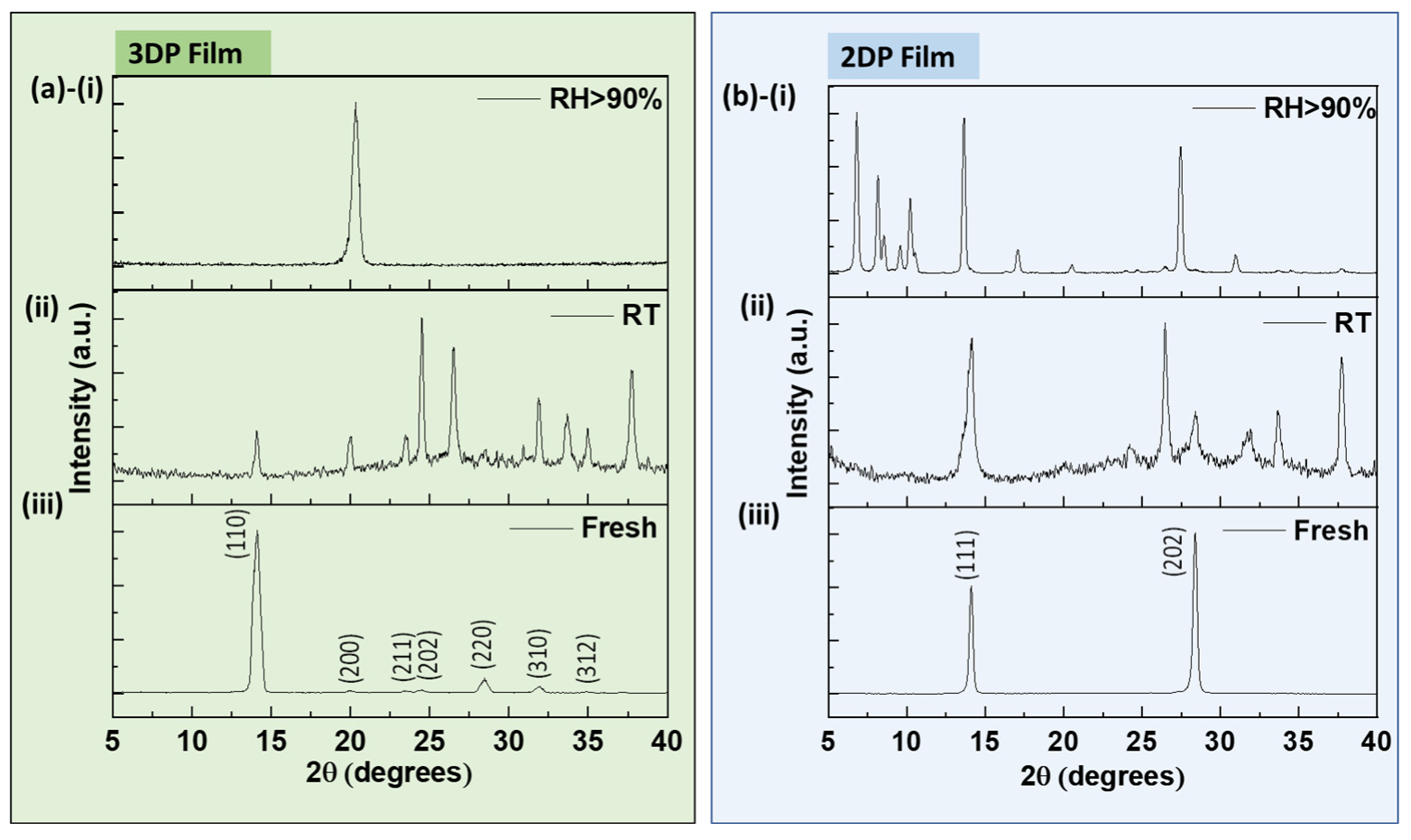
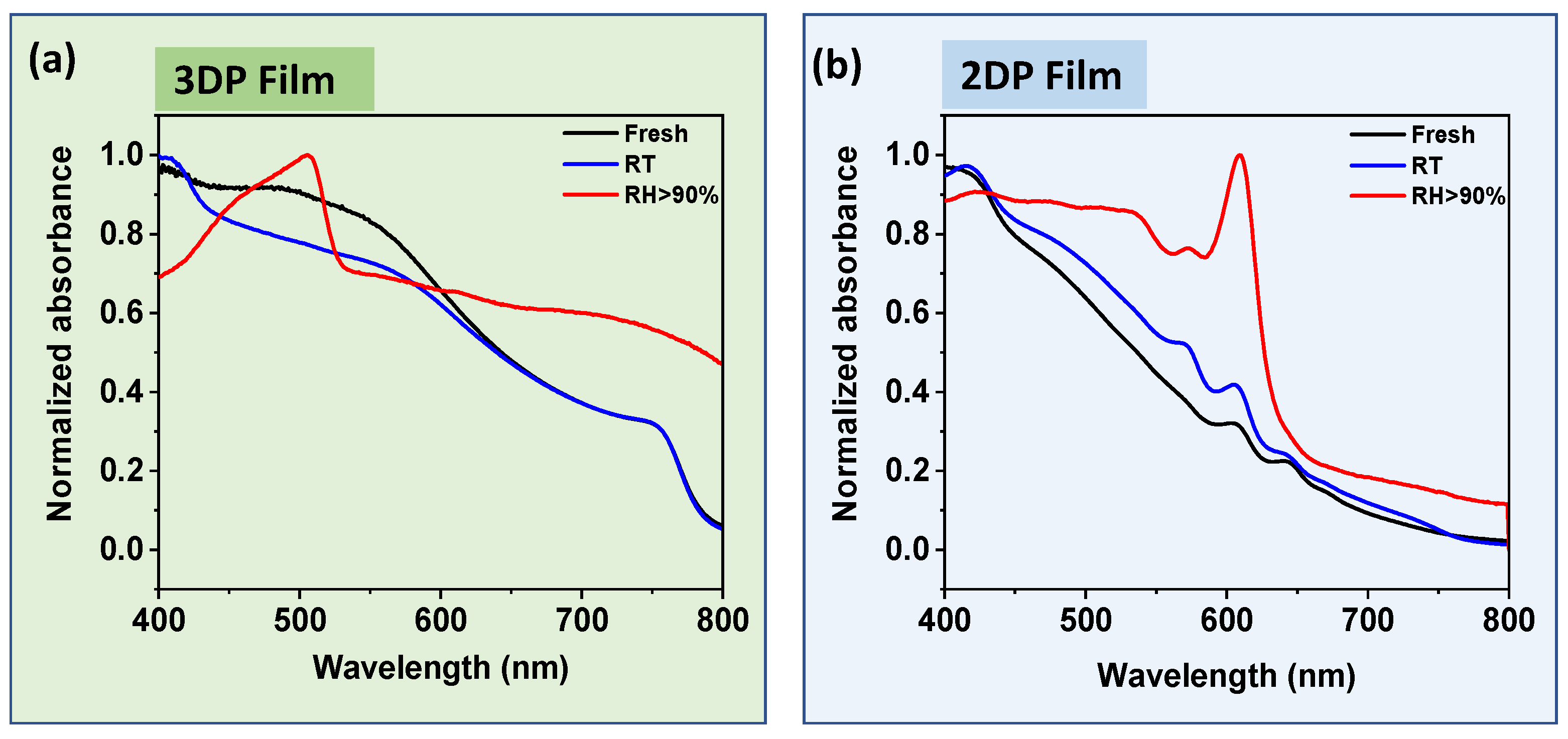
2.3. Stability Comparison of 3DP and 2DP Absorbers
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, W.J.; Shi, T.; Yan, Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv. Mater. 2014, 26, 4653. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Mende, L.; Dyakonov, V.; Olthof, S.; Ünlü, F.; Lê, K.M.T.; Mathur, S.; Karabanov, A.D.; Lupascu, D.C.; Herz, L.M.; Hinderhofer, A.; et al. Roadmap on organic inorganic hybrid perovskite semiconductors and devices. APL Mater. 2021, 9, 109202. [Google Scholar] [CrossRef]
- Savenije, T.J.; Ponseca, C.S.; Kunneman, L.; Abdellah, M.; Zheng, K.; Tian, Y.; Zhu, Q.; Canton, S.E.; Scheblykin, I.G.; Pullerits, T.; et al. Thermally Activated Exciton Dissociation and Recombination Control the Carrier Dynamics in Organometal Halide Perovskite. J. Phys. Chem. Lett. 2014, 5, 2189. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozzaand, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967. [Google Scholar] [CrossRef]
- Wetzelaer, G.J.A.H.; Scheepers, M.; Sempere, A.M.; Momblona, C.; Ávila, J.; Bolink, H.J. Trap-Assisted Non-Radiative Recombination in Organic–Inorganic Perovskite Solar Cells. Adv. Mater. 2015, 27, 1837. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Snaith, H. Methylammonium lead triiodide perovskite solar cells: A new paradigm in photovoltaics. MRS Bull. 2015, 40, 641. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, X.; Chen, X.; Wang, B.; Chen, Z.; Hu, Y.; Guo, Y.; Zhang, L.; Ma, Y.; Gao, L.; et al. Carrier lifetime enhancement in halide perovskite via remote epitaxy. Nat. Commun. 2019, 10, 4145. [Google Scholar] [CrossRef]
- Alarousu, E.; El-Zohry, A.M.; Yin, J.; Zhumekenov, A.A.; Yang, C.; Alhabshi, E.; Gereige, I.; AlSaggaf, A.; Malko, A.V.; Bakr, O.M.; et al. Ultralong Radiative States in Hybrid Perovskite Crystals: Compositions for Submillimeter Diffusion Lengths. J. Phys. Chem. Lett. 2017, 8, 4386. