Growth and Diet of Northern Pike (Esox lucius) in Boreal Lakes: Implications for Ecosystem Management
Abstract
1. Introduction
- (1)
- The growth rates (von Bertalanffy growth parameters) of northern pike will differ between Steepbank and Wappau due to differences in environmental conditions (e.g., lake size, depth, aquatic vegetation, and prey availability).
- (2)
- Northern pike in Wappau will exhibit a higher relative body condition compared to those in Steepbank due to a more diverse and abundant prey base, based on a prey fish catch.
- (3)
- Prey selection by northern pike will differ between Steepbank and Wappau, with northern pike in Steepbank relying more on conspecific predation due to limited prey diversity and a potentially lower prey fish catch.
2. Materials and Methods
2.1. Study Lakes
2.2. Sampling and Study Data
2.3. Age and Growth Analysis
2.4. Condition Analysis
2.5. Diet and Stomach Contents Analysis
3. Results
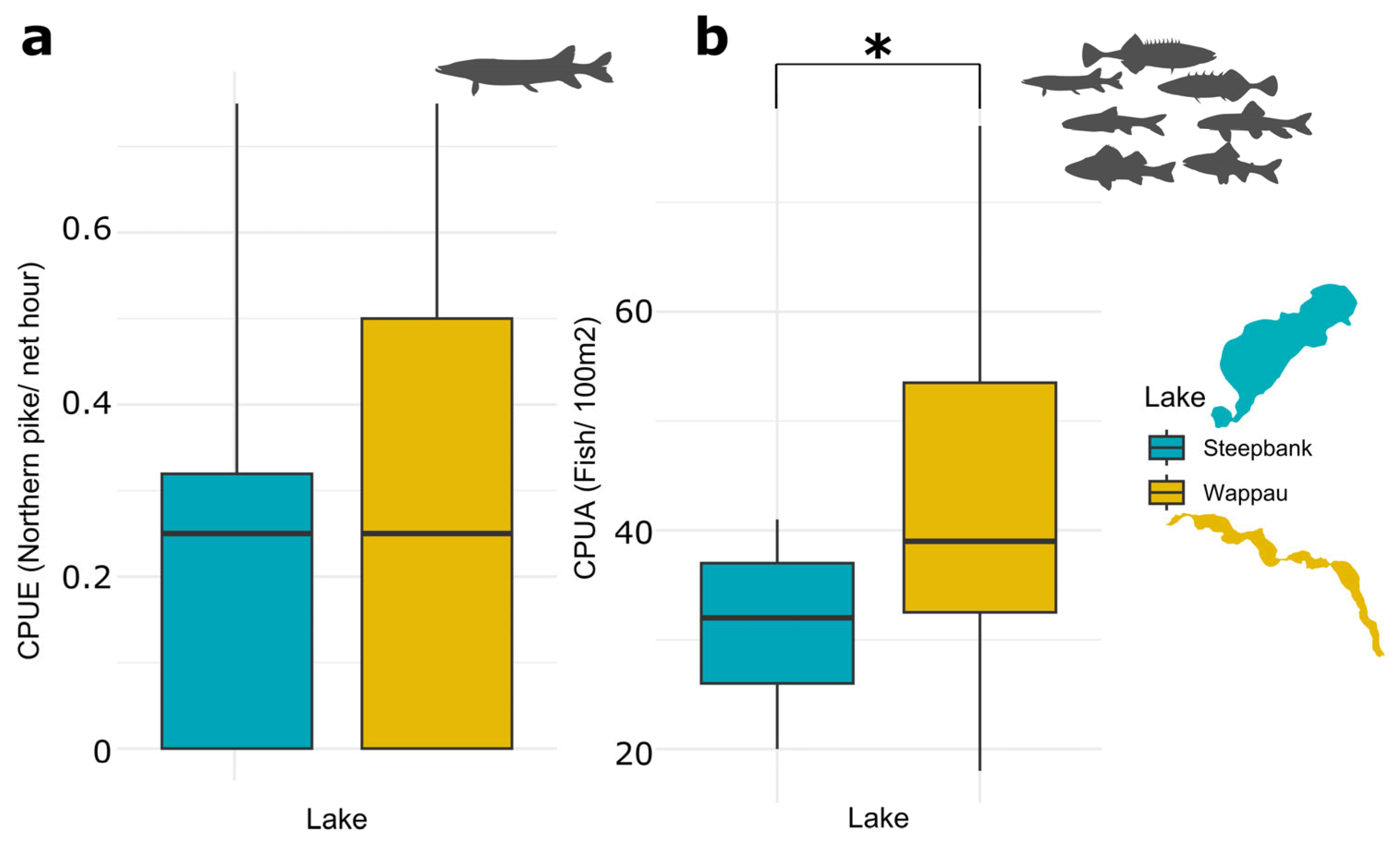
3.1. Northern Pike CPUE and Prey Fish CPUA
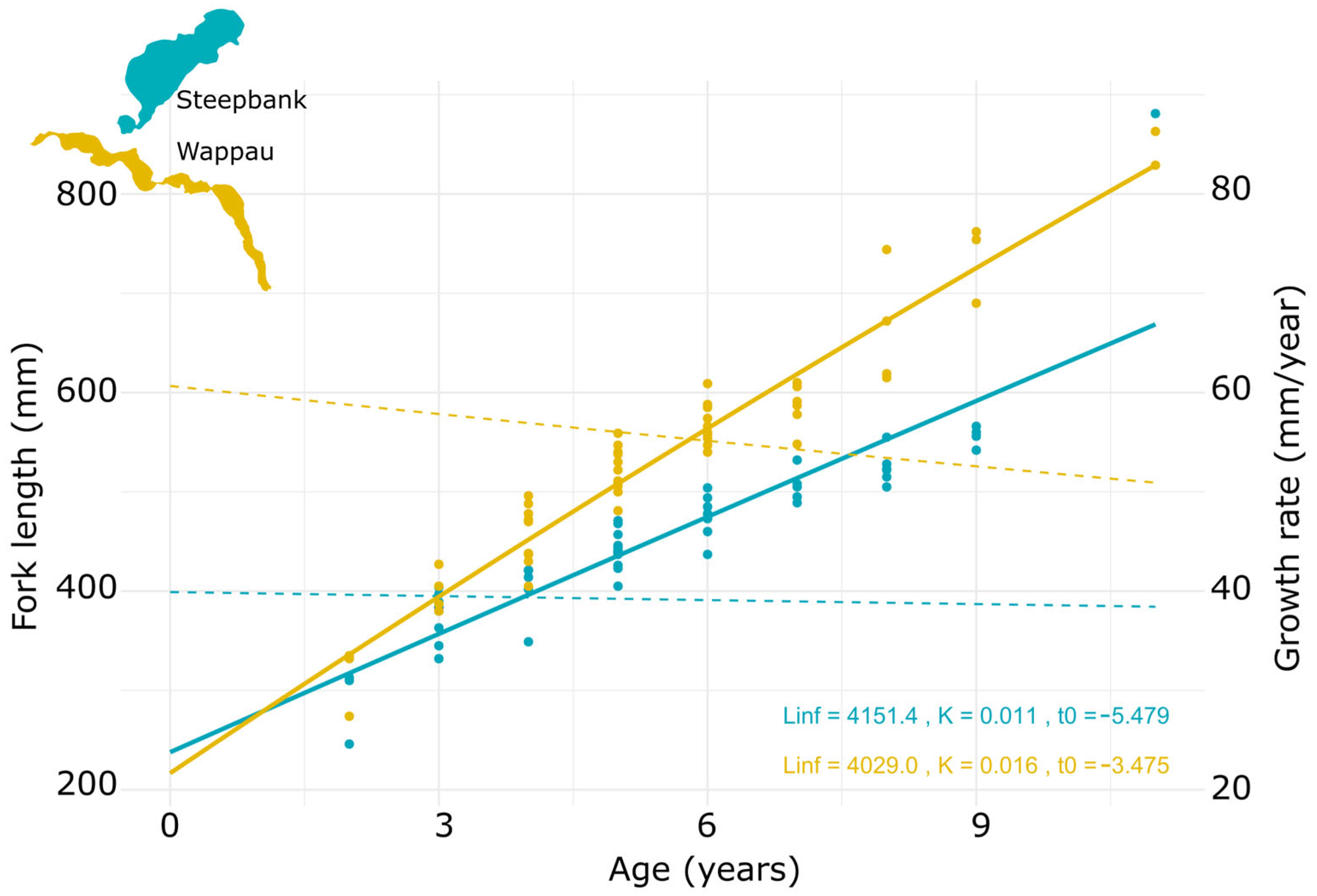
3.2. Age and Growth
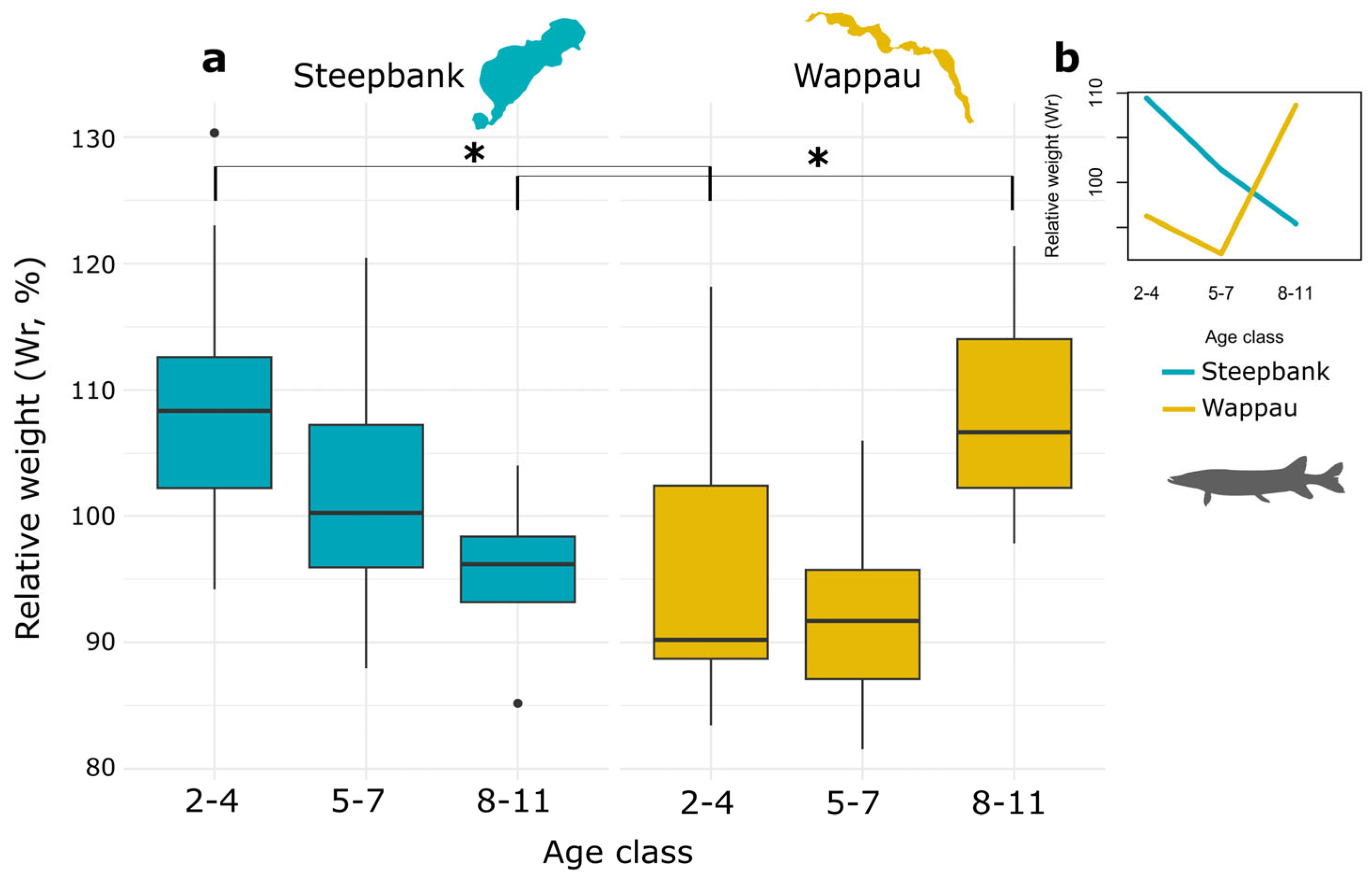
3.3. Condition
3.4. Diet and Stomach Contents
4. Discussion
4.1. Growth Rates, Condition, and Prey Choice
4.2. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desforges, J.E.; Clarke, J.; Harmsen, E.J.; Jardine, A.M.; Robichaud, J.A.; Serré, S.; Chakrabarty, P.; Bennett, J.R.; Hanna, D.E.L.; Smol, J.P.; et al. The alarming state of freshwater biodiversity in Canada. Can. J. Fish. Aquat. Sci. 2022, 79, 352–365. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- WWF. Living Planet Report 2020—Bending the Curve of Biodiversity Loss; Almond, R.E.A., Grooten, M., Petersen, T., Eds.; WWF: Gland, Switzerland, 2020. [Google Scholar]
- Dudgeon, D.; Strayer, D.L. Bending the curve of global freshwater biodiversity loss: What are the prospects? Biol. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Theis, S.; Castellanos-Acuña, D.; Hamann, A.; Poesch, M.S. Small-bodied fish species from the western United States will be under severe water stress by 2040. Conserv. Sci. Pract. 2023, 5, e12856. [Google Scholar] [CrossRef]
- Britton, J.R.; Lynch, A.J.; Bardal, H.; Bradbeer, S.J.; Coetzee, J.A.; Coughlan, N.E.; Dalu, T.; Tricarico, E.; Gallardo, B.; Lintermans, M. Preventing and controlling nonnative species invasions to bend the curve of global freshwater biodiversity loss. Environ. Rev. 2023, 31, 310–326. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kahru, A.; Nõges, P.; Tuvikene, A.; Vasemägi, A.; Mander, Ü.; Nõges, T. Environmental feedbacks in temperate aquatic ecosystems under global change: Why do we need to consider chemical stressors? Reg. Environ. Chang. 2017, 17, 2079–2096. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management: Fish Conservation in Freshwater and Marine Realms. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 838–857. [Google Scholar] [CrossRef]
