Bidirectional Regulation of Nitric Oxide and Endothelin-1 in Cerebral Vasospasm: Mechanisms and Therapeutic Perspectives
Abstract
1. Introduction
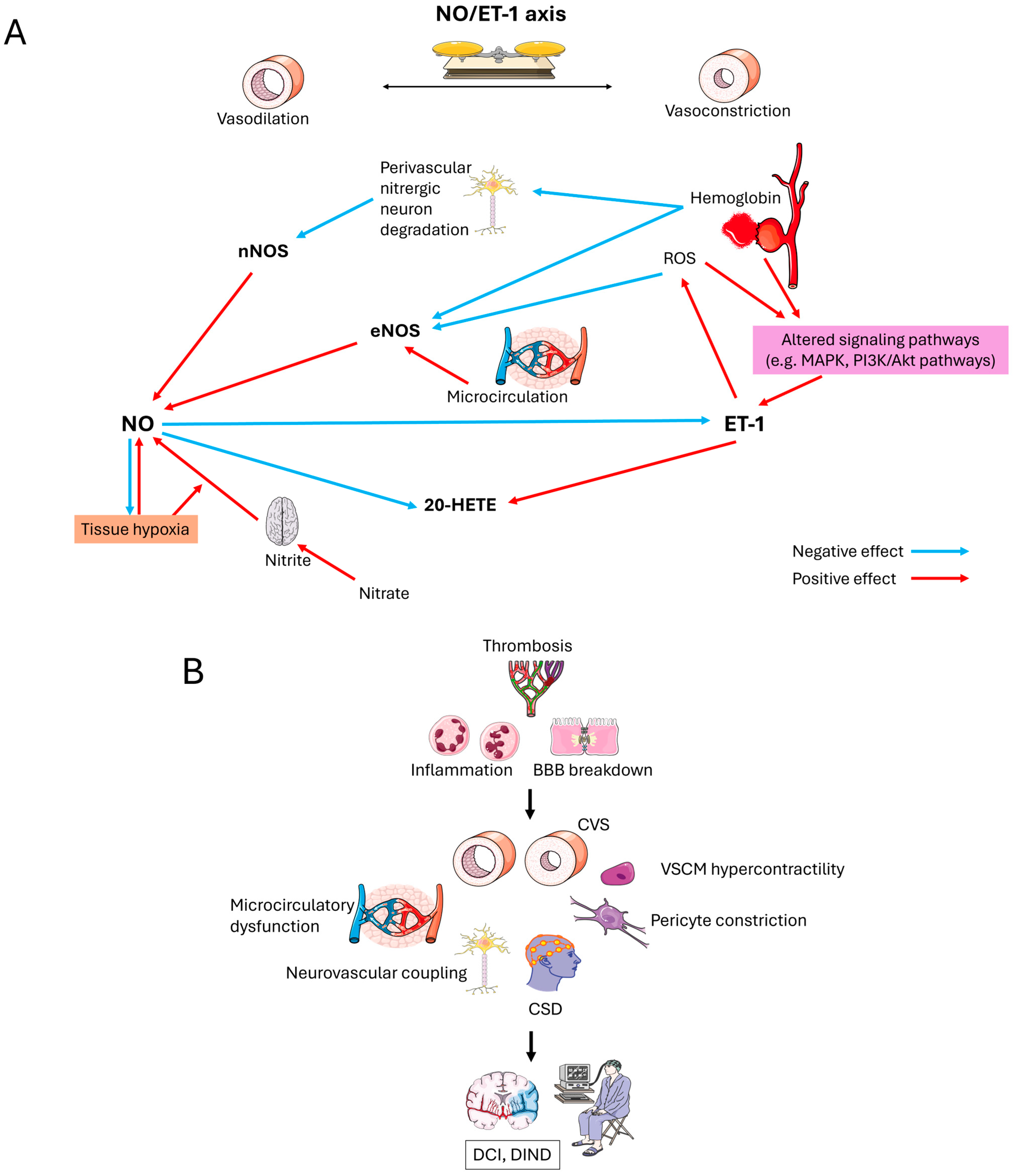
2. NO-Mediated Vasoregulation: Pharmacological Foundations
3. NO Dysregulation Post-SAH: Therapeutic Challenges
4. ET-1/NO Antagonism: Pharmacodynamic Interplay
5. Pharmacological Interventions: Targeting the NO/ET-1 Axis
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 20-HETE | 20-Hydroxyeicosatetraenoic Acid |
| α-SMA | alpha Smooth Muscle Actin |
| ARBs | Angiotensin Receptor Blockers |
| ACEi | Angiotensin-Converting Enzyme Inhibitors |
| AngII | Angiotensin II |
| ADMA | Asymmetric Dimethyl Arginine |
| BBB | Blood–Brain Barrier |
| CBF | Cerebral Blood Flow |
| cGMP | Cyclic Guanosine Monophosphate |
| CNS | Central Nervous System |
| CO2 | Carbon Dioxide |
| CSD | Cortical Spreading Depolarizations |
| CSI | Cortical Spreading Ischemia |
| CVS | Cerebral Vasospasm |
| CTH | Transit Time Heterogeneity |
| CYPA4 | Cytochrome P450 |
| DCC | Deleted in Colorectal Cancer |
| DCI | Delayed Cerebral Ischemia |
| DIND | Delayed Ischemic Neurological Deficit |
| eNOS | Endothelial NOS |
| ERK | Extracellular Signal Regulated Kinase |
| ET-1 | Endothelin-1 |
| HO-1 | Heme Oxygenase-1 |
| iNOS | Inducible NOS |
| I/R | Ischemia/Reperfusion |
| KCa channels | Calcium-Activated K+ Channels |
| L-NAME | L-NG-Nitro Arginine Methyl Ester |
| MAPK | Mitogen Activated Protein Kinase |
| metHb | Methaemoglobin |
| MMP9 | Matrix Metalloproteinase 9 |
| MnSOD | Mangan Superoxide Dismutase |
| MTT | Mean Transit Time |
| NADPH | Nicotinamide Dinucleotide Phosphate |
| NF-κB | Nuclear Factor Kappa B |
| NMDA | N-Methyl-D-Aspartate |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| nNOS | Neuronal NOS |
| O2− | Superoxide |
| ONOO− | Peroxynitrite |
| oxyHb | Oxygenated Hemoglobin |
| PAH | Pulmonary Arterial Hypertension |
| PARP | Poly(Adenosine Diphosphate-Ribose)-Polymerase |
| PDE | Phosphodiesterase |
| PI3K/Akt | Phosphatidylinositol-3-Kinase/Protein Kinase B |
| PKG | Protein Kinase G |
| ROCK | Rho-Associated Protein Kinase |
| ROS | Reactive Oxygen Species |
| SAH | Subarachnoid Hemorrhage |
| sGC | Soluble Guanylate Cyclase |
| TMP | Tetramethylpyrazine |
| tPA | Tissue Plasminogen Activator |
| VEGF | Vascular Endothelial Growth Factor |
| VSMC | Vascular Smooth Muscle Cell |
References
- Vajkoczy, P.; Horn, P.; Thome, C.; Munch, E.; Schmiedek, P. Regional cerebral blood flow monitoring in the diagnosis of delayed ischemia following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2003, 98, 1227–1234. [Google Scholar] [CrossRef]
- Gross, B.A.; Lin, N.; Frerichs, K.U.; Du, R. Vasospasm after spontaneous angiographically negative subarachnoid hemorrhage. Acta Neurochir. 2012, 154, 1127–1133. [Google Scholar] [CrossRef]
- Hui, F.K.; Tumialán, L.M.; Tanaka, T.; Cawley, C.M.; Zhang, Y.J. Clinical Differences Between Angiographically Negative, Diffuse Subarachnoid Hemorrhage and Perimesencephalic Subarachnoid Hemorrhage. Neurocritical Care 2009, 11, 64–70. [Google Scholar] [CrossRef]
- Grubb, R.L.; Raichle, M.E.; Eichling, J.O.; Gado, M.H. Effects of subarachnoid hemorrhage on cerebral blood volume, blood flow, and oxygen utilization in humans. J. Neurosurg. 1977, 46, 446–453. [Google Scholar] [CrossRef]