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, V.B.; Kraus, H.; Kapustianyk, V.; Kim, H.J.; Mercere, P.; Rudko, M.; da Silva, P.; Antonyak, O.; Dendebera, M. Bright and fast scintillations of an inorganic halide perovskite CsPbBr3 crystal at cryogenic temperatures. Sci. Rep. 2020, 10, 8601. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, H.; Fang, Y.; Stieg, A.Z.; Song, T.B.; Wang, H.H.; Xu, X.; Liu, Y.; Lu, S.; You, J.; et al. The optoelectronic role of chlorine in CH3NH3PbI3 (Cl)-based perovskite solar cells. Nat. Commun. 2015, 6, 7269. [Google Scholar] [CrossRef] [PubMed]
- Best Research-Cell Efficiencies. Available online: www.nrel.gov/ncpv/images/efficiencychart.jpg (accessed on 30 June 2022).
- Pacchioni, G. Highly efficient perovskite LEDs. Nat. Rev. Mater. 2021, 6, 108. [Google Scholar] [CrossRef]
- Fan, J.; Du, W.; Mi, Y.; Liu, X. Perovskite-based lasers: An introductory survey. In Nanoscale Semiconductor Lasers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–47. [Google Scholar]
- Deng, W.; Zhang, X.; Huang, L.; Xu, X.; Wang, L.; Wang, J.; Shang, Q.; Lee, S.T.; Jie, J. Aligned Single-Crystalline Perovskite Microwire Arrays for High-Performance Flexible Image Sensors with Long-Term Stability. Adv. Mater. 2016, 28, 2201. [Google Scholar] [CrossRef]
- Li, C.H.A.; Zhou, Z.; Vashishtha, P.; Halpert, J.E. The Future Is Blue (LEDs): Why Chemistry Is the Key to Perovskite Displays. Chem. Mater. 2019, 31, 6003. [Google Scholar] [CrossRef]
- Kennard, R.M.; Dahlman, C.J.; Morgan, E.E.; Chung, J.; Cotts, B.L.; Kincaid, J.R.; DeCrescent, R.A.; Stone, K.H.; Panuganti, S.; Mohtashami, Y.; et al. Enhancing and Extinguishing the Different Emission Features of 2D (EA1−xFAx)4Pb3Br10 Perovskite Films. Adv. Opt. Mater. 2022. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Y.; Wang, Z.; Xu, Z.Q.; Zheng, C.; Weber, B.; Song, J.; Wang, Y.; Lu, Y.; Zhang, Y.; et al. Two-dimensional CH3NH3PbI3 perovskite: Synthesis and optoelectronic application. ACS Nano 2016, 10, 3536. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Y.; Ou, Q.; Ha, S.T.; Qiu, C.W.; Zhang, H.; Bao, Q.; Cheng, Y.B.; Xiong, Q. Photonics and optoelectronics of 2D metal-halide perovskites. Small 2018, 14, 1800682. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured ogranometal halide perovskites. Science 2012, 338, 643. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, H.; Heo, J.H.; Kim, T.S.; Myoung, N.; Lee, C.L.; Im, S.H.; Lee, T.W. Multicolored organic/inorganic hybrid perovskite light-emitting diodes. Adv. Mater. 2015, 27, 1248. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Hultqvist, A.; García-Fernández, A.; Anand, A.; Al-Ashouri, A.; Hagfeldt, A.; Crovetto, A.; Abate, A.; Ricciardulli, A.G.; Vijayan, A.; et al. An open-access database and analysis tool for perovskite solar cells based on the FAIR data principles. Nat. Energy 2022, 7, 107–115. [Google Scholar] [CrossRef]
- Sherafatipour, G.; Benduhn, J.; Patil, B.R.; Ahmadpour, M.; Spoltore, D.; Rubahn, H.G.; Vandewal, K.; Madsen, M. Degradation pathways in standard and inverted DBP-C70 based organic solar cells. Sci. Rep. 2019, 9, 4024. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.K.; Xue, J.; Yang, Y. A review of perovskites solar cell stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M.; Tsai, M.C.; Chen, L.H.; Dubale, A.A.; Hwang, B.J.; et al. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.M.; Chang, W.H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75. [Google Scholar] [CrossRef]
- Wali, Q.; Iftikhar, F.J.; Khan, M.E.; Ullah, A.; Iqbal, Y.; Jose, R. Advances in stability of perovskite solar cells. Org. Electron. 2020, 78, 105590. [Google Scholar] [CrossRef]
- He, R.; Ren, S.; Chen, C.; Yi, Z.; Luo, Y.; Lai, H.; Wang, W.; Zeng, G.; Hao, X.; Wang, Y.; et al. Wide-bandgap organic–inorganic hybrid and all-inorganic perovskite solar cells and their application in all-perovskite tandem solar cells. Energy Environ. Sci. 2021, 14, 5723–5759. [Google Scholar] [CrossRef]