- Pardini, R.; Nichols, E.; Püttker, T. Biodiversity Response to Habitat Loss and Fragmentation. In Encyclopedia of the Anthropocene; Elsevier: Amsterdam, The Netherlands, 2018; pp. 229–239. ISBN 978-0-12-813576-1. [Google Scholar]
- Abell, R.; Vigerstol, K.; Higgins, J.; Kang, S.; Karres, N.; Lehner, B.; Sridhar, A.; Chapin, E. Freshwater biodiversity conservation through source water protection: Quantifying the potential and addressing the challenges. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1022–1038. [Google Scholar] [CrossRef]
- Droste, N.; Alkan Olsson, J.; Hanson, H.; Knaggård, Å.; Lima, G.; Lundmark, L.; Thoni, T.; Zelli, F. A global overview of biodiversity offsetting governance. J. Environ. Manag. 2022, 316, 115231. [Google Scholar] [CrossRef] [PubMed]
- Latham, R.E.; Craig, L.S.; Abs, D.J.V. Land Stewardship and Freshwater Outcomes: An Overview of Practice and Results. Nat. Areas J. 2019, 39, 6. [Google Scholar] [CrossRef]
- Cooke, S.J.; Harrison, I.; Thieme, M.L.; Landsman, S.J.; Birnie-Gauvin, K.; Raghavan, R.; Creed, I.F.; Pritchard, G.; Ricciardi, A.; Hanna, D.E. Is it a new day for freshwater biodiversity? Reflections on outcomes of the Kunming-Montreal Global Biodiversity Framework. PLoS Sustain. Transform. 2023, 2, e0000065. [Google Scholar] [CrossRef]
- French McCay, D.; Rowe, J. Habitat restoration as mitigation for lost production at multiple trophic levels. Mar. Ecol. Prog. Ser. 2003, 264, 233–247. [Google Scholar] [CrossRef]
- Theis, S.; Ruppert, J.L.W.; Roberts, K.N.; Minns, C.K.; Koops, M.; Poesch, M.S. Compliance with and ecosystem function of biodiversity offsets in North American and European freshwaters. Conserv. Biol. 2019, 34, 41–53. [Google Scholar] [CrossRef]
- Allen, M.S.; Tugend, K.I.; Mann, M.J. Largemouth Bass Abundance and Angler Catch Rates following a Habitat Enhancement Project at Lake Kissimmee, Florida. N. Am. J. Fish. Manag. 2003, 23, 845–855. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Riepe, C.; Theis, S.; Pagel, T.; Fujitani, M. Dysfunctional information feedbacks cause the emergence of management panaceas in social-ecological systems: The case of fish stocking in inland recreational fisheries. J. Outdoor Recreat. Tour. 2022, 38, 100475. [Google Scholar] [CrossRef]
- Woodward, R.T.; Griffin, W.L. Size and Bag Limits in Recreational Fisheries: Theoretical and Empirical Analysis. Mar. Resour. Econ. 2003, 18, 239–262. [Google Scholar] [CrossRef]
- Evans, M.S. The large lake ecosystems of northern Canada. Aquat. Ecosyst. Health Manag. 2000, 3, 65–79. [Google Scholar] [CrossRef]
