Ternary Planar Heterojunction Organic Solar Cells Based on the Ternary Active Layers: α-6T/AlPcCl/C60
Abstract
1. Introduction
2. Materials and Methods
2.1. Realization of the Solar Cells
2.2. Characterizations Techniques
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouich, A.; Marí-Guaita, J.; Sahraoui, B.; Palacios, P.; Marí1, B. Tetrabutylammonium (TBA)-Doped Methylammonium Lead Iodide: High Quality and Stable Perovskite Thin Films. Front. Energy Res. 2022, 10, 840817. [Google Scholar] [CrossRef]
- Sampaio, P.G.V.; González, M.O.A. A review on organic photovoltaic cell. Int. J. Energy Res. 2022. [Google Scholar] [CrossRef]
- Chang, D.W.; Choi, H.J.; Filer, A.; Baek, J.B. Graphene in photovoltaic applications: Organic photovoltaic cells (OPVs) and dye-sensitized solar cells (DSSCs). J. Mater. Chem. A 2014, 2, 12136–12149. [Google Scholar] [CrossRef]
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular semiconductors in organic photovoltaic cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Q.; Gao, J.; Ma, X.; Son, J.H.; Jeong, S.Y.; Hu, Z.; Niu, L.; Woo, H.Y.; Zhang, J.; et al. Ternary Organic Photovoltaic Cells Exhibiting 17.59%, Efficiency with Two Compatible Y6 Derivations as Acceptor. Solar Rrl 2021, 5, 2100007. [Google Scholar] [CrossRef]
- Ma, X.; Zeng, A.; Gao, J.; Hu, Z.; Xu, C.; Son, J.H.; Jeong, S.Y.; Zhang, C.; Li, M.; Wang, K.; et al. Approaching 18% efficiency of ternary organic photovoltaics with wide bandgap polymer donor and well compatible Y6: Y6-1O as acceptor. Natl. Sci. Rev. 2021, 8, nwaa305. [Google Scholar] [CrossRef]
- Ram, K.S.; Singh, J. Over 20% Efficient and Stable Non-Fullerene-Based Ternary Bulk-Heterojunction Organic Solar Cell with WS2 Hole-Transport Layer and Graded Refractive Index Antireflection Coating. Adv. Theory Simul. 2020, 3, 2000047. [Google Scholar] [CrossRef]