- Kim, M.; Motti, S.G.; Sorrentino, R.; Petrozza, A. Enhanced solar cell stability by hygroscopic polymer passivation of metal halide perovskite thin film. Energy Environ. Sci. 2018, 11, 2609. [Google Scholar] [CrossRef]
- Abdelmageed, G.; Mackeen, C.; Hellier, K.; Jewell, L.; Seymour, L.; Tingwald, M.; Carter, S. Effect of temperature on light induced degradation in methylammonium lead iodide perovskite thin films and solar cells. Sol. Energy Mater. Sol. Cells 2018, 174, 566. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, Q.; Li, H.; Niu, Y.; Han, D.; Yang, Q.; Feng, L. Dopant free mixture of Spiro-OMeTAD and PTAA with tunable wettability as hole transport layer enhancing performance of inverted CsPbI₂Br perovskite solar cells. Org. Electron. 2020, 86, 105873. [Google Scholar] [CrossRef]
- Zhou, C.; Tarasov, A.B.; Goodilin, E.A.; Chen, P.; Wang, H.; Chen, Q. Recent strategies to improve moisture stability in metal halide perovskites materials and devices. J. Energy Chem. 2022, 65, 219. [Google Scholar] [CrossRef]
- Gao, P.; Yusoff, A.R.B.M.; Nazeeruddin, M.K. Dimensionality engineering of hybrid halide perovskite light absorbers. Nat. Commun. 2018, 9, 5028. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Stoumpos, C.C.; Zhu, M.; Mao, L.; Spanopoulos, I.; Liu, J.; Kontsevoi, O.Y.; Chen, M.; Sarma, D.; Zhang, Y.; et al. Enhanced photovoltaic performance and stability with a new type of hollow 3D perovskite {en} FASnI3. Sci. Adv. 2017, 3, e1701293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ren, X.; Chen, X.; Mao, J.; Cheng, J.; Zhao, Y.; Liu, Y.; Milic, J.; Yin, W.J.; Grätzel, M.; et al. Improving the stability and performance of perovskite solar cells via off-the-shelf post-device ligand treatment. Energy Environ. Sci. 2018, 11, 2253. [Google Scholar] [CrossRef]
- Ren, A.; Lai, H.; Hao, X.; Tang, Z.; Xu, H.; Jeco, B.M.F.Y.; Watanabe, K.; Wu, L.; Zhang, J.; Sugiyama, M.; et al. Efficient perovskite solar modules with minimized nonradiative recombination and local carrier transport losses. Joule 2020, 4, 1263–1277. [Google Scholar] [CrossRef]
