Enhanced Electrical Properties of Alkali-Doped ZnO Thin Films with Chemical Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Deposition
2.2. Characterization Techniques
3. Results and Discussion
3.1. Crystallographic Properties of M-Doped ZnO (M = Na, K) Thin Films
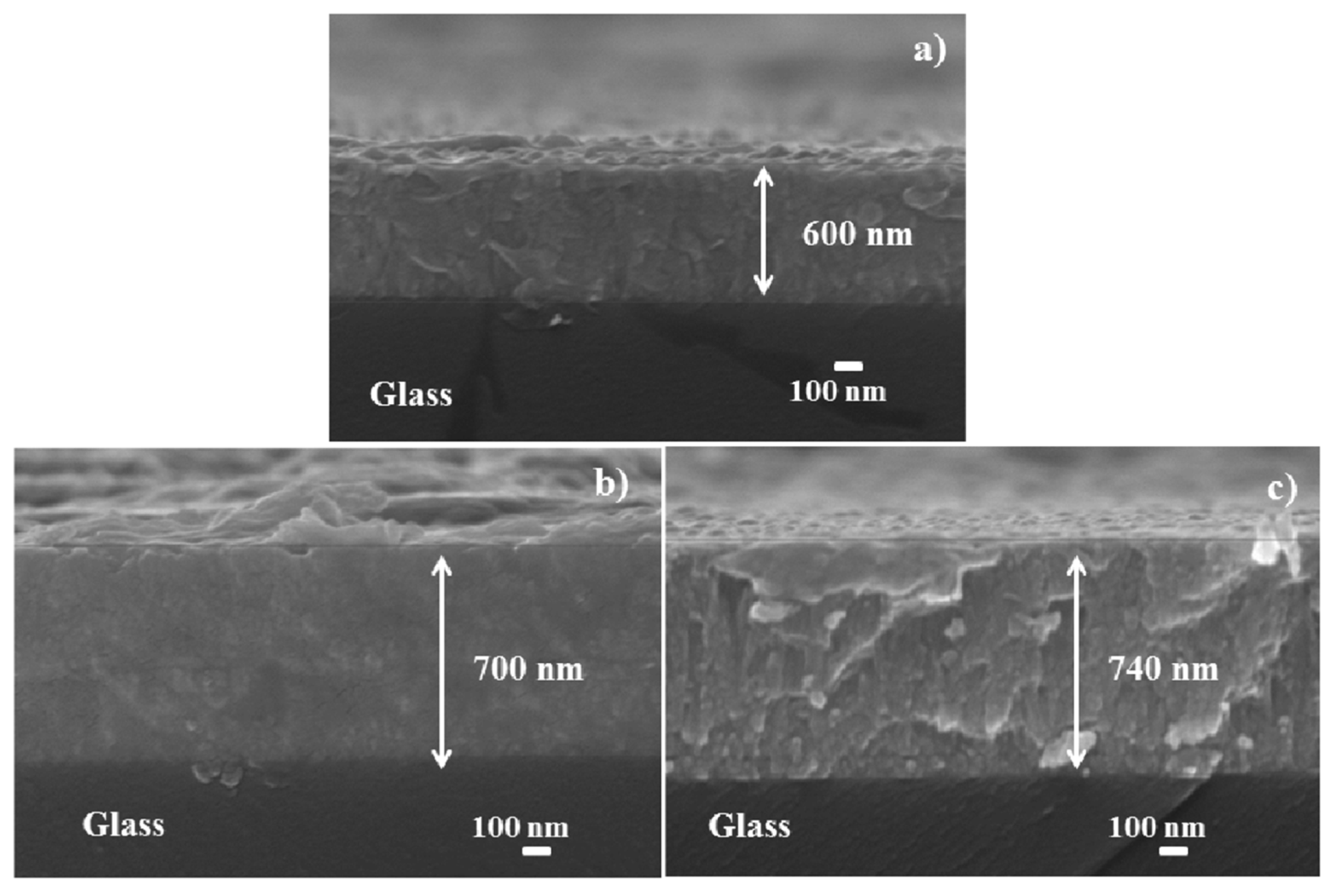
3.2. Morphological and Structural Properties of M-Doped ZnO (M = Na, K) Thin Films
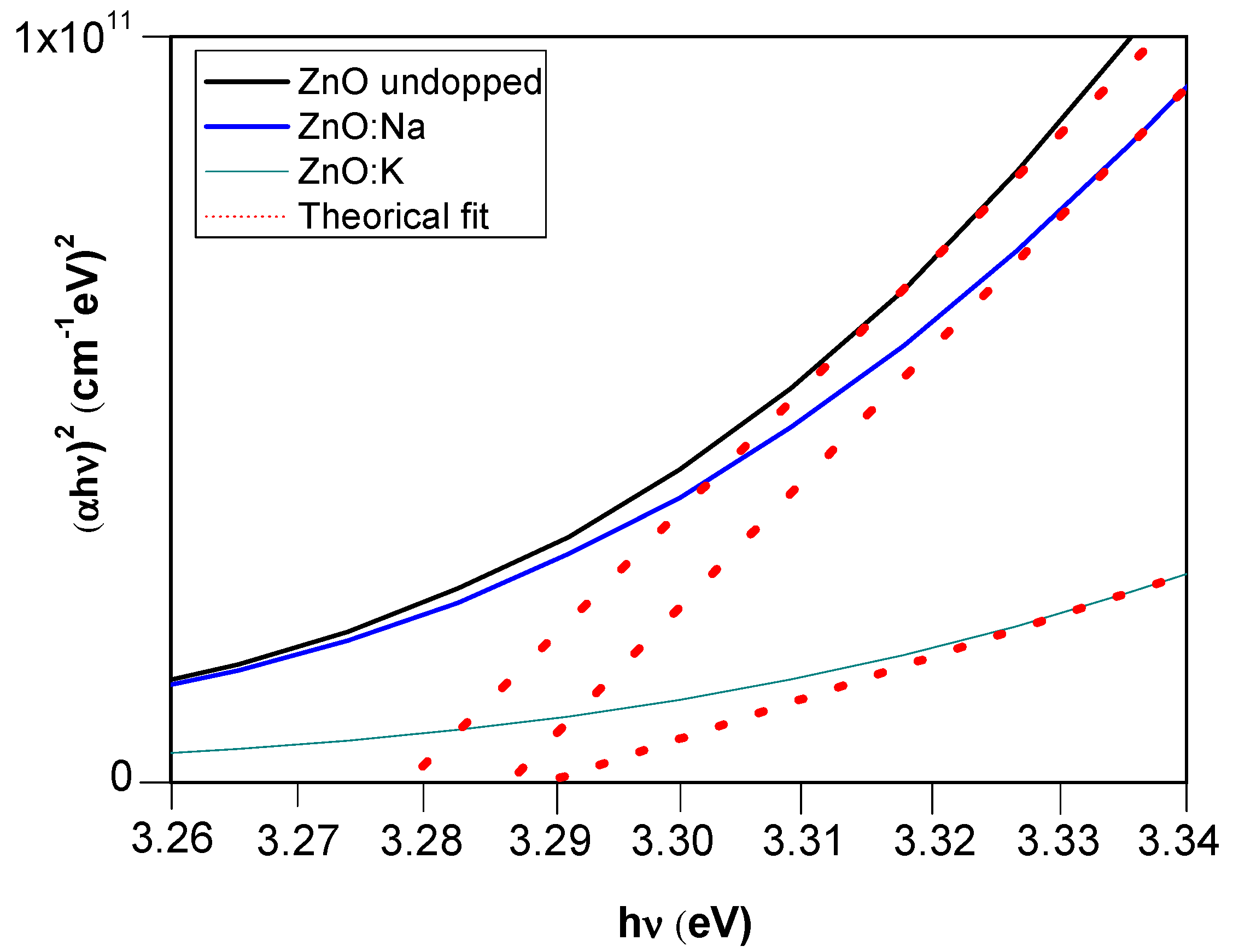
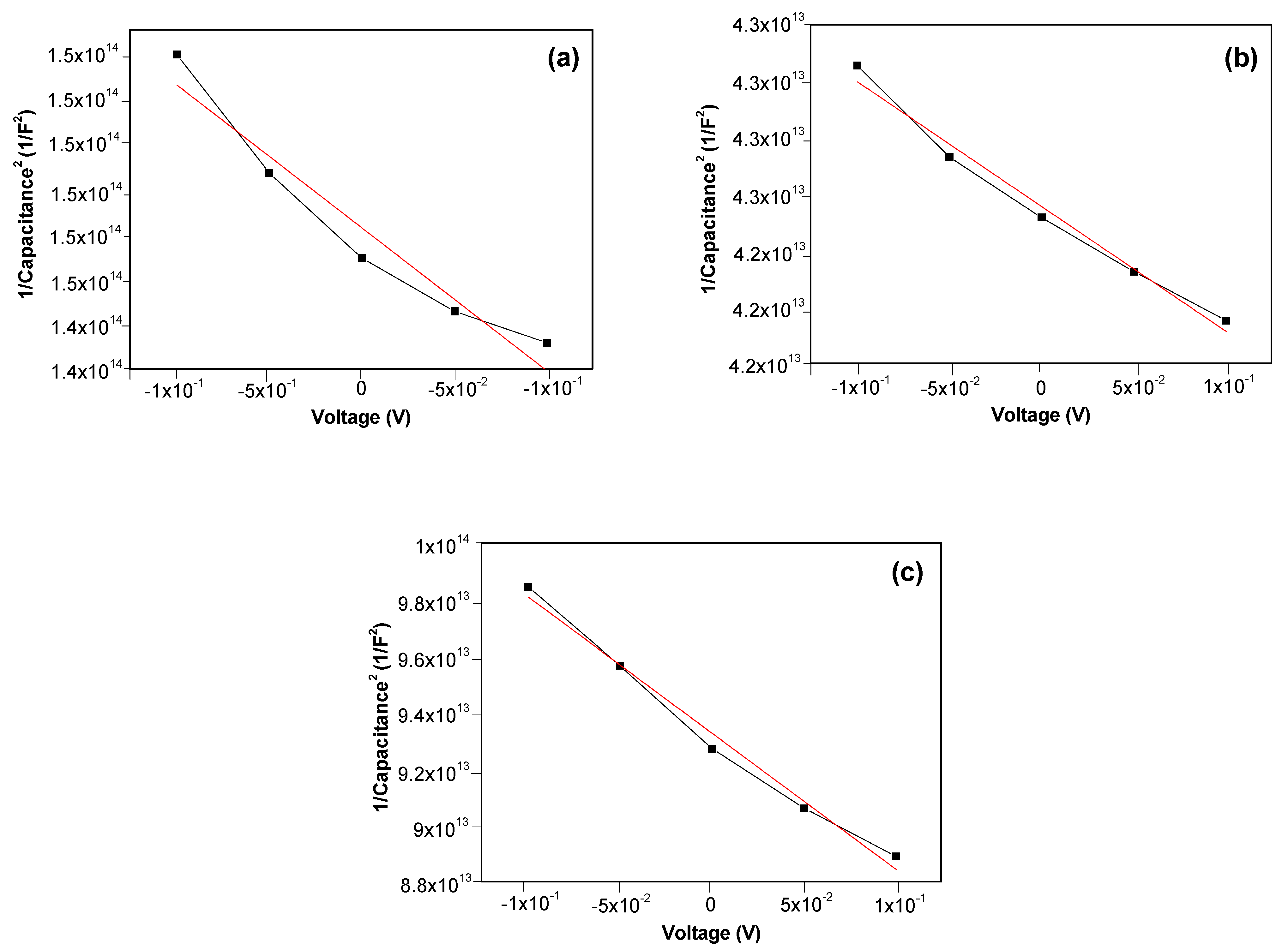
3.3. Optical and Electrical Properties of M-Doped ZnO (M = Na, K) Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferhati, H.; Djeffal, F.; Kacha, K.; Bendjerad, A.; Benhaya, A. Influence of TCO intermediate thin-layers on the electrical and thermal properties of metal/TCO/p-Si Schottky structure fabricated via RF magnetron sputtering. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 106, 25–30. [Google Scholar] [CrossRef]
- Cruz, A.; Wang, E.C.; Morales-Vilches, A.B.; Meza, D.; Neubert, S.; Szyszka, B.; Schlatmann, R.; Stannowski, B. Effect of front TCO on the performance of rear-junction silicon heterojunction solar cells: Insights from simulations and experiments. Sol. Energy Mater. Sol. Cells 2019, 195, 339–345. [Google Scholar] [CrossRef]
- Çolak, H.; Karaköse, E. Tm-DOPED ZnO NANORODS as a TCO for PV Applications. J. Rare Earths 2018, 36, 1067–1073. [Google Scholar] [CrossRef]
- Todorov, T.K.; Bishop, D.M.; Lee, Y.S. Materials perspectives for next-generation low-cost tandem solar cells. Sol. Energy Mater. Sol. Cells 2018, 180, 350–357. [Google Scholar] [CrossRef]
- Aouaj, M.A.; Diaz, R.; Belayachi, A.; Rueda, F.; Abd-Lefdil, M. Comparative study of ITO and FTO thin films grown by spray pyrolysis. Mater. Res. Bull. 2009, 44, 1458–1461. [Google Scholar] [CrossRef]
- Thampy, A.S.; Dhamodharan, S.K. Performance analysis and comparison of ITO-and FTO-based optically transparent terahertz U-shaped patch antennas. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 66, 52–58. [Google Scholar] [CrossRef]
