Long-Term Changes in Fish Community Composition of a Coregonid Dominated Oligotrophic Lake
Abstract
1. Introduction
2. Materials and Methods
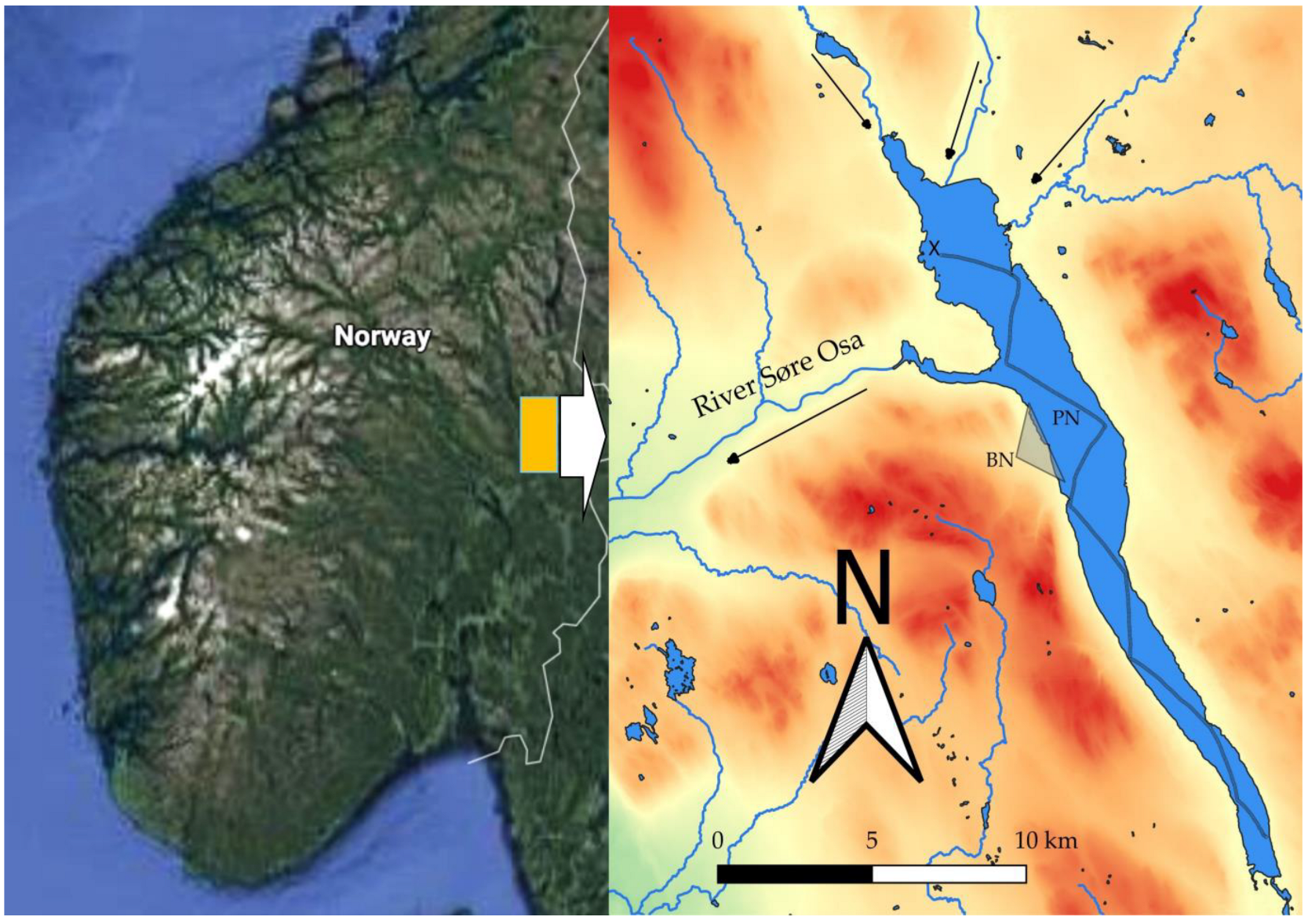
2.1. Study Area
2.2. Sampling
2.3. Data Treatment and Statistical Analysis
Model 2: y = factor(Year)*Species + Fish length;
Model 3: y = factor(Year)*Fish length + Species;
Model 4: y = factor(Year) + Fish length + Species.