- Roghabadi, F.A.; Alidaei, M.; Mousavi, S.M.; Ashjari, T.; Tehrani, A.S.; Ahmadi, V.; Sadrameli, S.M. Stability progress of perovskite solar cells dependent on the crystalline structure: From 3D ABX 3 to 2D Ruddlesden–Popper perovskite absorbers. J. Mater. Chem. A 2019, 7, 5898. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Z.; Chueh, C.C.; Liu, H.; Peng, B.; Petrone, A.; Li, X.; Wamg, L.; Jen, A.K.Y. Mixed cation FAxPEA1–xPbI3 with enhanced phase and ambient stability toward high-performance perovskite solar cells. Adv. Energy Mater. 2017, 7, 1601307. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Kanatzidis, M.G. The renaissance of halide perovskites and their evolution as emerging semiconductors. Acc. Chem. Res. 2015, 48, 2791. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Mohite, A.D. High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells. Nature 2016, 536, 312. [Google Scholar] [CrossRef] [PubMed]
- Blancon, J.C.; Tsai, H.; Nie, W.; Stoumpos, C.C.; Pedesseau, L.; Katan, C.; Kepenekian, M.; Soe, C.M.M.; Appavoo, K.; Sfeir, M.Y.; et al. Extremely efficient internal exciton dissociation through edge states in layered 2D perovskites. Science 2017, 355, 1288. [Google Scholar] [CrossRef]
- Li, W.; Sidhik, S.; Traore, B.; Asadpour, R.; Hou, J.; Zhang, H.; Fehr, A.; Essman, J.; Wang, Y.; Hoffman, J.M.; et al. Light-activated interlayer contraction in two-dimensional perovskites for high-efficiency solar cells. Nat. Nanotechnol. 2022, 17, 45. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.B. Two-dimensional layered materials: Structure, properties, and prospects for device applications. J. Mater. Res. 2014, 29, 348. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Peng, J.; Tang, J.; Zheng, K.; Liang, Z. 2D Ruddlesden–Popper Perovskites for Optoelectronics. Adv. Mater. 2018, 30, 1703487. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden–Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852. [Google Scholar] [CrossRef]