- Han, C.H.; Han, S.D.; Gwak, J.; Khatkar, S.P. Synthesis of indium tin oxide (ITO) and fluorine-doped tin oxide (FTO) nano-powder by sol-gel combustion hybrid method. Mater. Lett. 2007, 61, 1701–1703. [Google Scholar] [CrossRef]
- Bisht, H.; Eun, H.T.; Mehrtens, A.; Aegerter, M.A. Comparison of spray pyrolyzed FTO, ATO and ITO coatings for flat and bent glass substrates. Thin Solid Films 1999, 351, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T.; Matsui, H.; Tanabe, N. New transparent conductive films: FTO coated ITO. Thin Solid Films 2003, 445, 241–244. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, Y.B.; Kim, D.E.; Kim, K.S.; Yoon, Y.D.; Kang, W.J.; Lee, W.; Cho, H.K.; Kim, Y.H. The pH-dependent corrosion behavior of ternary oxide semiconductors and common metals and its application for solution-processed oxide thin film transistors circuit integration. J. Alloy. Compd. 2017, 714, 572–582. [Google Scholar] [CrossRef]
- Aydogan, S.; Yilmaz, M. Crystallographic disorders depending on monovalent cations addition and their effects on ZnO’s characteristics. Ceram. Int. 2020, 46, 8420–8430. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Mahmood, A.; Naeem, A. Sol-Gel-Derived Doped ZnO Thin Films: Processing, Properties, and Applications. In Recent Applications in Sol-Gel Synthesis; Intech Open: London, UK, 2017; pp. 169–193. [Google Scholar] [CrossRef] [Green Version]
- Kegel, J.; Povey, I.M.; Pemble, M.E. Zinc oxide for solar water splitting: A brief review of the material’s challenges and associated opportunities. Nano Energy 2018, 54, 409–428. [Google Scholar] [CrossRef]
- Marouf, S.; Beniaiche, A.; Kardarian, K.; Mendes, M.J.; Sanchez-Sobrado, O.; Águas, H.; Fortunato, E.; Martins, R. Low-temperature spray-coating of high-performing ZnO:Al films for transparent electronics. J. Anal. Appl. Pyrolysis 2017, 127, 299–308. [Google Scholar] [CrossRef]