3. Results
3.1. Fish Density and Spatial Distribution
3.2. Fish Size
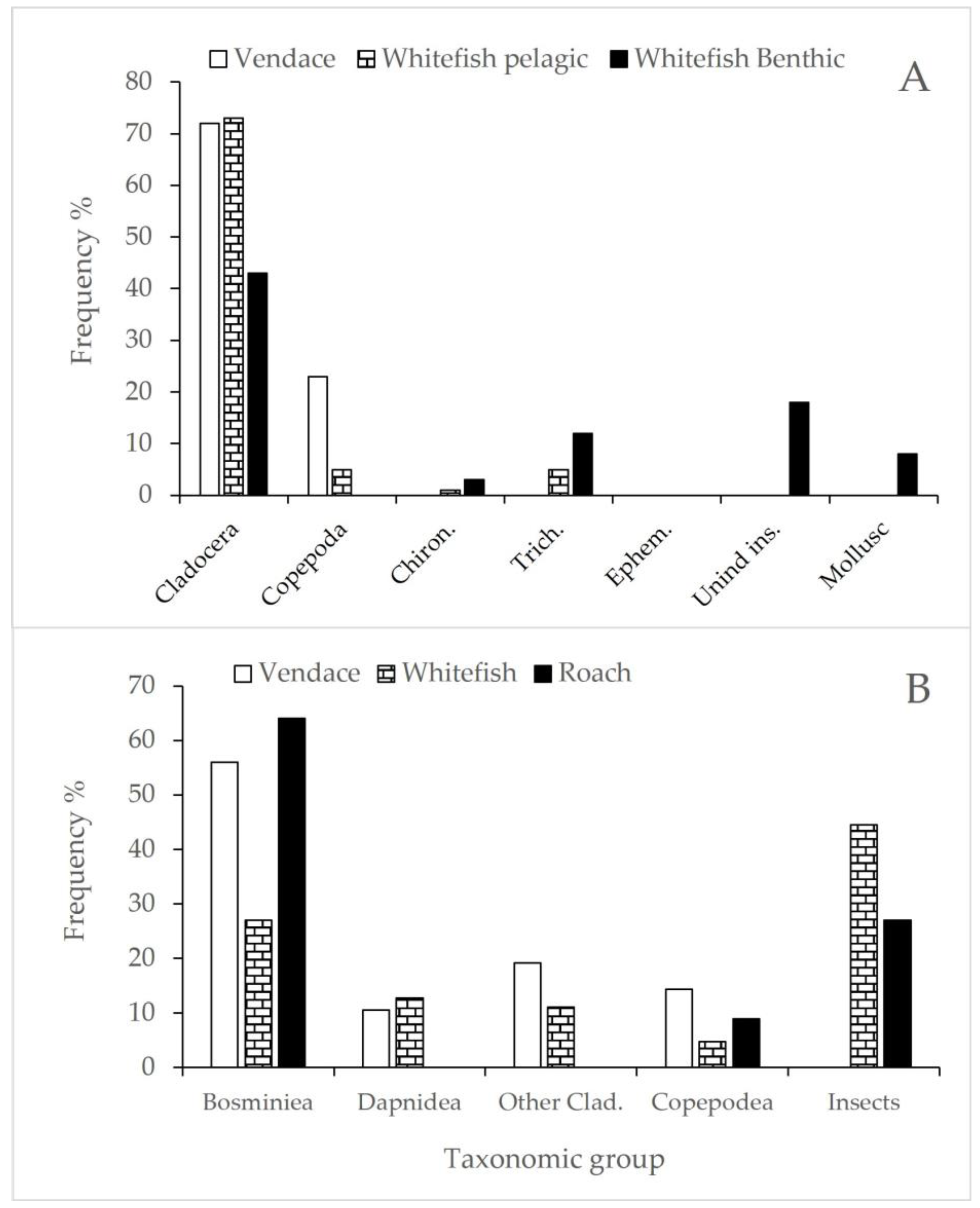
3.3. Diet
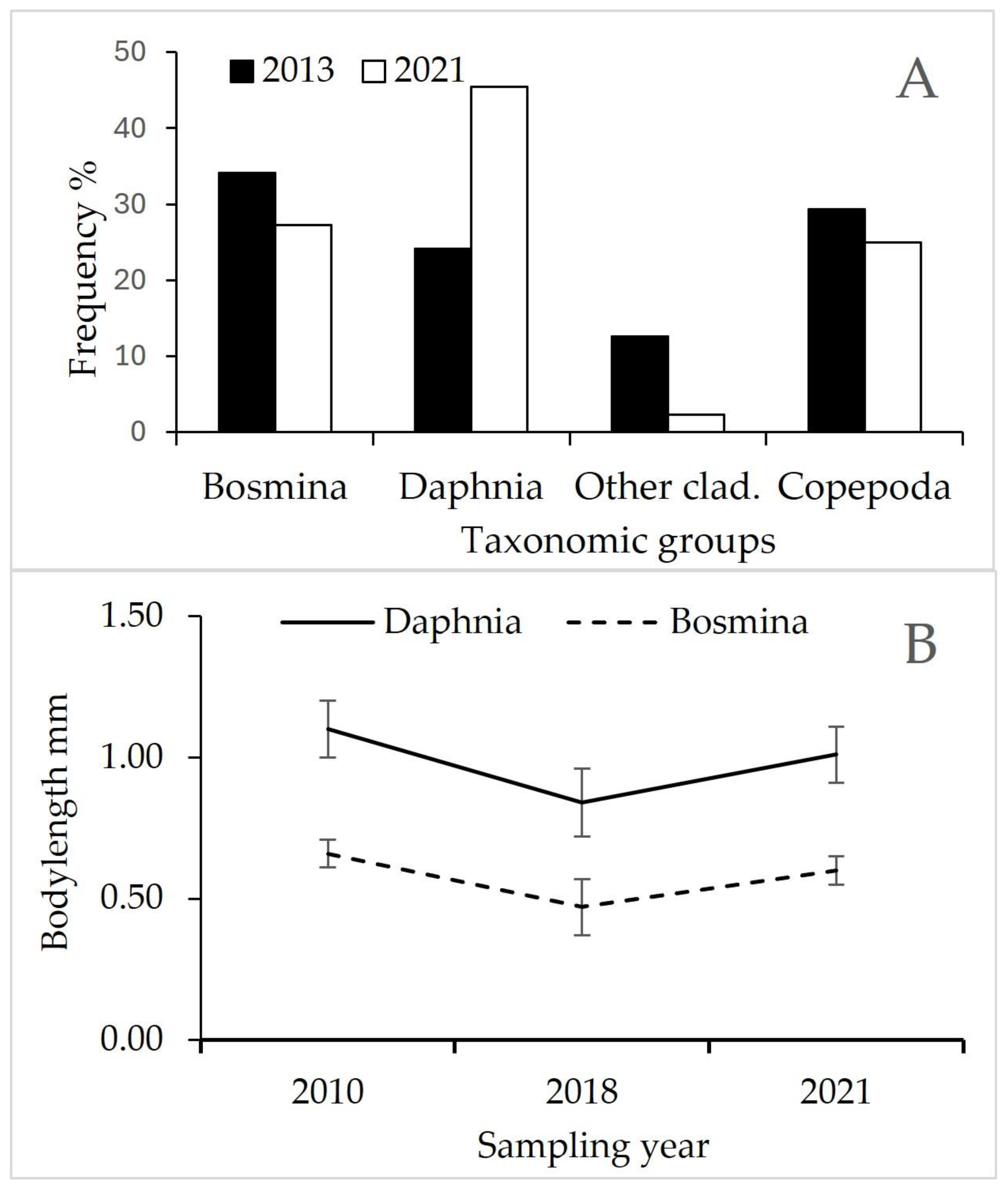
3.4. Zooplankton
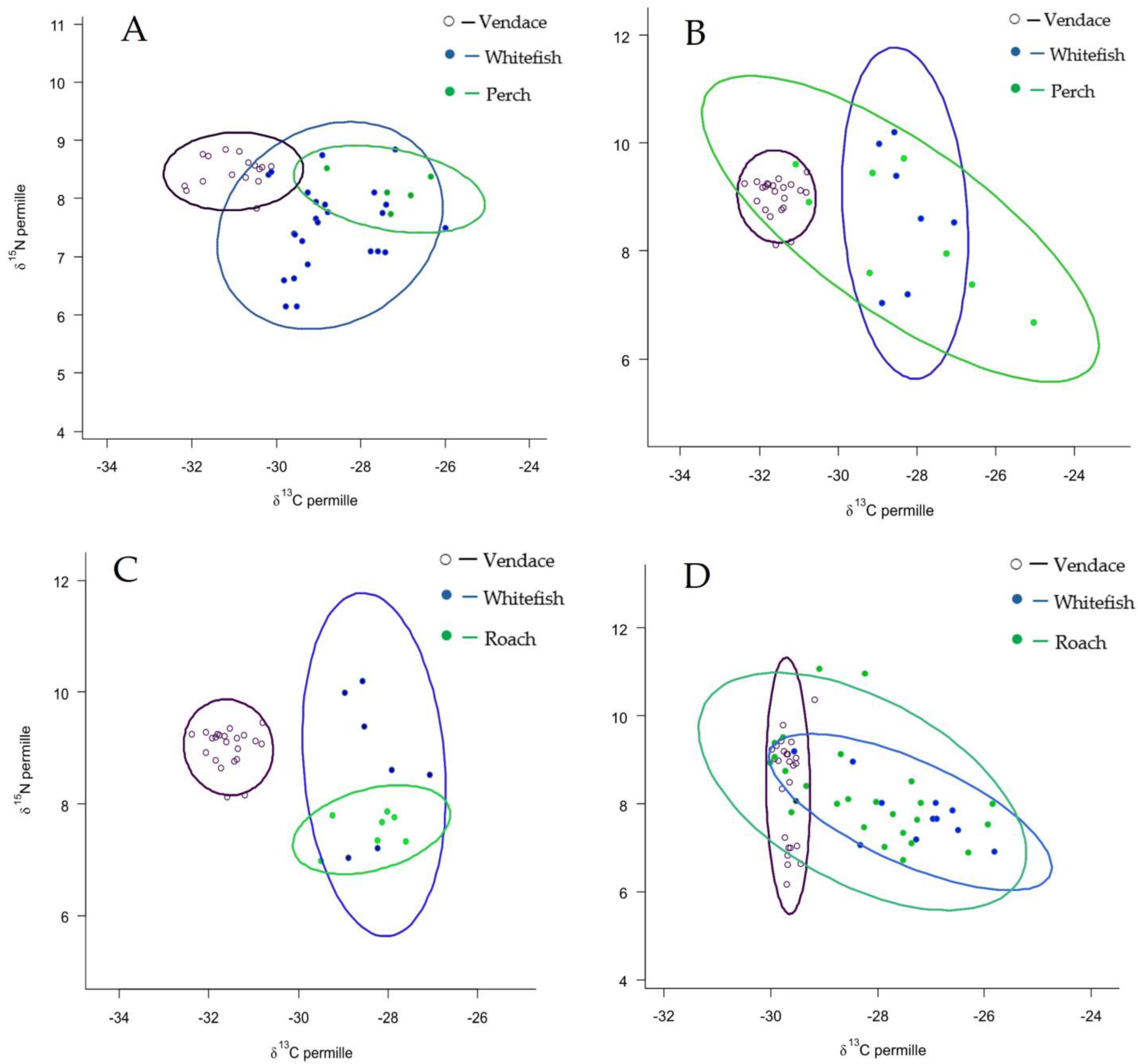
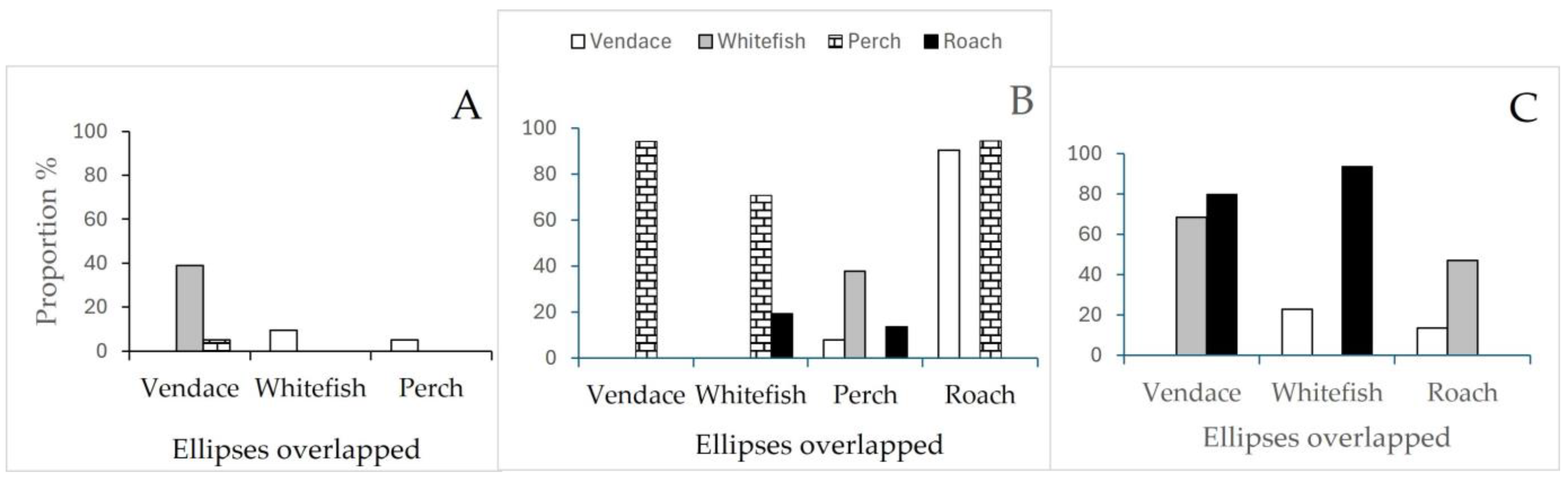
3.5. SIA
4. Discussion
4.1. Abundance and Spatial Distribution of Fish
4.2. Food and Niches
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Heino, J.; Alahuhta, J.; Bini, L.M.; Cai, Y.; Heiskanen, A.-S.; Hellsten, S.; Kortelainen, P.; Kotamäki, N.; Tolonen, K.T.; Vihervaara, P.; et al. Lakes in the era of global change: Moving beyond single-lake thinking in maintaining biodiversity and ecosystem services. Biol. Rev. 2021, 96, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [PubMed]
- Heino, J.; Culp, J.M.; Erkinaro, J.; Goedkoop, W.; Lento, J.; Rühland, K.M.; Smol, J.P. Abruptly and irreversibly changing Arctic freshwaters urgently require standardized monitoring. J. Appl. Ecol. 2020, 57, 1192–1198. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; Gonzalez-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.J.; De Meester, L.; Sondergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar]
- Rolls, R.J.; Hayden, B.; Kahilainen, K.K. Conceptualising the interactive effects of climate change and biological invasions on subarctic freshwater fish. Ecol. Evol. 2017, 7, 4109–4128. [Google Scholar]
- Byström, P.; Karlsson, J.; Nilsson, P.; Van Kooten, T.; Ask, J.; Olofsson, F. Substitution of top predators: Effects of pike invasion in a subarctic lake. Freshw. Biol. 2007, 52, 1271–1280. [Google Scholar]
- Muhlfeld, C.C.; Cline, T.J.; Finstad, A.G.; Hessen, D.O.; Perrin, S.; Thaulow, J.; Whited, D.; Vøllestad, L.A. Climate change vulnerability of Arctic char across Scandinavia. Glob. Change Biol. 2024, 30, e17387. [Google Scholar] [CrossRef]
- Linløkken, A.N. Effects of Lake Productivity on Density and Size Structure of Pelagic Fish Estimated by Means of Echosounding in 17 Lakes in Southeast Norway. Sensors 2021, 21, 3391. [Google Scholar] [CrossRef]