- Kurki, M.I.; Häkkinen, S.-K.; Frösen, J.; Tulamo, R.; Von Und Zu Fraunberg, M.; Wong, G.; Tromp, G.; Niemelä, M.; Hernesniemi, J.; Jääskeläinen, J.E.; et al. Upregulated Signaling Pathways in Ruptured Human Saccular Intracranial Aneurysm Wall: An Emerging Regulative Role of Toll-Like Receptor Signaling and Nuclear Factor-κB, Hypoxia-Inducible Factor-1A, and ETS Transcription Factors. Neurosurgery 2011, 68, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Sehba, F.A.; Hou, J.; Pluta, R.M.; Zhang, J.H. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012, 97, 14–37. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, Y.; Yuan, F.; Chen, L.; He, C.; Bao, Y.; Chen, Z.; Lou, M.; Xia, W.; Yang, G.-Y.; et al. A Novel Intravital Method to Evaluate Cerebral Vasospasm in Rat Models of Subarachnoid Hemorrhage: A Study with Synchrotron Radiation Angiography. PLoS ONE 2012, 7, e33366. [Google Scholar] [CrossRef] [PubMed]
- Osaka, K. Prolonged vasospasm produced by the breakdown products of erythrocytes. J. Neurosurg. 1977, 47, 403–411. [Google Scholar] [CrossRef]
- Cetas, J.; Lee, D.; Alkayed, N.; Wang, R.; Iliff, J.; Heinricher, M. Brainstem control of cerebral blood flow and application to acute vasospasm following experimental subarachnoid hemorrhage. Neuroscience 2009, 163, 719–729. [Google Scholar] [CrossRef]
- Güresir, E.; Schuss, P.; Borger, V.; Vatter, H. Experimental Subarachnoid Hemorrhage: Double Cisterna Magna Injection Rat Model—Assessment of Delayed Pathological Effects of Cerebral Vasospasm. Transl. Stroke Res. 2015, 6, 242–251. [Google Scholar] [CrossRef]
- Jakobsen, M.; Overgaard, J.; Marcussen, E.; Enevoldsen, E.M. Relation between angiographic cerebral vasospasm and regional CBF in patients with SAH. Acta Neurol. Scand. 1990, 82, 109–115. [Google Scholar] [CrossRef]
- Friedrich, B.; Michalik, R.; Oniszczuk, A.; Abubaker, K.; Kozniewska, E.; Plesnila, N. CO2 Has no Therapeutic Effect on Early Micro Vasospasm after Experimental Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2014, 34, e1–e6. [Google Scholar] [CrossRef]
- Takeuchi, K.; Miyata, N.; Renic, M.; Harder, D.R.; Roman, R.J. Hemoglobin, NO, and 20-HETE interactions in mediating cerebral vasoconstriction following SAH. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R84–R89. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Aamand, R.; Karabegovic, S.; Tietze, A.; Blicher, J.U.; Mikkelsen, I.K.; Iversen, N.K.; Secher, N.; Engedal, T.S.; Anzabi, M.; et al. The Role of the Microcirculation in Delayed Cerebral Ischemia and Chronic Degenerative Changes after Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2013, 33, 1825–1837. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Li, B.; Luo, C.; Zuo, S.; Liu, X.; Zhang, J.H.; Ruan, H.; Feng, H. Hemoglobin induced NO/cGMP suppression Deteriorate Microcirculation via Pericyte Phenotype Transformation after Subarachnoid Hemorrhage in Rats. Sci. Rep. 2016, 6, 22070. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; Brough, D.; Robinson, E.M.; Girard, S.; Rothwell, N.J.; Allan, S.M. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis. Model. Mech. 2012, 5, 823–833. [Google Scholar] [CrossRef]
- Bradley, W.G.; Schmidt, P.G. Effect of methemoglobin formation on the MR appearance of subarachnoid hemorrhage. Radiology 1985, 156, 99–103. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lee, W.-S. Estradiol Reduces Ferrous Citrate Complex-Induced NOS2 Up-Regulation in Cerebral Endothelial Cells by Interfering the Nuclear Factor Kappa B Transactivation through an Estrogen Receptor β–Mediated Pathway. PLoS ONE 2013, 8, e84320. [Google Scholar] [CrossRef] [PubMed]