- Gong, X.; Tong, M.; Brunetti, F.G.; Seo, J.; Sun, Y.; Moses, D.; Wudl, F.; Heeger, A.J. Bulk Heterojunction Solar Cells with Large Open-Circuit Voltage: Electron Transfer with Small Donor-Acceptor Energy Offset. Adv. Mater. 2011, 23, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Baran, D.; Kirchartz, T.; Wheeler, S.; Dimitrov, S.; Abdelsamie, M.; Gorman, J.; Ashraf, R.S.; Holliday, S.; Wadsworth, A.; Gasparini, N.; et al. Reduced voltage losses yield 10% efficient fullerene free organic solar cells with >1 V open circuit voltages. Energy Environ. Sci. 2016, 9, 3783. [Google Scholar] [CrossRef]
- Bin, H.; Zhang, Z.-G.; Gao, L.; Chen, S.; Zhong, L.; Xue, L.; Yang, C.; Li, Y. Non-Fullerene Polymer Solar Cells Based on Alkylthio and Fluorine Substituted 2D-Conjugated Polymers Reach 9.5% Efficiency. J. Am. Chem. Soc. 2016, 138, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.; Singh, J. Study of the mechanism and rate of exciton dissociation at the donor-acceptor interface in bulk-heterojunction organic solar cells. J. Appl. Phys. 2013, 114, 073510. [Google Scholar] [CrossRef]
- Singh, J.; Narayan, M.; Ompong, D.; Zhu, F. Dissociation of charge transfer excitons at the donor–acceptor interface in bulk heterojunction organic solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 7095–7099. [Google Scholar] [CrossRef]
- Lorch, C.; Barnajee, R.; Dieterle, J.; Hinderhofer, A.; Gerlach, A.; Drnec, J.; Schreiber, F. Templating effects of a-sexithiophene in donor-acceptor organic thin films. J. Phys. Chem. C 2015, 119, 23211–23220. [Google Scholar] [CrossRef]
- Bernède, J.C. Organic photovoltaic cells: History, principle and techniques. J. Chil. Chem. Soc. 2008, 53, 1549–1564. [Google Scholar] [CrossRef]