- Berthelsen, A.; Skov, C.; Søndergaard, M.; Larsen, M.; Lauridsen, T. Ecological implications of fish removal: Insights from gut-content analysis of roach (Rutilus rutilus) and European perch (Perca fluviatilis) in a eutrophic shallow lake. J. Fish Biol. 2023, 103, 1321–1334. [Google Scholar] [CrossRef]
- Søndergaard, M.; Lauridsen, T.L.; Johansson, L.S.; Jeppesen, E. Repeated Fish Removal to Restore Lakes: Case Study of Lake Væng, Denmark—Two Biomanipulations during 30 Years of Monitoring. Water 2017, 9, 43. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.; Su, H.; Ma, X.; Wu, Z.; Shen, H.; Yu, J.; Liu, J.; Wu, Y.; Ding, G.; et al. Is Zooplankton Body Size an Indicator of Water Quality in (Sub)tropical Reservoirs in China? Ecosystems 2022, 25, 308–319. [Google Scholar]
- Nagano, M.; Yoshida, T. Size-selective predation accounts for intra- and inter-specific variation of inducible morphological defense of Daphnia. Ecosphere 2020, 11, e03192. [Google Scholar]
- Manca, M.; Vijverberg, J.; Polishchuk, L.; Voronov, D. Daphnia Body Size and Population Dynamics under Predation by Invertebrate and Fish Predators in Lago Maggiore: An Approach Based on Contribution Analysis. J. Limnol.—J. LIMNOL 2008, 67, 15–21. [Google Scholar]
- Jin, Q.; Wang, Y.; Zhang, K.; Li, G.; Chen, Y.; Hong, Y.; Cheng, H.; Deng, D. Morphological and life-history trait plasticity of two Daphnia species induced by fish kairomones. Ecol. Evol. 2024, 14, e11422. [Google Scholar]
- Eloranta, A.P.; Siwertsson, A.; Knudsen, R.; Amundsen, P.A. Dietary plasticity of Arctic charr (Salvelinus alpinus) facilitates coexistence with competitively superior European whitefish (Coregonus lavaretus). Ecol. Freshw. Fish 2011, 20, 558–568. [Google Scholar]
- Hayden, B.; Holopainen, T.; Amundsen, P.-A.; Eloranta, A.P.; Knudsen, R.; Præbel, K.; Kahilainen, K.K. Interactions between invading benthivorous fish and native whitefish in subarctic lakes. Freshw. Biol. 2013, 58, 1234–1250. [Google Scholar] [CrossRef]
- Harrod, C.; Mallela, J.; Kahilainen, K.K. Phenotype-environment correlations in a putative whitefish adaptive radiation. J. Anim. Ecol. 2010, 79, 1057–1068. [Google Scholar]