- Min, M.; Hossain, R.F.; Adhikari, N.; Kaul, A.B. Inkjet-Printed Organohalide 2D Layered Perovskites for High-Speed Photodetectors on Flexible Polyimide Substrates. ACS Appl. Mater. Interfaces 2020, 12, 10809. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.F.; Min, M.; Ma, L.C.; Sakri, S.R.; Kaul, A.B. Carrier photodynamics in 2D perovskites with solution-processed silver and graphene contacts for bendable optoelectronics. NPJ 2D Mater. Appl. 2021, 5, 34. [Google Scholar] [CrossRef]
- Liu, B.; Soe, C.M.M.; Stoumpos, C.C.; Nie, W.; Tsai, H.; Lim, K.; Mohite, A.D.; Kanatzidis, M.G.; Marks, T.J.; Singer, K.D. Optical properties and modeling of 2D perovskite solar cells. Sol. RRL 2017, 1, 1700062. [Google Scholar] [CrossRef]
- Leguy, A.M.; Azarhoosh, P.; Alonso, M.I.; Campoy-Quiles, M.; Weber, O.J.; Yao, J.; Bryant, D.; Weller, M.T.; Nelson, J.; Walsh, A.; et al. Experimental and theoretical optical properties of methylammonium lead halide perovskites. Nanoscale 2016, 8, 6317. [Google Scholar] [CrossRef]
- Liang, X.; Baker, R.W.; Wu, K.; Deng, W.; Ferdani, D.; Kubiak, P.S.; Marken, F.; Torrente-Murciano, L.; Cameron, P.J. Continuous low temperature synthesis of MAPbX3 perovskite nanocrystals in a flow reactor. React. Chem. Eng. 2018, 3, 640. [Google Scholar] [CrossRef]
- El-Henawey, M.I.; Gebhardt, R.S.; El-Tonsy, M.M.; Chaudhary, S. Organic solvent vapor treatment of lead iodide layers in the two-step sequential deposition of CH3NH3PbI3-based perovskite solar cells. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 1947. [Google Scholar] [CrossRef]
- Sidhik, S.; Li, W.; Samani, M.H.; Zhang, H.; Wang, Y.; Hoffman, J.; Mohite, A.D.; Fehr, A.K.; Wong, M.S.; Katan, C.; et al. Memory Seeds Enable High Structural Phase Purity in 2D Perovskite Films for High-Efficiency Devices. Adv. Mater. 2021, 33, 2007176. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, Y.; Askerka, M.; Quan, L.N.; Gong, X.; Zhao, W.; Zhao, J.; Tan, H.; Long, G.; Gao, L.; et al. Color-stable highly luminescent sky-blue perovskite light-emitting diodes. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Jia, E.D.; Lou, X.; Zhou, C.L.; Hao, W.C.; Wang, W.J. O3 fast and simple treatment-enhanced p-doped in Spiro-MeOTAD for CH3NH3I vapor-assisted processed CH3NH3PbI3 perovskite solar cells. Chin. Phys. B 2017, 26, 068803. [Google Scholar] [CrossRef]