- Sharp, F.R.; Zhan, X.; Liu, D.-Z. Heat Shock Proteins in the Brain: Role of Hsp70, Hsp 27, and HO-1 (Hsp32) and Their Therapeutic Potential. Transl. Stroke Res. 2013, 4, 685–692. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Zhang, J.-M. The Updated Role of Oxidative Stress in Subarachnoid Hemorrhage. Curr. Drug Deliv. 2016, 14, 832–842. [Google Scholar] [CrossRef]
- Echigo, R.; Shimohata, N.; Karatsu, K.; Yano, F.; Kayasuga-Kariya, Y.; Fujisawa, A.; Ohto, T.; Kita, Y.; Nakamura, M.; Suzuki, S.; et al. Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. J. Transl. Med. 2012, 10, 80. [Google Scholar] [CrossRef]
- Wirrig, C.; Hunter, I.; Mathieson, F.A.; Nixon, G.F. Sphingosylphosphorylcholine is a Proinflammatory Mediator in Cerebral Arteries. J. Cereb. Blood Flow Metab. 2011, 31, 212–221. [Google Scholar] [CrossRef]
- Handa, Y.; Kabuto, M.; Kobayashi, H.; Kawano, H.; Takeuchi, H.; Hayashi, M. The correlation between immunological reaction in the arterial wall and the time course of the development of cerebral vasospasm in a primate model. Neurosurgery 1991, 28, 542–549. [Google Scholar] [CrossRef]
- Zhou, C.; Yamaguchi, M.; Kusaka, G.; Schonholz, C.; Nanda, A.; Zhang, J.H. Caspase Inhibitors Prevent Endothelial Apoptosis and Cerebral Vasospasm in Dog Model of Experimental Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2004, 24, 419–431. [Google Scholar] [CrossRef]
- Kwon, M.S.; Woo, S.K.; Kurland, D.B.; Yoon, S.H.; Palmer, A.F.; Banerjee, U.; Iqbal, S.; Ivanova, S.; Gerzanich, V.; Simard, J.M. Methemoglobin Is an Endogenous Toll-Like Receptor 4 Ligand—Relevance to Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2015, 16, 5028–5046. [Google Scholar] [CrossRef]
- You, W.-C.; Wang, C.-X.; Pan, Y.-X.; Zhang, X.; Zhou, X.-M.; Zhang, X.-S.; Shi, J.-X.; Zhou, M.-L. Activation of Nuclear Factor-κB in the Brain after Experimental Subarachnoid Hemorrhage and Its Potential Role in Delayed Brain Injury. PLoS ONE 2013, 8, e60290. [Google Scholar] [CrossRef]
- Friedrich, V.; Bederson, J.B.; Sehba, F.A. Gender Influences the Initial Impact of Subarachnoid Hemorrhage: An Experimental Investigation. PLoS ONE 2013, 8, e80101. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.H.; Qin, X. Non-aneurysm subarachnoid hemorrhage in young adults. Acta. Neurochir. Suppl. 2011, 110, 209–213. [Google Scholar] [CrossRef]
- Satoh, M.; Parent, A.D.; Zhang, J.H. Inhibitory effect with antisense mitogen-activated protein kinase oligodeoxynucleotide against cerebral vasospasm in rats. Stroke 2002, 33, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yamaguchi, M.; Colohan, A.R.; Zhang, J.H. Role of p53 and Apoptosis in Cerebral Vasospasm after Experimental Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2005, 25, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Su, J.; Guo, B.; Wang, K.; Li, X.; Liang, G. Apigenin protects blood–brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int. Immunopharmacol. 2015, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, J.; Sun, C.; Qi, M.; Hang, C.; Gong, Y.; Han, X.; Shi, J. Potential role of JAK2 in cerebral vasospasm after experimental subarachnoid hemorrhage. Brain Res. 2008, 1214, 136–144. [Google Scholar] [CrossRef]
- Beg, S.S.; Hansen-Schwartz, J.A.; Vikman, P.J.; Xu, C.-B.; Edvinsson, L.I. Protein Kinase C Inhibition Prevents Upregulation of Vascular ETB and 5-HT1B Receptors and Reverses Cerebral Blood Flow Reduction after Subarachnoid Haemorrhage in Rats. J. Cereb. Blood Flow Metab. 2007, 27, 21–32. [Google Scholar] [CrossRef]
- Spallone, A. Cerebral vasospasm as a complication of aneurysmal subarachnoid hemorrhage: A brief review. Ital. J. Neurol. Sci. 1985, 6, 19–26. [Google Scholar] [CrossRef]