- Gao, F. A New Acceptor for Highly Efficient Organic Solar Cells. Joule 2019, 3, 908–919. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiona, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% efficiency organic solar cells. Sci. Commun. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Yang, W.; Luo, Y.; Guo, P.; Sun, H.; Yao, Y. Leakage Current Induced by Energetic Disorder in Organic Bulk Heterojunction Solar Cells: Comprehending the Ultrahigh Loss of Open-Circuit Voltage at Low Temperatures. Phys. Rev. Appl. 2017, 7, 044017. [Google Scholar] [CrossRef]
- Schaffer, C.J.; Palumbiny, C.M.; Niedermeier, M.A.; Jendrzejewski, C.; Santoro, G.; Roth, S.V.; Müller-Buschbaum, P. A Direct Evidence of Morphological Degradation on a Nanometer Scale in Polymer Solar Cells. Adv. Mater. 2013, 25, 6760–6764. [Google Scholar] [CrossRef]
- Wang, K.; Song, X.; Guo, X.; Wang, Y.; Lai, X.; Meng, F.; Du, M.; Fan, D.; Zhang, R.; Li, G.; et al. Efficient as-cast thick film small-molecule organic solar cell with less fluorination on the donor. Mater. Chem. Front. 2020, 4, 206. [Google Scholar] [CrossRef]
- He, X.; Yin, L.; Li, Y. Design of organic small molecules for photovoltaic application with high open-circuit voltage (Voc). J. Mater. Chem. C 2019, 7, 2487–2521. [Google Scholar] [CrossRef]
- Po, R.; Roncali, J. Beyond efficiency: Scalability of molecular donor materials for organic photovoltaics. Mater. Chem. C 2016, 4, 3677–3685. [Google Scholar] [CrossRef]
- Zhang, Q.; Kan, B.; Liu, F.; Long, G.; Wan, X.; Chen, X.; Zuo, Y.; Ni, W.; Zhang, H.; Li, M.; et al. Small-molecule solar cells with efficiency over 9%. Nat. Photonics 2015, 9, 35–41. [Google Scholar] [CrossRef]