- Ynineb, F.; Attaf, N.; Aida, M.S.; Bougdira, J.; Bouznit, Y.; Rinnert, H. Morphological and optoelectrical study of ZnO:In/p-Si heterojunction prepared by ultrasonic spray pyrolysis. Thin Solid Films 2017, 628, 36–42. [Google Scholar] [CrossRef]
- Mondal, P.; Das, D. Further improvements in conducting and transparent properties of ZnO:Ga films with perpetual c-axis orientation: Materials optimization and application in silicon solar cells. Appl. Surf. Sci. 2017, 411, 315–320. [Google Scholar] [CrossRef]
- Seawsakul, K.; Horprathum, M.; Eiamchai, P.; Pattantsetakul, V.; Limwichean, S.; Muthitamongkol, P.; Thanachayanont, C.; Songsiriritthigul, P. Glancing-angle pulsed dc magnetron sputtered AZO thin films for TCO applications. Mater. Today Proc. 2018, 5, 14166–14171. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Chen, F.; Shen, Q.; Zhang, L. Chemical evolution of target surfaces during RF magnetron sputtering and its effect on the performance of TCO films. Appl. Surf. Sci. 2019, 493, 665–672. [Google Scholar] [CrossRef]
- Bedia, A.; Bedia, F.Z.; Aillerie, M.; Maloufi, N. Structural, electrical and optical properties of Al–Sn codoped ZnO transparent conducting layer deposited by spray pyrolysis technique. Superlattices Microstruct. 2017, 111, 714–721. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, T.; Wu, S.; Ji, X.; Zhang, Q. Na-doped ZnO nanorods fabricated by chemical vapor deposition and their optoelectrical properties. J. Alloy. Compd. 2017, 690, 189–194. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; He, H.; Ye, Z.; Jiang, J.; Cao, L. Growth of Na doped p-type non-polar a-plane ZnO films by pulsed laser deposition. Mater. Lett. 2012, 76, 81–83. [Google Scholar] [CrossRef]
- Tay, C.B.; Chua, S.J.; Loh, K.P. Stable p-type doping of ZnO film in aqueous solution at low temperatures. J. Phys. Chem. C 2010, 114, 9981–9987. [Google Scholar] [CrossRef]
- Zhang, J.; Tse, K.; Wong, M.; Zhang, Y.; Zhu, J. A brief review of co-doping. Front. Phys. 2016, 11, 117405. [Google Scholar] [CrossRef] [Green Version]