- Chen, S.; Feng, H.; Sherchan, P.; Klebe, D.; Zhao, G.; Sun, X.; Zhang, J.; Tang, J.; Zhang, J.H. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog. Neurobiol. 2014, 115, 64–91. [Google Scholar] [CrossRef]
- Schebesch, K.-M.; Brawanski, A.; Bele, S.; Schödel, P.; Herbst, A.; Bründl, E.; Kagerbauer, S.M.; Martin, J.; Lohmeier, A.; Stoerr, E.-M.; et al. Neuropeptide Y—An early biomarker for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurol. Res. 2013, 35, 1038–1043. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Tian, X.; Shen, H.; Dou, Y.; Li, H.; Chen, G. Transient receptor potential channel 1/4 reduces subarachnoid hemorrhage-induced early brain injury in rats via calcineurin-mediated NMDAR and NFAT dephosphorylation. Sci. Rep. 2016, 6, srep33577. [Google Scholar] [CrossRef]
- TTokiyoshi, K.; Ohnishi, T.; Nii, Y. Efficacy and toxicity of thromboxane synthetase inhibitor for cerebral vasospasm after subarachnoid hemorrhage. Surg. Neurol. 1991, 36, 112–118. [Google Scholar] [CrossRef]
- Beg, S.A.; Hansen-Schwartz, J.A.; Vikman, P.J.; Xu, C.-B.; Edvinsson, L.I. ERK1/2 Inhibition Attenuates Cerebral Blood Flow Reduction and Abolishes ETB and 5-HT1B Receptor Upregulation after Subarachnoid Hemorrhage in Rat. J. Cereb. Blood Flow Metab. 2006, 26, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ayer, R.; Jadhav, V.; Chen, W.; Tsubokawa, T.; Zhang, J.H. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J. Neurosci. Res. 2008, 86, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Munakata, A.; Naraoka, M.; Katagai, T.; Shimamura, N.; Ohkuma, H. Role of Cyclooxygenase-2 in Relation to Nitric Oxide and Endothelin-1 on Pathogenesis of Cerebral Vasospasm After Subarachnoid Hemorrhage in Rabbit. Transl. Stroke Res. 2016, 7, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.J.; Renic, M.; Dunn, K.M.J.; Takeuchi, K.; Hacein-Bey, L. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurol. Res. 2006, 28, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Neuschmelting, V.; Marbacher, S.; Fathi, A.-R.; Jakob, S.M.; Fandino, J. Elevated level of endothelin-1 in cerebrospinal fluid and lack of nitric oxide in basilar arterial plasma associated with cerebral vasospasm after subarachnoid haemorrhage in rabbits. Acta Neurochir. 2009, 151, 795–802. [Google Scholar] [CrossRef]
- Takeuchi, K.; Renic, M.; Bohman, Q.C.; Harder, D.R.; Miyata, N.; Roman, R.J. Reversal of delayed vasospasm by an inhibitor of the synthesis of 20-HETE. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2203–H2211. [Google Scholar] [CrossRef]
- Leo, F.; Suvorava, T.; Heuser, S.K.; Li, J.; LoBue, A.; Barbarino, F.; Piragine, E.; Schneckmann, R.; Hutzler, B.; Good, M.E.; et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 2021, 144, 870–889. [Google Scholar] [CrossRef]
- Cseplo, P.; Vamos, Z.; Ivic, I.; Torok, O.; Toth, A.; Koller, A. The Beta-1-Receptor Blocker Nebivolol Elicits Dilation of Cerebral Arteries by Reducing Smooth Muscle [Ca2+]i. PLoS ONE 2016, 11, e0164010. [Google Scholar] [CrossRef]
- Petzold, G.C.; Haack, S.; Halbach, O.v.B.U.; Priller, J.; Lehmann, T.-N.; Heinemann, U.; Dirnagl, U.; Dreier, J.P. Nitric Oxide Modulates Spreading Depolarization Threshold in the Human and Rodent Cortex. Stroke 2008, 39, 1292–1299. [Google Scholar] [CrossRef]
- Chen, L.-C.; Hsu, C.; Chiueh, C.C.; Lee, W.-S. Ferrous Citrate Up-Regulates the NOS2 through Nuclear Translocation of NFκB Induced by Free Radicals Generation in Mouse Cerebral Endothelial Cells. PLoS ONE 2012, 7, e46239. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.H.; Stamer, W.D.; Bertrand, J.; Read, A.T.; Marando, C.M.; Ethier, C.R.; Overby, D.R. Role of nitric oxide in murine conventional outflow physiology. Am. J. Physiol. Physiol. 2015, 309, C205–C214. [Google Scholar] [CrossRef]