- Hayden, B.; Harrod, C.; Kahilainen, K.K. Lake morphometry and resource polymorphism determine niche segregation between cool- and cold-water-adapted fish. Ecology 2014, 95, 538–552. [Google Scholar]
- Sandlund, O.T.; Gjelland, K.Ø.; Bøhn, T.; Knudsen, R.; Amundsen, P.-A. Contrasting Population and Life History Responses of a Young Morph-Pair of European Whitefish to the Invasion of a Specialised Coregonid Competitor, Vendace. PLoS ONE 2013, 8, e68156. [Google Scholar]
- Sánchez-Hernández, J.; Hayden, B.; Harrod, C.; Kahilainen, K.K. Population niche breadth and individual trophic specialisation of fish along a climate-productivity gradient. Rev. Fish Biol. Fish. 2021, 31, 1025–1043. [Google Scholar] [CrossRef]
- Eloranta, A.; Kjærstad, G.; Power, M.; Lakka, H.-K.; Arnekleiv, J.; Finstad, A. Impacts of piscicide-induced fish removal on resource use and trophic diversity of lake invertebrates. Sci. Total Environ. 2022, 835, 155364. [Google Scholar]
- Kangosjärvi, H.; Amundsen, P.-A.; Byström, P.; Finstad, A.G.; Power, M.; Sánchez-Hernández, J.; Eloranta, A.P. Environmental drivers of food webs in charr and trout-dominated cold-water lakes. Fish Fish. 2024, 25, 858–875. [Google Scholar]
- Linløkken, A.N.; Sandlund, O.T. Recruitment of sympatric vendace (Coregonus albula) and whitefish (C. lavaretus) is affected by different environmental factors. Ecol. Freshw. Fish 2015, 25, 652–663. [Google Scholar]
- Sandlund, O.T.; Linløkken, A.N.; Gjelland, K.Ø.; Johnsen, S.I.; Rognerud, S.; Museth, J.; Dokk, J.G.; Garmo, Ø.; Walseng, B. Fiskesamfunnet i Osensjøen, Trysil og Åmot kommuner, Hedmark. Status og endringer siden 1970-åra; Norwegian Institute for Nature Research: Trondheim, Norway, 2014; p. 54. [Google Scholar]
- Løvik, J.E.; Eriksen, T.E.; Kile, M.R.; Schneider, S.; Skjelbred, B. Overvåking av Vassdrag i Hedmark i 2011; Norwegian Institute of Water Reserach: Oslo, Norway, 2012; p. 57. [Google Scholar]
- Sandlund, O.T.; Jonsson, B. Life history plasticity: Migration ceased in response to environmental change. Ecol. Freshw. Fish 2014, 25, 225–233. [Google Scholar] [CrossRef]