- Galindo, S.; Ahmadpour, M.; Gerling, L.G.; Marsal, A.; Voz, C.; Alcubilla, R.; Puigdollers, J. Influence of the density of states on the open-circuit voltage in small-molecule solar cells. Org. Electron. 2014, 15, 2553–2560. [Google Scholar] [CrossRef]
- Cnops, K.; Rand, B.P.; Cheyns, D.; Verreet, B.; Empl, M.A.; Heremans, P. 8.4% efficient fullerene-free organic solar cells exploiting long-range exciton energy transfer. Nat. Commun. 2014, 5, 3406. [Google Scholar] [CrossRef]
- Leliège, A.; Grolleau, J.; Allain, M.; Blanchard, P.; Demeter, D.; Rousseau, T.; Roncali, J. Small D-p-A systems with s-phenylenebridged accepting units as active materials for organic photovoltaics. Chem.—Eur. J. 2013, 19, 9948–9960. [Google Scholar] [CrossRef] [PubMed]
- Ilmin, R.; Haque, A.; Khan, M.S. High efficiency small molecule based donor materials for organic solar cells. Org. Electron. 2018, 58, 53–62. [Google Scholar] [CrossRef]
- Ftouhi, H.; Lamkaouane, H.; Louarn, G.; Diani, M.; Bernède, J.-C.; Addou, M.; Ftouhi, H.; Lamkaouane, H.; Louarn, G.; Diani, M.; et al. Investigation of aluminum phthalocyanine chloride as acceptor material in planar organic solar cells: Comparative study with fullerene. J. Mater. Sci. Mater. Electron. 2021, 32, 27710–27720. [Google Scholar] [CrossRef]
- El Jouad, Z.; Morsli, M.; Louarn, G.; Cattin, L.; Addou, M.; Bernède, J.-C. Improving the efficiency of subphthalocyanine based planar organic solar cells through the use of MoO3/CuI double anode buffer layer. Sol. Energy Mater. Sol. Cells 2015, 141, 429–435. [Google Scholar] [CrossRef]
- Lakhdar Toumi, A.; Khelil, A.; Tobel, K.; Makha, M.; Hernández, L.A.; Mouchaal, Y.; Cattin, L.; del Valle, M.A.; Diaz, F.R.; Bernède, J.-C. On the exciton blocking layer at the interface organic/cathode in planar multiheterojunction organic solar cells. Solid State Electron. 2015, 104, 1–5. [Google Scholar] [CrossRef]