- McLeod, D.S.; Hasegawa, T.; Baba, T.; Grebe, R.; D’AUriac, I.G.; Merges, C.; Edwards, M.; Lutty, G.A. From Blood Islands to Blood Vessels: Morphologic Observations and Expression of Key Molecules during Hyaloid Vascular System Development. Investig. Opthalmology Vis. Sci. 2012, 53, 7912–7927. [Google Scholar] [CrossRef]
- Yemisci, M.; Gursoy-Ozdemir, Y.; Vural, A.; Can, A.; Topalkara, K.; Dalkara, T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009, 15, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Starke, R.M.; Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. The Role of Oxidative Stress in Cerebral Aneurysm Formation and Rupture. Curr. Neurovascular Res. 2013, 10, 247–255. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Liy, P.M.; Puzi, N.N.A.; Jose, S.; Vidyadaran, S. Nitric oxide modulation in neuroinflammation and the role of mesenchymal stem cells. Exp. Biol. Med. 2021, 246, 2399–2406. [Google Scholar] [CrossRef]
- Sonar, S.A.; Lal, G. The iNOS Activity During an Immune Response Controls the CNS Pathology in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2019, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Qin, L.; Li, S.; Xu, K.; Geng, H. CT perfusion-derived mean transit time of cortical brain has a negative correlation with the plasma level of Nitric Oxide after subarachnoid hemorrhage. Acta Neurochir. 2014, 156, 527–533. [Google Scholar] [CrossRef]
- Lilla, N.; Hartmann, J.; Koehler, S.; Ernestus, R.-I.; Westermaier, T. Early NO-donor treatment improves acute perfusion deficit and brain damage after experimental subarachnoid hemorrhage in rats. J. Neurol. Sci. 2016, 370, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Luettich, A.; Franko, E.; Spronk, D.B.; Lamb, C.; Corkill, R.; Patel, J.; Ezra, M.; Pattinson, K.T.S. Beneficial Effect of Sodium Nitrite on EEG Ischaemic Markers in Patients with Subarachnoid Haemorrhage. Transl. Stroke Res. 2022, 13, 265–275. [Google Scholar] [CrossRef]
- Ezra, M.; Garry, P.; Rowland, M.J.; Mitsis, G.D.; Pattinson, K.T. Phase dynamics of cerebral blood flow in subarachnoid haemorrhage in response to sodium nitrite infusion. Nitric Oxide Biol. Chem. 2021, 106, 55–65. [Google Scholar] [CrossRef]
- Sehba, F.A.; Schwartz, A.Y.; Chereshnev, I.; Bederson, J.B. Acute Decrease in Cerebral Nitric Oxide Levels after Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2000, 20, 604–611. [Google Scholar] [CrossRef]
- Suzuki, Y.; Osuka, K.; Noda, A.; Tanazawa, T.; Takayasu, M.; Shibuya, M.; Yoshida, J. Nitric Oxide Metabolites in the Cisternal Cerebral Spinal Fluid of Patients with Subarachnoid Hemorrhage. Neurosurgery 1997, 41, 807–812. [Google Scholar] [CrossRef]
- Springborg, J.B.; Ma, X.; Rochat, P.; Knudsen, G.M.; Amtorp, O.; Paulson, O.B.; Juhler, M.; Olsen, N.V. A single subcutaneous bolus of erythropoietin normalizes cerebral blood flow autoregulation after subarachnoid haemorrhage in rats. Br. J. Pharmacol. 2002, 135, 823–829. [Google Scholar] [CrossRef]
- Koide, M.; Bonev, A.D.; Nelson, M.T.; Wellman, G.C. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca 2+ -activated K + (BK) channels. Proc. Natl. Acad. Sci. USA 2012, 109, E1387–E1395. [Google Scholar] [CrossRef]
- Richards, J.; El-Hamamsy, I.; Chen, S.; Sarang, Z.; Sarathchandra, P.; Yacoub, M.H.; Chester, A.H.; Butcher, J.T. Side-Specific Endothelial-Dependent Regulation of Aortic Valve Calcification. Am. J. Pathol. 2013, 182, 1922–1931. [Google Scholar] [CrossRef]