- Margenau, T.L.; AveLallemant, S.P.; Giehtbrock, D.; Schram, S.T. Ecology and management of northern pike in Wisconsin. Hydrobiologia 2008, 601, 111–123. [Google Scholar] [CrossRef]
- Paukert, C.P.; Klammer, J.A.; Pierce, R.B.; Simonson, T.D. An Overview of Northern Pike Regulations in North America. Fisheries 2001, 26, 6–13. [Google Scholar] [CrossRef]
- Casselman, J.M.; Lewis, C.A. Habitat requirements of northern pike ( Essox lucius ). Can. J. Fish. Aquat. Sci. 1996, 53, 161–174. [Google Scholar] [CrossRef]
- Grimm, M.P. Northern pike (Esox lucius L.) and aquatic vegetation, tools in the management of fisheries and water quality in shallow waters. Hydrobiol. Bull. 1989, 23, 59–65. [Google Scholar] [CrossRef]
- Craig, J.F. A short review of pike ecology. Hydrobiologia 2008, 601, 5–16. [Google Scholar] [CrossRef]
- Diana, J.S. Simulation of Mechanisms Causing Stunting in Northern Pike Populations. Trans. Am. Fish. Soc. 1987, 116, 612–617. [Google Scholar] [CrossRef]
- Skov, C.; Koed, A. Habitat use of 0+ year pike in experimental ponds in relation to cannibalism, zooplankton, water transparency and habitat complexity. J. Fish Biol. 2004, 64, 448–459. [Google Scholar] [CrossRef]
- Schindler, D.W. A Dim Future for Boreal Waters and Landscapes. BioScience 1998, 48, 157–164. [Google Scholar] [CrossRef]
- Gennaretti, F.; Arseneault, D.; Bégin, Y. Millennial stocks and fluxes of large woody debris in lakes of the North American taiga. J. Ecol. 2014, 102, 367–380. [Google Scholar] [CrossRef]
- Arzel, C.; Nummi, P.; Arvola, L.; Pöysä, H.; Davranche, A.; Rask, M.; Olin, M.; Holopainen, S.; Viitala, R.; Einola, E.; et al. Invertebrates are declining in boreal aquatic habitat: The effect of brownification? Sci. Total Environ. 2020, 724, 138199. [Google Scholar] [CrossRef] [PubMed]
- France, R.; Culbert, H.; Freeborough, C.; Peters, R. Leaching and early mass loss of boreal leaves and wood in oligotrophic water. Hydrobiologia 1997, 345, 209–214. [Google Scholar] [CrossRef]
- Theis, S.; Ruppert, J.L.W.; Poesch, M.S. Coarse woody habitat use by local fish species and structural integrity of enhancements over time in a shallow northern boreal lake assessed in a Bayesian modelling approach. Ecol. Solut. Evid. 2023, 4, e12200. [Google Scholar] [CrossRef]
- Weber, M.G.; Flannigan, M.D. Canadian boreal forest ecosystem structure and function in a changing climate: Impact on fire regimes. Environ. Rev. 1997, 5, 145–166. [Google Scholar] [CrossRef]
- Pickell, P.D.; Andison, D.W.; Coops, N.C. Characterizations of anthropogenic disturbance patterns in the mixedwood boreal forest of Alberta, Canada. For. Ecol. Manag. 2013, 304, 243–253. [Google Scholar] [CrossRef]
- Timoney, K.P.; Lee, P. Does the Alberta Tar Sands Industry Pollute? The Scientific Evidence. Open Conserv. Biol. J. 2009, 3, 65–81. [Google Scholar] [CrossRef]