- Bu, I.Y.Y. Direct formation of p-type ZnO by using potassium hydroxide. Optik 2018, 159, 87–93. [Google Scholar] [CrossRef]
- Kaneva, N.; Stambolova, I.; Blaskov, V.; Dimitriev, Y.; Bojinova, A.; Dushkin, C. A comparative study on the photocatalytic efficiency of ZnO thin films prepared by spray pyrolysis and sol-gel method. Surf. Coat. Technol. 2012, 207, 5–10. [Google Scholar] [CrossRef]
- Zhao, J.L.; Li, X.M.; Zhang, S.; Yang, C.; Gao, X.D.; Yu, W.D. Highly (002)-oriented ZnO film grown by ultrasonic spray pyrolysis on ZnO-seeded Si (100) substrate. J. Mater. Res. 2006, 21, 2185–2190. [Google Scholar] [CrossRef] [Green Version]
- Murti, D.K.; Bluhm, T.L. Preferred orientation of ZnO films controlled by r.f. sputtering. Thin Solid Films 1982, 87, 57–61. [Google Scholar] [CrossRef]
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Erhart, P.; Albe, K. First-principles study of migration mechanisms and diffusion of oxygen in zinc oxide. Phys. Rev. B 2006, 73, 115207. [Google Scholar] [CrossRef] [Green Version]
- Ilican, S. Effect of Na doping on the microstructures and optical properties of ZnO nanorods. J. Alloy. Compd. 2013, 553, 225–232. [Google Scholar] [CrossRef]
- Guan, S.; Watabe, T.; Hao, L.; Yoshida, H.; Cheng, Y.; Zhou, K.; Lu, Y. Enhanced photocatalytic activity of potassium-doped titania photocatalyst films with nanosheet structure. Mater. Lett. 2019, 242, 174–178. [Google Scholar] [CrossRef]
- Purbayanto, M.A.K.; Rusydi, A.; Darma, Y. The effect of crystallinity on the surface modification and optical properties of ZnO thin films. Phys. Chem. Chem. Phys. 2020, 22, 2010–2018. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Yu, Y.H.; Pei, Z.L.; Bai, X.D.; Sun, C.; Huang, R.F.; Wen, L.S. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Appl. Surf. Sci. 2000, 158, 134–140. [Google Scholar] [CrossRef]
- Hsieh, P.T.; Chen, Y.C.; Kao, K.S.; Wang, C.M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2008, 90, 317–321. [Google Scholar] [CrossRef]