- Sandlund, O.T. Sik og Lågåsild i Osensjøen, in Fiskeribiologiske Undersøkelser i Osenområdet. Rapport nr. 6; Norwegian Institute of Nature Research: Tronheim, Norway, 1979; p. 57. [Google Scholar]
- Sandlund, O.T.; Jonsson, B.; Næsje, T.F.; Aass, P. Year-class fluctuations in vendace, Coregonus albula (Linnaeus):—Who’s got the upper hand in intraspecific competition? J. Fish Biol. 1991, 38, 873–885. [Google Scholar]
- Sandlund, O.T. The Dynamics of Habitat Use in the Salmonid Genera Coregonus and Salvelinus: Ontogenetic Niche Shifts and Polymorphism. Ph.D. Thesis, University of Trondheim, Trondheim, Norway, 1991; p. 201. [Google Scholar]
- Lindem, T. Successes with conventional in situ determinations of fish target strength. In Symposium on Fisheries Acoustics No. 53; International Council for the Exploration of the Sea (ICES): Bergen, Norway, 1983. [Google Scholar]
- Kongsberg Maritime, A.S. SIMRAD EK15. Reference Manual; Kongsberg Maritime AS: Kongsberg, Norway, 2014; p. 309. [Google Scholar]
- Linløkken, A.N.; Næstad, F.; Langdal, K.; Østbye, K. Comparing Fish Density and Echo Strength Distribution Recorded by Two Generations of Single Beam Echo Sounders. Appl. Sci. 2019, 9, 2041. [Google Scholar] [CrossRef]
- Linløkken, A. Monitoring pelagic whitefish (Coregonus lavaretus) and vendace (Coregonus albula) in a hydroelectric reservoir using hydroacoustics. Regul. Rivers-Res. Manag. 1995, 10, 315–328. [Google Scholar] [CrossRef]
- Appelberg, M.; Berger, H.M.; Hesthagen, T.; Kleiven, E.; Kurkilahti, M.; Raitaniemi, J.; Rask, M. Development and intercalibration of methods in Nordic freshwater fish monitoring. Water Air Soil Pollut. 1995, 85, 401–406. [Google Scholar]
- Lindem, T. Hydro Acoustic Data Acquisition System Instruction Manual ver. 3.9; Lindem Data Acquisition: Oslo, Norway, 1990; p. 25. [Google Scholar]
- Balk, H.; Lindem, T. Sonar4 and Sonar5-Pro. Post Processing Systems Operator Manual Version 6.0.3. Available online: https://folk.universitetetioslo.no/hbalk/SonarX/1_downloads/SonarX-Manual_v608-2023.pdf (accessed on 23 April 2015).