- Ruppert, J.L.W.; Hogg, J.; Poesch, M.S. Community assembly and the sustainability of habitat offsetting targets in the first compensation lake in the oil sands region in Alberta, Canada. Biol. Conserv. 2018, 219, 138–146. [Google Scholar] [CrossRef]
- Blanchette, M.L.; Lund, M.A. Pit lakes are a global legacy of mining: An integrated approach to achieving sustainable ecosystems and value for communities. Curr. Opin. Environ. Sustain. 2016, 23, 28–34. [Google Scholar] [CrossRef]
- Johnson, E.A.; Miyanishi, K. Creating New Landscapes and Ecosystems. Ann. N. Y. Acad. Sci. 2008, 1134, 120–145. [Google Scholar] [CrossRef]
- Bouwes, N.; Bennett, S.; Wheaton, J. Adapting Adaptive Management for Testing the Effectiveness of Stream Restoration: An Intensively Monitored Watershed Example. Fisheries 2016, 41, 84–91. [Google Scholar] [CrossRef]
- Gulati, R.D.; Dionisio Pires, L.M.; Van Donk, E. Lake restoration studies: Failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 2008, 38, 233–247. [Google Scholar] [CrossRef]
- Hale, R.; Swearer, S.E. When good animals love bad restored habitats: How maladaptive habitat selection can constrain restoration. J. Appl. Ecol. 2016, 54, 1478–1486. [Google Scholar] [CrossRef]
- Koops, M.A.; Dey, C.J.; Fung, S.; Theis, S.; Tunney, T.D.; van der Lee, A.S. Estimation des Effets et des Mesures de Compensation de la Mort du Poisson; Secrétariat Canadien de Consultation Scientifique (SCCS): Montreal, QC, Canada, 2022. [Google Scholar]
- Theis, S.; Koops, M.A.; Poesch, M.S. A Meta-analysis on the Effectiveness of Offsetting Strategies to Address Harm to Freshwater Fishes. Environ. Manag. 2022, 70, 793–807. [Google Scholar] [CrossRef]
- Pereira, D.L.; Hansen, M.J. A Perspective on Challenges to Recreational Fisheries Management: Summary of the Symposium on Active Management of Recreational Fisheries. N. Am. J. Fish. Manag. 2003, 23, 1276–1282. [Google Scholar] [CrossRef]
- Sass, G.G.; Shaw, S.L. Catch-and-Release Influences on Inland Recreational Fisheries. Rev. Fish. Sci. Aquac. 2020, 28, 211–227. [Google Scholar] [CrossRef]
- Miller, L.L.; Rasmussen, J.B.; Palace, V.P.; Sterling, G.; Hontela, A. Selenium Bioaccumulation in Stocked Fish as an Indicator of Fishery Potential in Pit Lakes on Reclaimed Coal Mines in Alberta, Canada. Environ. Manag. 2013, 52, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Jurasinski, G. simba: A Collection of Functions for Similarity Analysis of Vegetation Data. 2012. Available online: http://www.r-project.org/ (accessed on 23 July 2023).
- Kalinowska, K.; Ulikowski, D.; Traczuk, P.; Kozłowski, M.; Kapusta, A. Fish species richness in polish lakes. Diversity 2023, 15, 164. [Google Scholar] [CrossRef]