- Dreier, J.P.; Major, S.; Manning, A.; Woitzik, J.; Drenckhahn, C.; Steinbrink, J.; Tolias, C.; Oliveira-Ferreira, A.I.; Fabricius, M.; Hartings, J.A.; et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009, 132, 1866–1881. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Pluta, R.M.; Zhang, J.H. Cerebral vasospasm after subarachnoid hemorrhage: The emerging revolution. Nat. Clin. Pract. Neurol. 2007, 3, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, Z.; Wang, X.; Chang, J.; Yang, M.; Zhong, H.; Hong, F.; Yang, S. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef]
- Saito, A.; Maier, C.M.; Narasimhan, P.; Nishi, T.; Song, Y.S.; Yu, F.; Liu, J.; Lee, Y.-S.; Nito, C.; Kamada, H.; et al. Oxidative Stress and Neuronal Death/Survival Signaling in Cerebral Ischemia. Mol. Neurobiol. 2005, 31, 105–116. [Google Scholar] [CrossRef]
- Shin, H.K.; Lee, J.H.; Kim, C.D.; Kim, Y.K.; Hong, J.Y.; Hong, K.W. Prevention of Impairment of Cerebral Blood Flow Autoregulation during Acute Stage of Subarachnoid Hemorrhage by Gene Transfer of Cu/Zn SOD-1 to Cerebral Vessels. J. Cereb. Blood Flow Metab. 2003, 23, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Date, I.; Nakajima, M.; Takahashi, K.; Iseda, K.; Tamiya, T.; Ohmoto, T.; Ninomiya, Y.; Asari, S. Inhibition of Poly(ADP-Ribose) Polymerase Attenuates Cerebral Vasospasm After Subarachnoid Hemorrhage in Rabbits. Stroke 2001, 32, 225–231. [Google Scholar] [CrossRef]
- Leng, L.Z.; Fink, M.E.; Iadecola, C. Spreading Depolarization. Arch. Neurol. 2011, 68, 31–36. [Google Scholar] [CrossRef]
- Sanicola, H.W.; Stewart, C.E.; Luther, P.; Yabut, K.; Guthikonda, B.; Jordan, J.D.; Alexander, J.S. Pathophysiology, Management, and Therapeutics in Subarachnoid Hemorrhage and Delayed Cerebral Ischemia: An Overview. Pathophysiology 2023, 30, 420–442. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Gomez, F.; Bischof, H.; Diedrich, D.; Laudanski, K. Cortical Spreading Depolarization and Delayed Cerebral Ischemia; Rethinking Secondary Neurological Injury in Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2023, 24, 9883. [Google Scholar] [CrossRef]
- Dawson, V.; Dawson, T.; Bartley, D.; Uhl, G.; Snyder, S. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J. Neurosci. 1993, 13, 2651–2661. [Google Scholar] [CrossRef]
- Belenichev, I.; Popazova, O.; Bukhtiyarova, N.; Savchenko, D.; Oksenych, V.; Kamyshnyi, O. Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection. Antioxidants 2024, 13, 504. [Google Scholar] [CrossRef]
- Solodov, A.A.; Petrikov, S.S.; Klychnnikova, E.V.; Tazina, E.V.; Krylov, V.V.; Godkov, M.A.; Khamidova, L.T. Effect of normobaric hyperoxia on cerebral oxygenation, metabolism and oxidative stress in patients with subarachnoid hemorrhage caused by intracranial aneurysm rupture. Anesteziol. Reanimatol. 2013, 4, 66–71. [Google Scholar]
- Sasaki, T.; Wakai, S.; Asano, T.; Watanabe, T.; Kirino, T.; Sano, K. The effect of a lipid hydroperoxide of arachidonic acid on the canine basilar artery. An experimental study on cerebral vasospasm. J. Neurosurg. 1981, 54, 357–365. [Google Scholar] [CrossRef]
- Lan, S.H.; Lai, W.T.; Zheng, S.Y.; Yang, L.; Fang, L.C.; Zhou, L.; Tang, B.; Duan, J.; Hong, T. Upregulation of Connexin 40 Mediated by Nitric Oxide Attenuates Cerebral Vasospasm After Subarachnoid Hemorrhage via the Nitric Oxide-Cyclic Guanosine Monophosphate-Protein Kinase G Pathway. World Neurosurg. 2020, 136, e476–e486. [Google Scholar] [CrossRef]