- Qin, P.; Fang, G.; Zeng, W.; Fan, X.; Zheng, Q.; Cheng, F.; Wan, J.; Zhao, X. Influence of thermal radiation during aluminium thermal evaporation on organic solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 3311. [Google Scholar] [CrossRef]
- Ftouhi, H.; El-Menyawy, E.M.; Lamkaouane, H.; Diani, M.; Louarn, G.; Bernède, J.-C.; Addou, M.; Cattin, L. Efficient planar heterojunction based on α-sexithiophene/fullerene through the use of MoO3/CuI anode buffer layer. Thin Solid Films 2022, 41, 139025. [Google Scholar] [CrossRef]
- Ballarotto, M.; Herman, W.N.; Romero, D.B. Low-bandgap small molecules for near-infrared photovoltaic applications. J. Photonics Energy 2011, 1, 011102. [Google Scholar] [CrossRef][Green Version]
- Marí-Guaita, J.; Bouich, A.; Marí, B. Shedding Light on Phase Stability and Surface Engineering of Formamidinium Lead Iodide (FaPbI3) Thin Films for Solar Cells. Eng. Proc. 2021, 12, 1. [Google Scholar] [CrossRef]
- Liu, D.; Fan, P.; Zhang, D.; Zhang, X.; Yu, J. Förster resonance energy transfer and improved charge mobility for high performance and low-cost ternary polymer solar cells. Sol. Energy 2019, 189, 186–193. [Google Scholar] [CrossRef]
- Coffey, D.C.; Ferguson, A.J.; Kopidakis, N.; Rumbles, G. Photovoltaic charge generation in organic semiconductors based on long-range energy transfer. ACS Nano 2010, 4, 5437–5445. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Narayan, M.R.; Ompong, D. Comparative contributions of singlet and triplet excitons in the performance of organic devices. Phys. Status Solidi 2016, 13, 77–80. [Google Scholar] [CrossRef]