- Marshall, E.M.; Larocque, S.M.; Reddick, D.T.; Midwood, J.D.; Doka, S.E. Temperature, Dissolved Oxygen, Fish, Vegetation, and Substrate Surveys in Lake Ontario Coastal Wetlands; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2021; Available online: https://publications.gc.ca/collections/collection_2021/mpo-dfo/Fs97-6-3385-eng.pdf (accessed on 26 July 2023).
- Murphy, S.C.; Collins, N.C.; Doka, S.E. Determinants of temperature in small coastal embayments of Lake Ontario. J. Gt. Lakes Res. 2012, 38, 600–609. [Google Scholar] [CrossRef]
- Quirino, B.A.; Søndergaard, M.; Lauridsen, T.L.; Johansson, L.S.; Fugi, R.; Thomaz, S.M.; Lansac-Tôha, F.M.; Jeppesen, E. Associations between submerged macrophytes and fish communities at two spatial scales in 88 temperate shallow lakes. Freshw. Biol. 2023, 68, 1211–1223. [Google Scholar] [CrossRef]
- Daněk, T.; Bouše, E.; Musil, J. Wind of change: Selective summer fish kill in an oxbow lake associated with windy weather. Environ. Biol. Fishes 2023, 106, 1815–1823. [Google Scholar] [CrossRef]
- Nodo, P.; Childs, A.-R.; Pattrick, P.; Lemley, D.; James, N. Response of demersal fishes to low dissolved oxygen events in two eutrophic estuaries. Estuar. Coast. Shelf Sci. 2023, 293, 108514. [Google Scholar] [CrossRef]
- Zanghi, C.; Ioannou, C.C. The impact of increasing turbidity on the predator–prey interactions of freshwater fishes. Freshw. Biol. 2024. [Google Scholar] [CrossRef]
- Klein, Z.; McCormick, J. Evaluation of the influence of correcting for gillnet selectivity on the estimation of population parameters. PLoS ONE 2023, 18, e0287434. [Google Scholar] [CrossRef]
- Altuntaş, C.; Tokaç, A.; Herrmann, B.; Mısır, D.S.; Dağtekin, M.; Cerbule, K. Effect of mesh size in monofilament and multifilament gillnets on catch efficiency in the Black Sea whiting (Merlangius merlangus) fishery. Estuar. Coast. Shelf Sci. 2024, 299, 108695. [Google Scholar] [CrossRef]
- Bonar, S.A.; Hubert, W.A. Standard Sampling of Inland Fish: Benefits, Challenges, and a Call for Action. Fisheries 2002, 27, 10–16. [Google Scholar] [CrossRef]
- Frost, W.E. The food of pike, Esox lucius L., in Windermere. J. Anim. Ecol. 1954, 23, 339–360. [Google Scholar] [CrossRef]
- Ostertagová, E.; Ostertag, O.; Kováč, J. Methodology and Application of the Kruskal-Wallis Test. Appl. Mech. Mater. 2014, 611, 115–120. [Google Scholar] [CrossRef]
- Oele, D.L.; Lawson, Z.J.; McIntyre, P.B. Precision and Bias in Aging Northern Pike: Comparisons among Four Calcified Structures. N. Am. J. Fish. Manag. 2015, 35, 1177–1184. [Google Scholar] [CrossRef]
- Ogle, D.H. Introductory Fisheries Analyses With R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018; ISBN 1-315-37198-7. [Google Scholar]
- Ogle, D. FSA: Simple Fisheries Stock Assessment Methods. 2023. Available online: https://fishr-core-team.github.io/FSA/ (accessed on 3 October 2023).