- Bosche, B.; Graf, R.; Ernestus, R.; Dohmen, C.; Reithmeier, T.; Brinker, G.; Strong, A.J.; Dreier, J.P.; Woitzik, J. Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann. Neurol. 2010, 67, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Keyrouz, S.G.; Diringer, M.N. Clinical review: Prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit. Care 2007, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Starke, R.M.; Kim, G.H.; Komotar, R.J.; Hickman, Z.L.; Black, E.M.; Rosales, M.B.; Kellner, C.P.; Hahn, D.K.; Otten, M.L.; Edwards, J. Endothelial Nitric Oxide Synthase Gene Single Nucleotide Polymorphism Predicts Cerebral Vasospasm following Aneurysmal Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2008, 28, 1204–1211. [Google Scholar] [CrossRef]
- Faraco, G.; Moraga, A.; Moore, J.; Anrather, J.; Pickel, V.M.; Iadecola, C. Circulating Endothelin-1 Alters Critical Mechanisms Regulating Cerebral Microcirculation. Hypertension 2013, 62, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.; Ai, J.; Knight, B.; Tariq, A.; Jeon, H.; Shang, X.; Marsden, P.A.; Macdonald, R.L. Uncoupling of Endothelial Nitric Oxide Synthase after Experimental Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2011, 31, 190–199. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Huang, X.; Zhang, Y.; Wang, D.; Wei, H.; Dong, J.; Jiang, R.; Zhang, J. Intravenous transfusion of endothelial colony-forming cells attenuates vascular degeneration after cerebral aneurysm induction. Brain Res. 2014, 1593, 65–75. [Google Scholar] [CrossRef]
- Chang, C.-Z.; Wu, S.-C.; Chang, C.-M.; Lin, C.-L.; Kwan, A.-L. Arctigenin, a Potent Ingredient of Arctium lappa L., Induces Endothelial Nitric Oxide Synthase and Attenuates Subarachnoid Hemorrhage-Induced Vasospasm through PI3K/Akt Pathway in a Rat Model. BioMed Res. Int. 2015, 2015, 490209. [Google Scholar] [CrossRef]
- Cho, H.G.; Shin, H.K.; Shin, Y.W.; Lee, J.H.; Hong, K.W. Role of nitric oxide in the CBF autoregulation during acute stage after subarachnoid haemorrhage in rat pial artery. Fundam. Clin. Pharmacol. 2003, 17, 563–573. [Google Scholar] [CrossRef]
- Maddahi, A.; Ansar, S.; Chen, Q.; Edvinsson, L. Blockade of the MEK/ERK Pathway with a Raf Inhibitor Prevents Activation of Pro-Inflammatory Mediators in Cerebral Arteries and Reduction in Cerebral Blood Flow after Subarachnoid Hemorrhage in a Rat Model. J. Cereb. Blood Flow Metab. 2011, 31, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Vikman, P.; Ansar, S.; Henriksson, M.; Stenman, E.; Edvinsson, L. Cerebral ischemia induces transcription of inflammatory and extracellular-matrix-related genes in rat cerebral arteries. Exp. Brain Res. 2007, 183, 499–510. [Google Scholar] [CrossRef]
- Griessenauer, C.J.; Starke, R.M.; Foreman, P.M.; Hendrix, P.; Harrigan, M.R.; Fisher, W.S.; Vyas, N.A.; Lipsky, R.H.; Lin, M.; Walters, B.C.; et al. Associations between endothelin polymorphisms and aneurysmal subarachnoid hemorrhage, clinical vasospasm, delayed cerebral ischemia, and functional outcome. J. Neurosurg. 2018, 128, 1311–1317. [Google Scholar] [CrossRef]
- Yeung, P.K.; Shen, J.; Chung, S.S.; Chung, S.K. Targeted over-expression of endothelin-1 in astrocytes leads to more severe brain damage and vasospasm after subarachnoid hemorrhage. BMC Neurosci. 2013, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Kassell, N.F.; Mayer, S.; Ruefenacht, D.; Schmiedek, P.; Weidauer, S.; Frey, A.; Roux, S.; Pasqualin, A. CONSCIOUS-1 Investigators Clazosentan to Overcome Neurological Ischemia and Infarction Occurring After Subarachnoid Hemorrhage (CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 2008, 39, 3015–3021. [Google Scholar] [CrossRef]