- Szörényi, T.; Laude, L.D.; Bertóti, I.; Kántor, Z.; Geretovszky, Z. Excimer laser processing of indium-tin-oxide films: An optical investigation. J. Appl. Phys. 1995, 78, 6211–6219. [Google Scholar] [CrossRef]
- Ratana, T.; Amornpitoksuk, P.; Ratana, T.; Suwanboon, S. The wide band gap of highly oriented nanocrystalline Al doped ZnO thin films from sol-gel dip coating. J. Alloy. Compd. 2009, 470, 408–412. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, B.; Zhang, H.; Yu, C.; Li, F. Insights into Working Mechanism of Alkali Metal Fluorides as Dopants of ZnO Films in Inverted Polymer Solar Cells. J. Phys. Chem. C 2018, 122, 24542–24549. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement of Sheet Resistivities with the Four-Point Probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, S.A.; Lee, C.H.; Lee, H.J.; Jeong, S.Y.; Cho, C.R. The structural and optical behaviors of K-doped ZnO/Al2O3 (0001) films. Appl. Phys. Lett. 2004, 85, 419–421. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-band potential of a semiconductor: Using the Mott-Schottky equation. J. Chem. Educ. 2007, 84, 685–688. [Google Scholar] [CrossRef]
- Pedemonte, M.M. Estudios Fisicoquímicos Sobre Materiales Basados enel TiO2 Relacionados con la Foto-Descomposición de Agua y el Desarrollo de Celdas Solares Foto-Electroquímicas. Ph.D. Thesis, Universidad Nacional de La Plata, Provincia de Buenos Aires, Argentina, 2011. [Google Scholar]
- Singh, B.K.; Tripathi, S. Performance analysis of Schottky diodes based on Bi doped p-ZnO thin films. Superlattices Microstruct. 2018, 120, 288–297. [Google Scholar] [CrossRef]
- Le Bourhis, E. Glass: Mechanics and Technology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Reeber, R.R. Lattice parameters of zno from 4.2 to 296 k. J. Appl. Phys. 1970, 41, 5063–5066. [Google Scholar] [CrossRef]