- Wilcoxon, F.; Katti, S.; Wilcox, R.A. Critical values and probability levels for the Wilcoxon rank sum test and the Wilcoxon signed rank test. Sel. Tables Math. Stat. 1970, 1, 171–259. [Google Scholar]
- Blackwell, B.G.; Brown, M.L.; Willis, D.W. Relative Weight (Wr) Status and Current Use in Fisheries Assessment and Management. Rev. Fish. Sci. 2000, 8, 1–44. [Google Scholar] [CrossRef]
- Buckland, A.; Baker, R.; Loneragan, N.; Sheaves, M. Standardising fish stomach content analysis: The importance of prey condition. Fish. Res. 2017, 196, 126–140. [Google Scholar] [CrossRef]
- Shafer, G. Conditional probability. Int. Stat. Rev. Int. Stat. 1985, 53, 261–275. [Google Scholar] [CrossRef]
- Venturelli, P.A.; Tonn, W.M. Diet and Growth of Northern Pike in the Absence of Prey Fishes: Initial Consequences for Persisting in Disturbance-Prone Lakes. Trans. Am. Fish. Soc. 2006, 135, 1512–1522. [Google Scholar] [CrossRef]
- Cvetkovic, M.; Wei, A.; Chow-Fraser, P. Relative importance of macrophyte community versus water quality variables for predicting fish assemblages in coastal wetlands of the Laurentian Great Lakes. J. Gt. Lakes Res. 2010, 36, 64–73. [Google Scholar] [CrossRef]
- Randall, R.G.; Minns, C.K.; Cairns, V.W.; Moore, J.E. The relationship between an index of fish production and submerged macrophytes and other habitat features at three littoral areas in the Great Lakes. Can. J. Fish. Aquat. Sci. 1996, 53, 35–44. [Google Scholar] [CrossRef]
- Sass, G.G.; Shaw, S.L.; Fenstermacher, C.C.; Porreca, A.P.; Parkos III, J.J. Structural habitat in lakes and reservoirs: Physical and biological considerations for implementation. N. Am. J. Fish. Manag. 2023, 43, 290–303. [Google Scholar] [CrossRef]
- Sammons, S.M.; Scalet, C.G.; Neumann, R.M. Seasonal and Size-Related Changes in the Diet of Northern Pike from a Shallow Prairie Lake. J. Freshw. Ecol. 1994, 9, 321–329. [Google Scholar] [CrossRef]
- Findlay, D.L.; Vanni, M.J.; Paterson, M.; Mills, K.H.; Kasian, S.E.M.; Findlay, W.J.; Salki, A.G. Dynamics of a Boreal Lake Ecosystem during a Long-Term Manipulation of Top Predators. Ecosystems 2005, 8, 603–618. [Google Scholar] [CrossRef]
- Soupir, C.A.; Brown, M.L.; Kallemeyn, L.W. Trophic ecology of largemouth bass and northern pike in allopatric and sympatric assemblages in northern boreal lakes. Can. J. Zool. 2000, 78, 1759–1766. [Google Scholar] [CrossRef]
- Pierce, R.B.; Carlson, A.J.; Carlson, B.M.; Hudson, D.; Staples, D.F. Depths and Thermal Habitat Used by Large versus Small Northern Pike in Three Minnesota Lakes. Trans. Am. Fish. Soc. 2013, 142, 1629–1639. [Google Scholar] [CrossRef]
- Pierce, R.B.; Tomcko, C.M. Density and Biomass of Native Northern Pike Populations in Relation to Basin-Scale Characteristics of North-Central Minnesota Lakes. Trans. Am. Fish. Soc. 2005, 134, 231–241. [Google Scholar] [CrossRef]
- Anders Nilsson, P. Avoid your neighbours: Size-determined spatial distribution patterns among northern pike individuals: Avoid your neighbours. Oikos 2006, 113, 251–258. [Google Scholar] [CrossRef]
- Lepak, J.M.; Fetherman, E.R.; Pate, W.M.; Craft, C.D.; Gardunio, E.I. An experimental approach to determine esocid prey preference in replicated pond systems. Lake Reserv. Manag. 2012, 28, 224–231. [Google Scholar] [CrossRef]
- Radinger, J.; Matern, S.; Klefoth, T.; Wolter, C.; Feldhege, F.; Monk, C.T.; Arlinghaus, R. Ecosystem-based management outperforms species-focused stocking for enhancing fish populations. Science 2023, 379, 946–951. [Google Scholar] [CrossRef]
- Hühn, D.; Gwinn, D.C.; Shaw, S.L.; Alós, J.; Allen, M.S.; Pagel, T.; Skov, C.; Arlinghaus, R. Density-and size-dependent mechanisms modulate the outcome of stocking in a naturally recruiting freshwater piscivore (northern pike, Esox lucius): A replicated whole-lake experiment. Fish. Res. 2023, 267, 106799. [Google Scholar] [CrossRef]
- Ahrens, R.N.M.; Allen, M.S.; Walters, C.; Arlinghaus, R. Saving large fish through harvest slots outperforms the classical minimum-length limit when the aim is to achieve multiple harvest and catch-related fisheries objectives. Fish Fish. 2020, 21, 483–510. [Google Scholar] [CrossRef]