- Elliot, J.M. Some Methods for the Statistical Analysis of Samples of Benthic Invertabrates, 2nd ed.; No. 25; Publications, S., Ed.; Titus Wilson & Son Ltd.: Kendal, UK, 1979. [Google Scholar]
- R-Core-Team. R—A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 10 January 2023).
- Crawley, M.J. The R Book; Wiley: Chichester, UK, 2007. [Google Scholar]
- Schoener, T.W. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Plummer, M. rjags: Bayesian Graphical Models Using MCMC. R Package Version 4-17. [cited 2025; Interface to the JAGS MCMC library]. 2025. Available online: https://CRAN.R-project (accessed on 24 March 2025).
- Jackson, A.L. Introduction to SIBER. R Package 2023. Available online: https://cran.r-project.org/web/packages/SIBER/vignettes/Introduction-to-SIBER.html (accessed on 7 July 2024).
- Salojärvi, K. Recruitment mechanisms of the vendace (Coregonus albula (L.)) in Lake Oulujarvi, northern Finland. Aqua Fenn. 1991, 21, 163–173. [Google Scholar]
- Böhling, P.; Hudd, R.; Lehtonen, H.; Karås, P.; Neuman, E.; Thoresson, G. Variations in year-class strength of different perch (Perca fluviatilis) populations in the Baltic Sea with special reference to temperature and pollution. Can. J. Fish. Aquat. Sci. 1991, 48, 1181–1187. [Google Scholar]
- Karås, P. Recruitment of perch (Perca fluviatilis L.) from Baltic coastal waters. Arch. Für Hydrobiol. 1996, 138, 99–121. [Google Scholar]
- Linløkken, A.N. Temperature Effects on Recruitment and Individual Growth of Two Antagonistic Fish Species, Perch Perca fluviatilis and Roach Rutilus rutilus, from a Climate Change Perspective. Fishes 2023, 8, 295. [Google Scholar] [CrossRef]
- Britton, R.; Cowx, I.; Axford, S.; Frear, P. An overview of recruitment patterns of roach Rutilus rutilus (L.) between 1969 and 2001 in the rivers of England and their influence on population abundance. Ecohydrol. Hydrobiol. 2004, 4, 91–102. [Google Scholar]
- Grenouillet, G.; Hugueny, B.; Carrel, G.; Olivier, J.M.; Pont, D. Large-scale synchrony and inter-annual variability in roach recruitment in the Rhone River: The relative role of climatic factors and density-dependent processes. Freshw. Biol. 2001, 46, 11–26. [Google Scholar]
- Persson, L.; Byström, P.; Wahlström, E. Cannibalism and competition in Eurasian perch: Population dynamics of an ontogenetic omnivore. Ecology 2000, 81, 1058–1071. [Google Scholar]
- Haakana, H.; Huuskonen, H.; Karjalainen, J. Predation of perch on vendace larvae: Diet composition in an oligotrophic lake and digestion time of the larvae. J. Fish Biol. 2007, 70, 1171–1184. [Google Scholar]
- Amundsen, P.-A.; Bøhn, T.; Popova, O.A.; Staldvik, F.J.; Reshetnikov, Y.S.; Kashulin, N.A.; Lukin, A.A. Ontogenetic niche shifts and resource partitioning in a subarctic piscivore fish guild. Hydrobiologia 2003, 497, 109–119. [Google Scholar] [CrossRef]
- Kelly, B.; Amundsen, P.-A.; Power, M. Trophic niche segregation among native whitefish and invasive vendace in a north Norwegian lake system. Ecol. Freshw. Fish 2022, 31, 143–153. [Google Scholar]
- Bøhn, T.; Amundsen, P.-A. Effects of invading vendace (Coregonus albula L.) on species composition and body size in two zooplankton communities of the Pasvik River System, northern Norway. J. Plankton Res. 1998, 20, 243–256. [Google Scholar]
- Sarvala, J.; Helminen, H.; Karjalainen, J.; Marjomäki, T.J.; Forsman, T.; Anttila, L. Long-term decline of whitefish (Coregonus lavaretus) population in the boreal lake Pyhajarvi, southwest Finland, relative to simultaneous abiotic and biotic changes. Int. J. Limnol. 2024, 60, 16. [Google Scholar] [CrossRef]
- Brucet, S.; Pédron, S.; Mehner, T.; Lauridsen, T.L.; Argillier, C.; Winfield, I.J.; Volta, P.; Emmrich, M.; Hesthagen, T.; Holmgren, K.; et al. Fish diversity in European lakes: Geographical factors dominate over anthropogenic pressures. Freshw. Biol. 2013, 58, 1779–1793. [Google Scholar]
- Hayden, B.; Myllykangas, J.-P.; Rolls, R.J.; Kahilainen, K.K. Climate and productivity shape fish and invertebrate community structure in subarctic lakes. Freshw. Biol. 2017, 62, 990–1003. [Google Scholar]