- Mani, A.; Schoonman, J.; Goossens, A. Photoluminescence study of sexithiophene thin films. J. Phys. Chem. B 2005, 109, 4829–4836. [Google Scholar] [CrossRef]
- Mendil, N.; Daoudi, M.; Berkai, Z.; Belghachi, A. Disorder effect on carrier mobility in Fullerene organic semiconductor. J. Phys. Conf. Ser. 2015, 647, 012057. [Google Scholar] [CrossRef]
- Benduhn, J.; Tvingstedt, K.; Piersimoni, F.; Ullbrich, S.; Fan, Y.; Tropiano, M.; McGarry, K.A.; Zeika, O.; Riede, M.K.; Douglas, C.J.; et al. Intrinsic non-radiative voltage losses in fullerene-based organic solar cells. Nat. Energy 2017, 2, 17053. [Google Scholar] [CrossRef]
- Karki, A.; Vollbrecht, J.; Gillett, A.J.; Xiao, S.S.; Yang, Y.; Peng, Z.; Schopp, N.; Dixon, A.L.; Yoon, S.; Schrock, M.; et al. The role of bulk and interfacial morphology in charge generation, recombination, and extraction in non-fullerene acceptor organic solar cells. Energy Environ. Sci. 2020, 13, 3679–3692. [Google Scholar] [CrossRef]
- Cattin, L.; El Jouad, Z.; Siad, M.B.; Mohammed Krarroubi, A.; Neculqueo, G.; Arzel, L.; Stephant, N.; Mastropasqua Talamo, M.; Martinez, F.; Addou, M.; et al. Highlighting the possibility of parallel mechanism in planar ternary photovoltaic cells. AIP Adv. 2018, 8, 115329. [Google Scholar] [CrossRef]
- Zhan, L.; Li, S.; Lau, T.K.; Cui, Y.; Lu, X.; Shi, M.; Li, C.Z.; Li, H.; Hou, J.; Chen, H. Over 17% efficiency ternary organic solar cells enabled by two non-fullerene acceptors working in an alloy-like model. Energy Environ. Sci. 2020, 13, 635–645. [Google Scholar] [CrossRef]
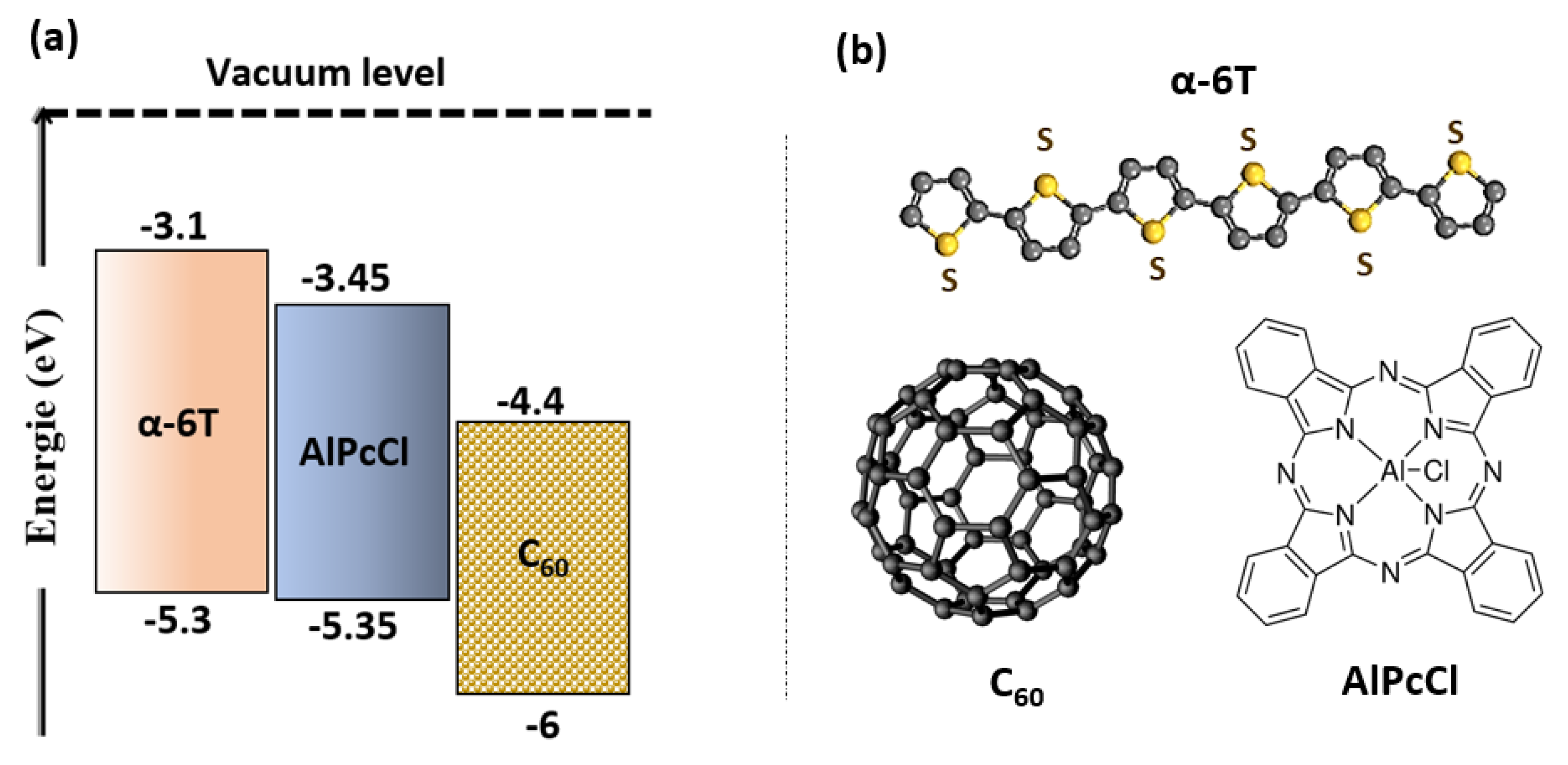


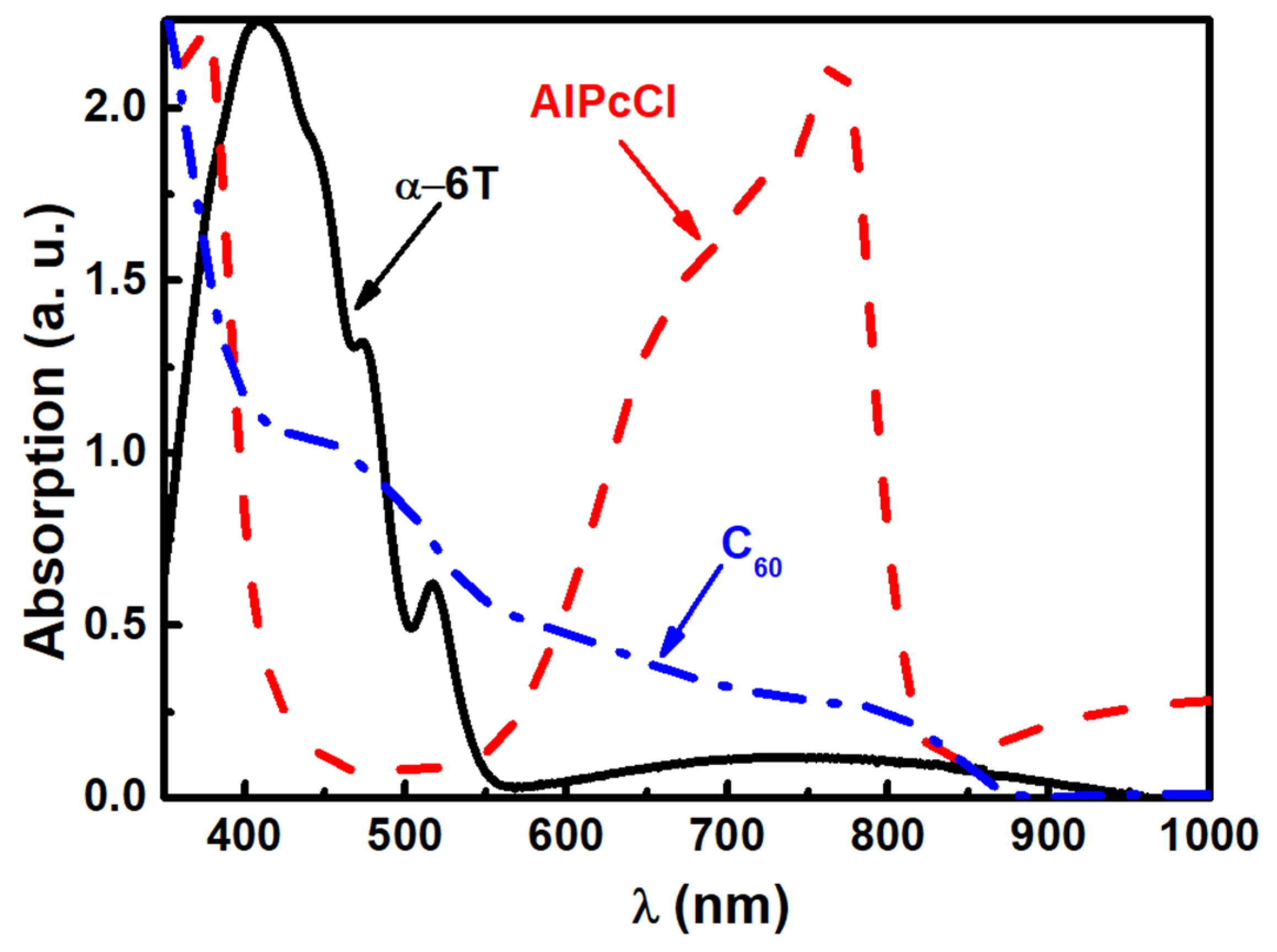
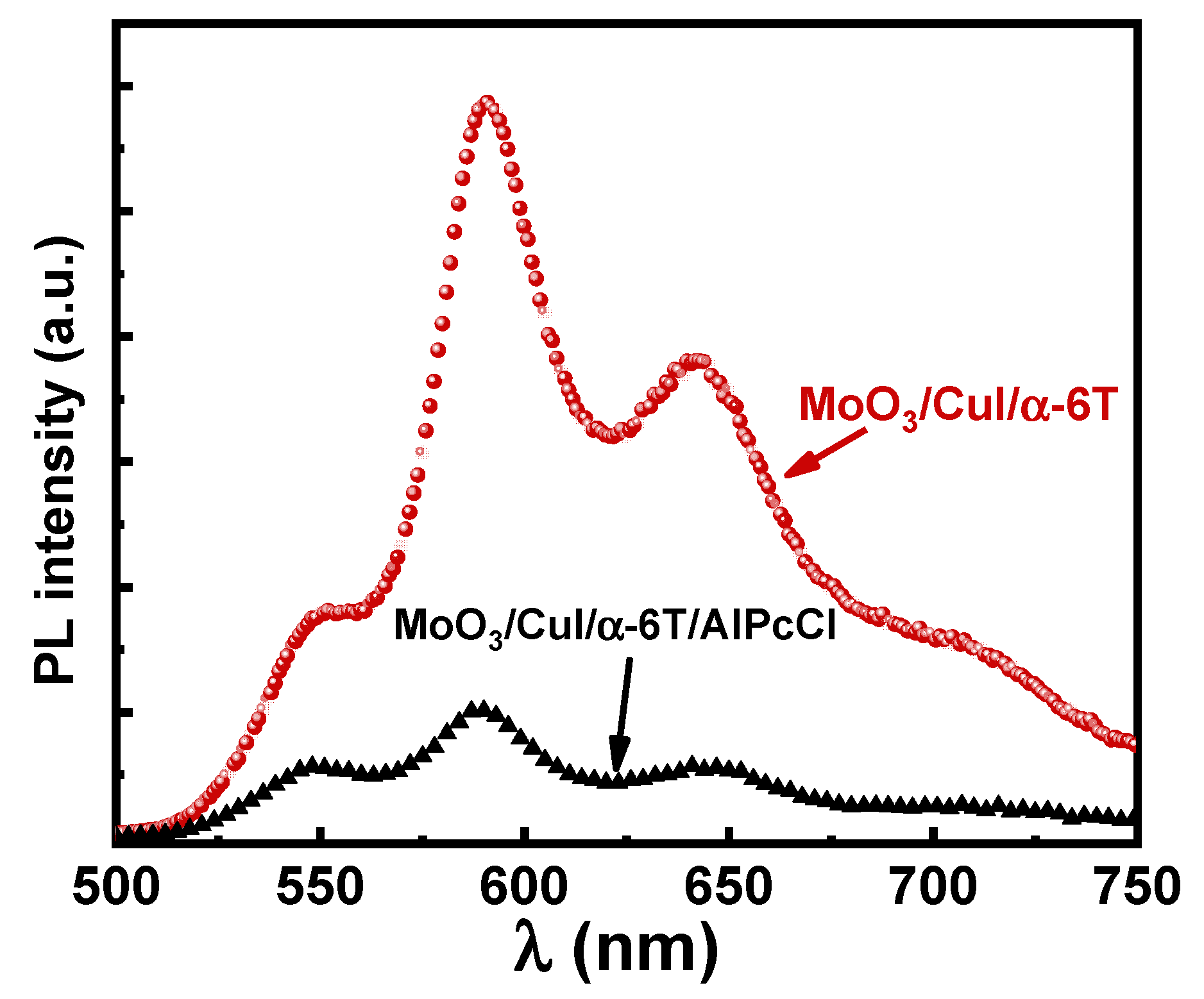
| Layer Thickness (nm) | Voc (V) | Jsc (mA/cm2) | FF (%) | η (%) | ||
|---|---|---|---|---|---|---|
| α-6T | AlPcCl | C60 | ||||