- Gwinn, D.C.; Allen, M.S.; Johnston, F.D.; Brown, P.; Todd, C.R.; Arlinghaus, R. Rethinking length-based fisheries regulations: The value of protecting old and large fish with harvest slots. Fish Fish. 2015, 16, 259–281. [Google Scholar] [CrossRef]
- Bond, N.R.; Lake, P.S. Local habitat restoration in streams: Constraints on the effectiveness of restoration for stream biota. Ecol. Manag. Restor. 2003, 4, 193–198. [Google Scholar] [CrossRef]
- Theis, S.; Ruppert, J.L.W.; Shirton, J.R.; Poesch, M.S. Measuring beta diversity components and beneficial effects of coarse woody habitat introduction on invertebrate and macrophyte communities in a shallow northern boreal lake; implications for offsetting. Aquat. Ecol. 2022, 56, 793–814. [Google Scholar] [CrossRef]
- Angradi, T.R.; Schweiger, E.W.; Bolgrien, D.W.; Ismert, P.; Selle, T. Bank stabilization, riparian land use and the distribution of large woody debris in a regulated reach of the upper Missouri River, North Dakota, USA. River Res. Appl. 2004, 20, 829–846. [Google Scholar] [CrossRef]
- Jones, E.B.D.; Helfman, G.S.; Harper, J.O.; Bolstad, P.V. Effects of Riparian Forest Removal on Fish Assemblages in Southern Appalachian Streams. Conserv. Biol. 1999, 13, 1454–1465. [Google Scholar] [CrossRef]
- Lane, J.A.; Portt, C.B.; Minns, C.K. Nursery Habitat Characteristics of Great Lakes Fishes; Canadian Manuscript Report of Fisheries and Aquatic Sciences No. 2338; Fisheries and Oceans Canada: Ottawa, ON, Canada, 1996. [Google Scholar]
- Yazicioğlu, O.; Yazici, R.; Yağci, A.; Yilmaz, M. Diet and Feeding Strategy of Northern Pike, Esox lucius L., 1758 Inhabiting A Deep Dam Lake from Located Central Anatolia, Türkiye. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Benedek, I.; Molnár, T. Size preference of live fish prey in the pellet-consuming pikeperch. Appl. Sci. 2023, 13, 2259. [Google Scholar] [CrossRef]
- Venturelli, P.A.; Tonn, W.M. Invertivory by northern pike (Esox lucius) structures communities of littoral macroinvertebrates in small boreal lakes. J. N. Am. Benthol. Soc. 2005, 24, 904–918. [Google Scholar] [CrossRef]
- Eklöv, P. Effects of Behavioural Flexibility and Habitat Complexity on Predator-Prey Interactions in Fish Communities; Department of Animal Ecology, Umeå University: Umeå, Sweden, 1995. [Google Scholar]
- Cutler, L.M.; Chipps, S.R.; Blackwell, B.G.; Coulter, A.A. Importance of a Lake-Wetland Complex for a Resilient Walleye Fishery. Wetlands 2024, 44, 69. [Google Scholar] [CrossRef]
- Laiveling, A.R.; Lorentz, C.N.; Booth, M.T. River connectivity increases the diversity of fish communities in gravel pit lakes. Trans. Am. Fish. Soc. 2023, 152, 550–576. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Hollingsworth, T.N.; Chapin, F.S.; Mack, M.C. Changes in fire regime break the legacy lock on successional trajectories in Alaskan boreal forest. Glob. Chang. Biol. 2010, 16, 1281–1295. [Google Scholar] [CrossRef]
- Danylchuk, A.J.; Tonn, W.M. Natural Disturbances and Fish: Local and Regional Influences on Winterkill of Fathead Minnows in Boreal Lakes. Trans. Am. Fish. Soc. 2003, 132, 289–298. [Google Scholar] [CrossRef]
- Tonn, W.M.; Langlois, P.W.; Prepas, E.E.; Danylchuk, A.J.; Boss, S.M. Winterkill cascade: Indirect effects of a natural disturbance on littoral macroinvertebrates in boreal lakes. J. N. Am. Benthol. Soc. 2004, 23, 237–250. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Kuris, A.M. How environmental stress affects the impacts of parasites. Limnol. Oceanogr. 1999, 44, 925–931. [Google Scholar] [CrossRef]
- Grosbois, G.; Mou, T.A.; Girona, M.M. Cyanobacteria in winter: Seasonal dynamics of harmful algal blooms and their driving factors in boreal lakes. Heliyon 2024, 10, e40687. [Google Scholar] [CrossRef]
- Richter, I.A.; Smokorowski, K.E.; Blanchfield, P.J. Seasonal habitat use of white sucker Catostomus commersonii in a small Boreal lake. Environ. Biol. Fishes 2024, 1–17. [Google Scholar] [CrossRef]
- Uzarski, D.G.; Burton, T.M.; Cooper, M.J.; Ingram, J.W.; Timmermans, S.T.A. Fish Habitat Use Within and Across Wetland Classes in Coastal Wetlands of the Five Great Lakes: Development of a Fish-based Index of Biotic Integrity. J. Gt. Lakes Res. 2005, 31, 171–187. [Google Scholar] [CrossRef]