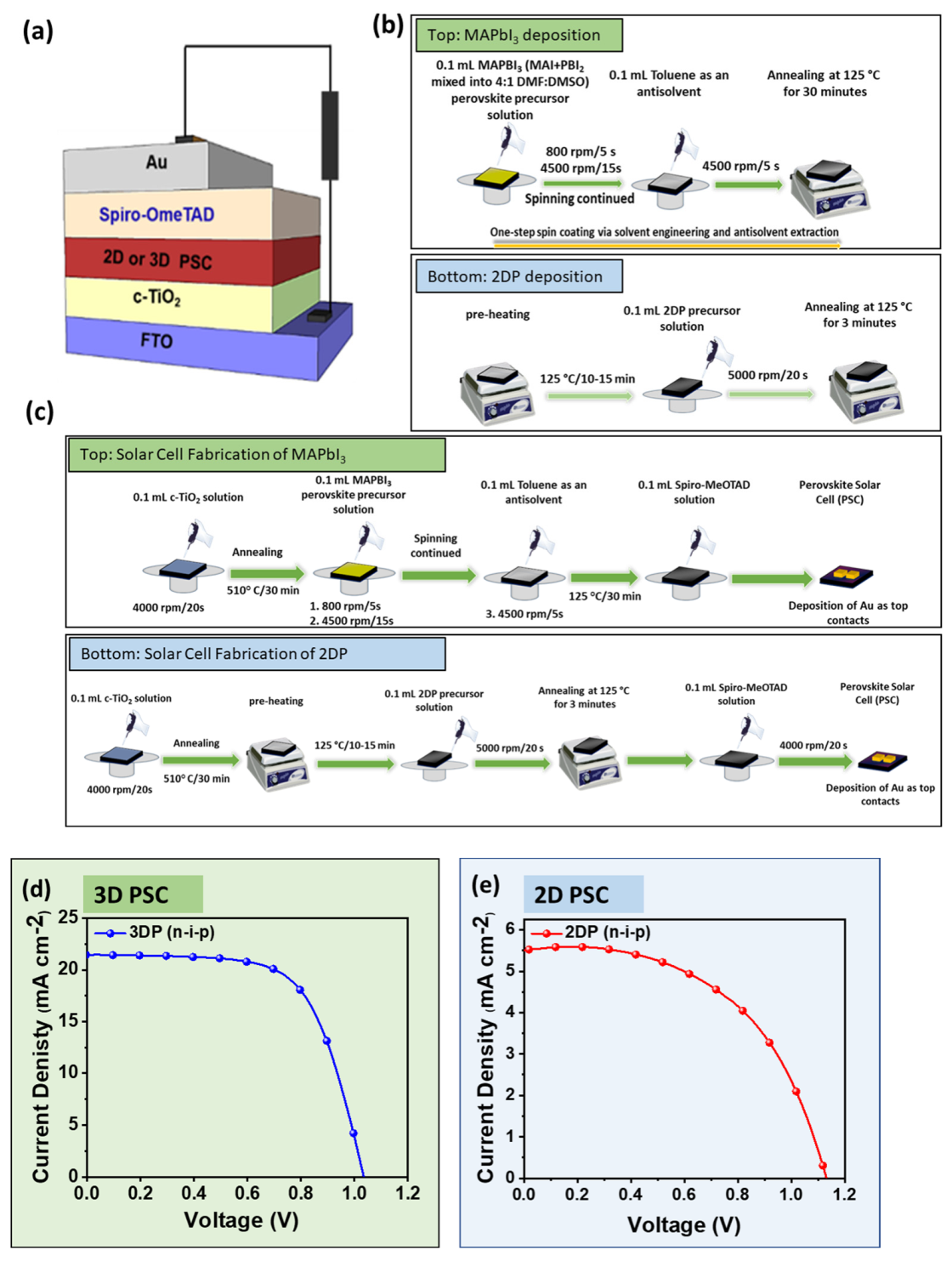
| PSC Absorbers | Voc (V) | Jsc (mA-cm−2) | FF (%) | PCE (%) | Rsh (kΩ-cm2) | Rs (Ω-cm2) |
|---|---|---|---|---|---|---|
| 3DP | 1.06 | 21.89 | 67.50 | 15.69 | 913.54 | 7.53 |
| 2DP | 1.13 | 5.5 | 53.51 | 3.33 | 1159.42 | 43.47 |
| Films | θ (Degrees) | β (Radian) | S (nm) |
|---|---|---|---|
| 3DP-Fresh | 7.05 | 0.00919 | 15.20 |
| 3DP-RT | 7.03 | 0.00464 | 30.10 |
| 2DP-Fresh | 7.05 | 0.00475 | 29.41 |
| 2DP-RT | 7.08 | 0.00863 | 16.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranthiraja, K.; Aryal, S.; Temsal, M.; Sharma, M.; Kaul, A.B. Optical Property and Stability Study of CH3(CH2)3NH3)2(CH3NH3)3Pb4I13 Ruddlesden Popper 2D Perovskites for Photoabsorbers and Solar Cells and Comparison with 3D MAPbI3. Solar 2022, 2, 385-400. https://doi.org/10.3390/solar2040023
Kranthiraja K, Aryal S, Temsal M, Sharma M, Kaul AB. Optical Property and Stability Study of CH3(CH2)3NH3)2(CH3NH3)3Pb4I13 Ruddlesden Popper 2D Perovskites for Photoabsorbers and Solar Cells and Comparison with 3D MAPbI3. Solar. 2022; 2(4):385-400. https://doi.org/10.3390/solar2040023
Chicago/Turabian StyleKranthiraja, Kakaraparthi, Sujan Aryal, Mahdi Temsal, Mohin Sharma, and Anupama B. Kaul. 2022. "Optical Property and Stability Study of CH3(CH2)3NH3)2(CH3NH3)3Pb4I13 Ruddlesden Popper 2D Perovskites for Photoabsorbers and Solar Cells and Comparison with 3D MAPbI3" Solar 2, no. 4: 385-400. https://doi.org/10.3390/solar2040023
APA StyleKranthiraja, K., Aryal, S., Temsal, M., Sharma, M., & Kaul, A. B. (2022). Optical Property and Stability Study of CH3(CH2)3NH3)2(CH3NH3)3Pb4I13 Ruddlesden Popper 2D Perovskites for Photoabsorbers and Solar Cells and Comparison with 3D MAPbI3. Solar, 2(4), 385-400. https://doi.org/10.3390/solar2040023