| 22 | 10 | 45 | 0.49 | 11.70 | 43 | 2.50 |
| 22 | 12 | 45 | 0.59 | 11.70 | 53 | 3.70 |
| 22 | 13 | 45 | 0.61 | 11.77 | 43 | 3.10 |
| 22 | 15 | 45 | 0.62 | 14.60 | 48 | 4.33 |
| 22 | 17.5 | 45 | 0.63 | 12.50 | 47 | 3.70 |
| Sample | Thickness (nm) | Voc (V) | Jsc (mA/cm2) | FF (%) | η (%) | ||
|---|---|---|---|---|---|---|---|
| α-6T | AlPcCl | C60 | |||||
| α-6T/C60 | 22 | *** | 45 | 0.51 | 6.10 | 58 | 1.77 |
| α-6T/AlPcCl | 22 | 25 | *** | 0.61 | 7.98 | 51 | 2.48 |
| AlPcCl/C60 | *** | 26 | 45 | 0.70 | 9.61 | 59.5 | 3.97 |
| α-6T/AlPcCl/C60 | 22 | 15 | 45 | 0.62 | 14.60 | 48 | 4.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ftouhi, H.; Lamkaouane, H.; Diani, M.; Louarn, G.; Arzel, L.; Bernède, J.-C.; Addou, M.; Cattin, L. Ternary Planar Heterojunction Organic Solar Cells Based on the Ternary Active Layers: α-6T/AlPcCl/C60. Solar 2022, 2, 375-384. https://doi.org/10.3390/solar2030022
Ftouhi H, Lamkaouane H, Diani M, Louarn G, Arzel L, Bernède J-C, Addou M, Cattin L. Ternary Planar Heterojunction Organic Solar Cells Based on the Ternary Active Layers: α-6T/AlPcCl/C60. Solar. 2022; 2(3):375-384. https://doi.org/10.3390/solar2030022
Chicago/Turabian StyleFtouhi, Hajar, Hind Lamkaouane, Mustapha Diani, Guy Louarn, Ludovic Arzel, Jean-Christian Bernède, Mohammed Addou, and Linda Cattin. 2022. "Ternary Planar Heterojunction Organic Solar Cells Based on the Ternary Active Layers: α-6T/AlPcCl/C60" Solar 2, no. 3: 375-384. https://doi.org/10.3390/solar2030022
APA StyleFtouhi, H., Lamkaouane, H., Diani, M., Louarn, G., Arzel, L., Bernède, J.-C., Addou, M., & Cattin, L. (2022). Ternary Planar Heterojunction Organic Solar Cells Based on the Ternary Active Layers: α-6T/AlPcCl/C60. Solar, 2(3), 375-384. https://doi.org/10.3390/solar2030022