- Brooks, J.L.; Boston, C.; Doka, S.; Gorsky, D.; Gustavson, K.; Hondorp, D.; Isermann, D.; Midwood, J.D.; Pratt, T.C.; Rous, A.M.; et al. Use of Fish Telemetry in Rehabilitation Planning, Management, and Monitoring in Areas of Concern in the Laurentian Great Lakes. Environ. Manag. 2017, 60, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Martins, E.G.; Struthers, D.P.; Gutowsky, L.F.G.; Power, M.; Doka, S.E.; Dettmers, J.M.; Crook, D.A.; Lucas, M.C.; Holbrook, C.M.; et al. A moving target—Incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations. Environ. Monit. Assess. 2016, 188, 239. [Google Scholar] [CrossRef] [PubMed]
- Radinger, J.; Britton, J.R.; Carlson, S.M.; Magurran, A.E.; Alcaraz-Hernández, J.D.; Almodóvar, A.; Benejam, L.; Fernández-Delgado, C.; Nicola, G.G.; Oliva-Paterna, F.J.; et al. Effective monitoring of freshwater fish. Fish Fish. 2019, 20, 729–747. [Google Scholar] [CrossRef]
- Diller, S.N.; Harrison, A.M.; Kowalski, K.P.; Brady, V.J.; Ciborowski, J.J.H.; Cooper, M.J.; Dumke, J.D.; Gathman, J.P.; Ruetz, C.R.; Uzarski, D.G.; et al. Influences of seasonality and habitat quality on Great Lakes coastal wetland fish community composition and diets. Wetl. Ecol. Manag. 2022, 30, 439–460. [Google Scholar] [CrossRef]
- Bowen, K.L.; Currie, W.J.; Niblock, H.; Ward, C.L.; Metcalfe, B.; Cuddington, K.M.D.; Johnson, T.B.; Koops, M.A. Importance of long-term intensive monitoring programs for understanding multiple drivers influencing Lake Ontario zooplankton communities. J. Gt. Lakes Res. 2022, 48, 717–733. [Google Scholar] [CrossRef]
- Lennox, R.J.; Westrelin, S.; Souza, A.T.; Šmejkal, M.; Říha, M.; Prchalová, M.; Nathan, R.; Koeck, B.; Killen, S.; Jarić, I. A role for lakes in revealing the nature of animal movement using high dimensional telemetry systems. Mov. Ecol. 2021, 9, 40. [Google Scholar] [CrossRef]
- Midwood, J.D.; Gutowsky, L.F.G.; Hlevca, B.; Portiss, R.; Wells, M.G.; Doka, S.E.; Cooke, S.J. Tracking bowfin with acoustic telemetry: Insight into the ecology of a living fossil. Ecol. Freshw. Fish 2018, 27, 225–236. [Google Scholar] [CrossRef]
- Axler, R.; Yokom, S.; Tikkanen, C.; McDonald, M.; Runke, H.; Wilcox, D.; Cady, B. Restoration of a mine pit lake from aquacultural nutrient enrichment. Restor. Ecol. 1998, 6, 1–19. [Google Scholar] [CrossRef]
- Hoyle, J.A.; Bowlby, J.N.; Brousseau, C.M.; Johnson, T.B.; Morrison, B.J.; Randall, R.G. Fish community structure in the Bay of Quinte, Lake Ontario: The influence of nutrient levels and invasive species. Aquat. Ecosyst. Health Manag. 2012, 15, 370–384. [Google Scholar] [CrossRef]


| Lake | Size ha | Max Depth m | Mean Summer pH | Mean Summer Temperature °C (1 m Steps) | Littoral % Aquatic Vegetation | Mean DO mg/L | Secchi Depth m |
|---|---|---|---|---|---|---|---|
| Steepbank | 185.4 | 16 | 8.23 ± 0.47 | 18.5 ± 0.68 | 6.38 ± 8.95 | 8.16 ± 0.09 | 2.25 |
| Wappau | 576.6 | 6 | 8.66 ± 0.03 | 18.4 ± 0.71 | 18.04 ± 16.94 | 8.87 ± 1.57 | 1.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theis, S.; Shirton, J.; Barbeau, M.; Ruppert, J.L.W.; Poesch, M.S. Growth and Diet of Northern Pike (Esox lucius) in Boreal Lakes: Implications for Ecosystem Management. Hydrobiology 2025, 4, 1. https://doi.org/10.3390/hydrobiology4010001
Theis S, Shirton J, Barbeau M, Ruppert JLW, Poesch MS. Growth and Diet of Northern Pike (Esox lucius) in Boreal Lakes: Implications for Ecosystem Management. Hydrobiology. 2025; 4(1):1. https://doi.org/10.3390/hydrobiology4010001
Chicago/Turabian StyleTheis, Sebastian, Jesse Shirton, Michael Barbeau, Jonathan L. W. Ruppert, and Mark S. Poesch. 2025. "Growth and Diet of Northern Pike (Esox lucius) in Boreal Lakes: Implications for Ecosystem Management" Hydrobiology 4, no. 1: 1. https://doi.org/10.3390/hydrobiology4010001
APA StyleTheis, S., Shirton, J., Barbeau, M., Ruppert, J. L. W., & Poesch, M. S. (2025). Growth and Diet of Northern Pike (Esox lucius) in Boreal Lakes: Implications for Ecosystem Management. Hydrobiology, 4(1), 1. https://doi.org/10.3390/hydrobiology4010001