- Jeppesen, E.; Jensen, J.P.; Sondergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar]
- Allen, K.R. The food and migration of perch (Perca fluviatilis) in Windermere. J. Anim. Ecol. 1935, 4, 264–273. [Google Scholar]
- Persson, L. Effects of intraspecific and interspecific competition on dynamics and size structure of a perch Perca fluviatilis and a roach Rutilus rutilus population. Oikos 1983, 41, 126–132. [Google Scholar] [CrossRef]
- Sandlund, O.T.; Næsje, T.F.; Kjellberg, G. The size selection of Bosmina longispina and Daphnia galeata by co-occuring cisco (Coregonus albula), whitefish (C. lavaretus) and smelt (Osmerus eperlanus). Arch. Für Hydrobiol. 1987, 110, 357–363. [Google Scholar]
- Kerfoot, W.C. Combat between predatory copepods and their prey: Cyclops, Epischura, and Bosmina1. Limnol. Oceanogr. 1978, 23, 1089–1102. [Google Scholar]
- Prati, S.; Henriksen, E.H.; Smalås, A.; Knudsen, R.; Klemetsen, A.; Sánchez-Hernández, J.; Amundsen, P.-A. The effect of inter- and intraspecific competition on individual and population niche widths: A four-decade study on two interacting salmonids. Oikos 2021, 130, 1679–1691. [Google Scholar]
- Giller, P.S. Competition and the niche; the effect on niche width. In Community Structure and the Niche; Giller, P.S., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 22–39. [Google Scholar]
- Roughgarden, J. Species packing and the competition function with illustrations from coral reef fish. Theor. Popul. Biol. 1974, 5, 163–186. [Google Scholar]
- Eloranta, A.; Finstad, A.; Sandlund, O.; Knudsen, R.; Kuparinen, A.; Amundsen, P.-A. Species interactions, environmental gradients and body size shape population niche width. J. Anim. Ecol. 2021, 91, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Brabrand, A.; Faafeng, B.A.; Nilssen, J.P.M. Relative importance of phosphorus supply to phytoplankton production—Fish excretion versus external loading. Can. J. Fish. Aquat. Sci. 1990, 47, 364–372. [Google Scholar] [CrossRef]
- Persson, A.; Hamrin, S.F. Effects of cyprinids on the release of phosphorus from lake sediment. Int. Ver. Theor. Und Angew. Limnol. Verhandlungen 1994, 25, 2124–2127. [Google Scholar]
- Tarvainen, M.; Sarvala, J.; Helminen, H. The role of phosphorus release by roach Rutilus rutilus (L.) in the water quality changes of a biomanipulated lake. Freshw. Biol. 2002, 47, 2325–2336. [Google Scholar] [CrossRef]
- Eloranta, A.; Johnsen, S.; Bærum, K.; Sandlund, O.; Finstad, A.; Rognerud, S.; Museth, J. Introduced European smelt (Osmerus eperlanus) affects food web and fish community in a large Norwegian lake. Biol. Invasions 2019, 21, 85–98. [Google Scholar] [CrossRef]
- Jensen, H.; Kahilainen, K.; Amundsen, P.-A.; Gjelland, K.; Tuomaala, A.; Malinen, T.; Bøhn, T. Predation by brown trout (Salmo trutta) along a diversifying prey community gradient. Can. J. Fish. Aquat. Sci. 2008, 65, 1831–1841. [Google Scholar] [CrossRef]


| Year | Species | n | δ13C (‰) | δ15N (‰) | TA | SEAc | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | S.D. | Mean | Range | S.D. | |||||
| 2010 | Vendace | 16 | −31.0 | −32.2–−30.1 | 0.7 | 8.5 | 10.4–10.6 | 0.3 | 1.39 | 0.62 |
| Whitefish | 25 | −28.7 | −30.2–−26.0 | 1.1 | 7.5 | 6.1–8.8 | 0.7 | 7.23 | 2.56 | |
| Roach | 1 | −27.8 | - | - | 7.8 | - | - | - | - | |
| Perch | 5 | −27.3 | −28.8–−26.4 | 0.9 | 8.2 | 7.7–8.5 | 0.3 | 0.87 | 1.12 | |
| Brown trout | 2 | −27.8 | −28.0–−27.7 | 0.2 | 8.7 | 8.1–9.4 | 0.9 | - | - | |
| Grayling | 1 | −25.1 | - | - | 7.8 | - | - | - | - | |
| Burbot | 2 | −28.4 | −28.4–−28.4 | 0.0 | 10.5 | 10.4–10.6 | 0.1 | - | - | |
| Pike | 3 | −28.4 | −28.5–−28.3 | 0.1 | 9.1 | 8.6–9.5 | 0.5 | - | - | |
| 2013 | Vendace | 22 | −31.6 | −32.4–−30.8 | 0.4 | 9.0 | 8.1–9.5 | 0.3 | 1.28 | 0.47 |
| Whitefish | 7 | −28.3 | −29.0–−27.1 | 0.7 | 8.7 | 7.0–10.2 | 1.3 | 3.54 | 3.05 | |
| Roach | 7 | −28.4 | −29.5–−27.6 | 0.7 | 7.5 | 7.0–7.9 | 0.3 | 1.09 | 0.82 | |