- Van De Walle, C.G. Hydrogen as a cause of doping in zinc oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamataki, M.; Tsamakis, D.; Xanthakis, J.P.; Ali, H.A.; Esmaili-Sardari, S.; Iliadis, A.A. Electrical characterization of Cr Schottky contacts on undoped and Ni-doped p-ZnO films grown by pulsed laser deposition on Si (1 0 0) substrates. Microelectron. Eng. 2013, 104, 95–99. [Google Scholar] [CrossRef]
- Akcan, D.; Gungor, A.; Arda, L. Structural and optical properties of Na-doped ZnO films. J. Mol. Struct. 2018, 1161, 299–305. [Google Scholar] [CrossRef]
- Zhang, G.; Pan, C.; Zhou, Q. Effects of doping Na on the structure and physical properties of La2/3Ca1/3MnO3. Solid State Commun. 2007, 141, 471–473. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Yuan, J. Effect of K-doping on structural and optical properties of ZnO thin films. Superlattices Microstruct. 2008, 44, 276–281. [Google Scholar] [CrossRef]



| Dopants (at 3%) | Resistivity (Ωcm) | Mobility (cm2 /Vs) | Carrier Concentration (cm−3) | Conduction Type |
|---|---|---|---|---|
| Undoped ZnO | 1.03 × 10−1 | 1 × 102 | 5 × 1017 | n |
| Na | 3.18 × 10−2 | 1 × 102 | 1 × 1018 | n |
| K | 5.64 × 10−2 | 9 × 101 | 1 × 1018 | n |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuadra, J.G.; Porcar, S.; Fraga, D.; Stoyanova-Lyubenova, T.; Carda, J.B. Enhanced Electrical Properties of Alkali-Doped ZnO Thin Films with Chemical Process. Solar 2021, 1, 30-40. https://doi.org/10.3390/solar1010004
Cuadra JG, Porcar S, Fraga D, Stoyanova-Lyubenova T, Carda JB. Enhanced Electrical Properties of Alkali-Doped ZnO Thin Films with Chemical Process. Solar. 2021; 1(1):30-40. https://doi.org/10.3390/solar1010004
Chicago/Turabian StyleCuadra, Jaime G., Samuel Porcar, Diego Fraga, Teodora Stoyanova-Lyubenova, and Juan B. Carda. 2021. "Enhanced Electrical Properties of Alkali-Doped ZnO Thin Films with Chemical Process" Solar 1, no. 1: 30-40. https://doi.org/10.3390/solar1010004
APA StyleCuadra, J. G., Porcar, S., Fraga, D., Stoyanova-Lyubenova, T., & Carda, J. B. (2021). Enhanced Electrical Properties of Alkali-Doped ZnO Thin Films with Chemical Process. Solar, 1(1), 30-40. https://doi.org/10.3390/solar1010004