| Perch | 8 | −28.4 | −31.1–−25.0 | 2.1 | 8.4 | 6.7–9.7 | 1.2 | 7.96 | 5.72 | |
| Brown trout | 12 | −28.0 | −31.2–−26.5 | 1.3 | 8.9 | 7.7–10.2 | 0.8 | - | - | |
| Grayling | 12 | −26.0 | −28.1–25.2 | 0.7 | 7.8 | 6.9–8.7 | 0.6 | - | - | |
| Burbot | 12 | −28.0 | −34.3–−25.6 | 1.3 | 8.9 | 5.7–10.7 | 0.8 | - | - | |
| Pike | 4 | −29.2 | −31.2–−27.6 | 1.6 | 8.3 | 7.4–9.2 | 0.9 | - | - | |
| 2021 | Vendace | 25 | −29.7 | −32.4–−25.9 | 0.2 | 8.4 | 6.2–10.4 | 1.2 | 1.91 | 0.65 |
| Whitefish | 11 | −27.4 | −29.6–−25.8 | 1.1 | 7.8 | 6.9–10.2 | 0.7 | 3.84 | 1.94 | |
| Roach | 26 | −28.3 | −30.0–25.9 | 1.3 | 8.3 | 6.7–11.1 | 1.1 | 11.22 | 3.85 | |
| Year | Item Group | n | δ13C | δ15N |
|---|---|---|---|---|
| 2010 | ZooB bulk | 1 | −25.93 | 5.32 |
| ZooP bulk | 1 | −29.89 | 3.71 | |
| 2013 | Bosmina/Daphnia mixture | 1 | −33.01 | 1.68 |
| Bosmina | 1 | −32.59 | 2.09 | |
| D. cristata | 1 | −31.14 | −0.87 | |
| Daphnia sp. | 1 | −32.51 | 2.79 | |
| ZooP bulk. | 1 | −32.57 | 1.82 | |
| 2021 | Annelida | 1 | −26.36 | 7.23 |
| Coleoptera | 1 | −26.61 | 15.13 | |
| Chironomidae | 1 | −27.62 | 11.52 | |
| Ephemer. | 1 | −27.26 | 12.64 | |
| Trichopt | 1 | −26.93 | 12.32 | |
| Daphnia | 1 | −28.29 | 4.77 | |
| Bosmina | 1 | −26.52 | 3.11 |
| Coefficients | δ13C | δ15N | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | S.E. | t | p | Estimate | S.E. | t | p | |
| Intercept (Pch 2010) | −29.03 | 0.63 | −46.31 | <0.001 | 5.96 | 4.35 | 1.37 | >0.05 |
| 2013 | −0.66 | 0.52 | −1.28 | >0.05 | −0.22 | 4.46 | −0.05 | >0.05 |
| 2021 | 2.06 | 0.38 | 5.46 | <0.001 | 5.72 | 2.37 | 2.41 | <0.05 |
| Roach | −2.40 | 0.56 | −4.28 | <0.001 | −3.88 | 5.00 | −0.78 | >0.05 |
| Vendace | −3.51 | 0.45 | −7.75 | <0.001 | 2.06 | 4.99 | 0.41 | >0.05 |
| Whitefish | −1.85 | 0.45 | −4.13 | <0.001 | −0.62 | 4.40 | −0.14 | >0.05 |
| Fish length | 0.01 | 0.00 | 3.50 | <0.001 | 0.01 | 0.02 | 0.51 | >0.05 |
| 2013:Roach | 2.98 | 0.74 | 4.03 | <0.001 | 4.65 | 5.20 | 0.89 | >0.05 |
| 2021:Roach | NA | NA | NA | NA | NA | NA | NA | NA |
| 2013:Vendace | 0.30 | 0.58 | 0.52 | >0.05 | 0.65 | 5.55 | 0.12 | >0.05 |
| 2021:Vendace | −0.07 | 0.42 | −0.16 | >0.05 | −9.02 | 3.44 | −2.62 | <0.01 |
| 2013:Whitefish | 1.19 | 0.63 | 1.87 | >0.05 | 0.00 | 4.73 | 0.00 | >0.05 |
| 2021:Whitefish | NA | NA | NA | NA | NA | NA | NA | NA |
| 2013:Fish length | 0.01 | 0.02 | 0.31 | >0.05 | ||||
| 2021:Fish length | −0.02 | 0.01 | −2.04 | <0.05 | ||||
| Roach:Fish length | 0.01 | 0.02 | 0.70 | >0.05 | ||||
| Vendace:Fish Length | −0.01 | 0.02 | −0.33 | >0.05 | ||||
| Whitefish:Fish length | 0.00 | 0.02 | −0.11 | >0.05 | ||||
| 2013:Roach:Fish length | −0.02 | 0.02 | −0.83 | >0.05 | ||||
| 2021:Roach:Fish length | NA | NA | NA | NA | ||||
| 2013:Vendace:Fish length | 0.00 | 0.02 | −0.20 | >0.05 | ||||
| 2021:Vendace:Fish length | 0.046 | 0.02 | 3.08 | <0.001 | ||||
| 2013:Whitefish:Fish length | 0.00 | 0.02 | −0.03 | >0.05 | ||||
| 2021:Whitefish:Fish length | NA | NA | NA | NA | ||||
| Model statistics | F = 35.2, d.f. = 10,128, p < 0.001, R2 = 0.73 | F = 8.9, d.f. = 19,119, p < 0.001, R2 = 0.59 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linløkken, A.N.; Grimsgaard, A.B.; Eloranta, A.P. Long-Term Changes in Fish Community Composition of a Coregonid Dominated Oligotrophic Lake. Hydrobiology 2025, 4, 10. https://doi.org/10.3390/hydrobiology4020010
Linløkken AN, Grimsgaard AB, Eloranta AP. Long-Term Changes in Fish Community Composition of a Coregonid Dominated Oligotrophic Lake. Hydrobiology. 2025; 4(2):10. https://doi.org/10.3390/hydrobiology4020010
Chicago/Turabian StyleLinløkken, Arne N., Aslak B. Grimsgaard, and Antti P. Eloranta. 2025. "Long-Term Changes in Fish Community Composition of a Coregonid Dominated Oligotrophic Lake" Hydrobiology 4, no. 2: 10. https://doi.org/10.3390/hydrobiology4020010
APA StyleLinløkken, A. N., Grimsgaard, A. B., & Eloranta, A. P. (2025). Long-Term Changes in Fish Community Composition of a Coregonid Dominated Oligotrophic Lake. Hydrobiology, 4(2), 10. https://doi.org/10.3390/hydrobiology4020010