- Galiè, N.; Olschewski, H.; Oudiz, R.J.; Torres, F.; Frost, A.; Ghofrani, H.A.; Badesch, D.B.; McGoon, M.D.; McLaughlin, V.V.; Roecker, E.B.; et al. Ambrisentan for the Treatment of Pulmonary Arterial Hypertension. Circulation 2008, 117, 3010–3019. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, H.; Chung, Y.; Lee, S.U.; Park, W.; Park, J.C.; Ahn, J.S.; Lee, S. Efficacy of Acetylcysteine and Selenium in Aneurysmal Subarachnoid Hemorrhage Patients: A Prospective, Multicenter, Single Blind Randomized Controlled Trial. J. Korean Med. Sci. 2023, 38, e161. [Google Scholar] [CrossRef]
- Tsai, I.J.; Croft, K.D.; Puddey, I.B.; Beilin, L.J.; Barden, A. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am. J. Physiol. Circ. Physiol. 2011, 300, H1194–H1200. [Google Scholar] [CrossRef]
- Miyata, N.; Seki, T.; Tanaka, Y.; Omura, T.; Taniguchi, K.; Doi, M.; Bandou, K.; Kametani, S.; Sato, M.; Okuyama, S.; et al. Beneficial Effects of a New 20-Hydroxyeicosatetraenoic Acid Synthesis Inhibitor, TS-011 [N-(3-Chloro-4-morpholin-4-yl) Phenyl-N′-hydroxyimido Formamide], on Hemorrhagic and Ischemic Stroke. J. Pharmacol. Exp. Ther. 2005, 314, 77–85. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; de Haan, R.J.; Vermeulen, M.; Roos, Y.B. Statin Treatment and the Occurrence of Hemorrhagic Stroke in Patients With a History of Cerebrovascular Disease. Stroke 2008, 39, 497–502. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; de Haan, R.J.; Vermeulen, M.; Roos, Y.B. Effect of Statin Treatment on Vasospasm, Delayed Cerebral Ischemia, and Functional Outcome in Patients With Aneurysmal Subarachnoid Hemorrhage. Stroke 2010, 41, e47–e52. [Google Scholar] [CrossRef] [PubMed]
- Khurana, V.G.; Smith, L.A.; Baker, T.A.; Eguchi, D.; O’brien, T.; Katusic, Z.S. Protective Vasomotor Effects of In Vivo Recombinant Endothelial Nitric Oxide Synthase Gene Expression in a Canine Model of Cerebral Vasospasm. Stroke 2002, 33, 782–789. [Google Scholar] [CrossRef]
- Zhao, Y.D.; Courtman, D.W.; Deng, Y.; Kugathasan, L.; Zhang, Q.; Stewart, D.J. Rescue of Monocrotaline-Induced Pulmonary Arterial Hypertension Using Bone Marrow–Derived Endothelial-Like Progenitor Cells: Efficacy of combined cell and eNOS gene therapy in established disease. Circ. Res. 2005, 96, 442–450. [Google Scholar] [CrossRef]
- Taneja, G.; Sud, A.; Pendse, N.; Panigrahi, B.; Kumar, A.; Sharma, A.K. Nano-medicine and Vascular Endothelial Dysfunction: Options and Delivery Strategies. Cardiovasc. Toxicol. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Dutta, P.; Hoyer, F.F.; Grigoryeva, L.S.; Sager, H.B.; Leuschner, F.; Courties, G.; Borodovsky, A.; Novobrantseva, T.; Ruda, V.M.; Fitzgerald, K.; et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J. Exp. Med. 2015, 212, 497–512. [Google Scholar] [CrossRef]
- Stoodley, M.; Weihl, C.C.; Zhang, Z.-D.; Lin, G.; Johns, L.M.; Kowalczuk, A.; Ghadge, G.; Roos, R.P.; Macdonald, R.L. Effect of Adenovirus-mediated Nitric Oxide Synthase Gene Transfer on Vasospasm after Experimental Subarachnoid Hemorrhage. Neurosurgery 2000, 46, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Onoue, H.; Tsutsui, M.; Smith, L.; Stelter, A.; O’bRien, T.; Katusic, Z.S. Expression and Function of Recombinant Endothelial Nitric Oxide Synthase Gene in Canine Basilar Artery After Experimental Subarachnoid Hemorrhage. Stroke 1998, 29, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Medana, C.; Di Stilo, A.; Visentin, S.; Fruttero, R.; Gasco, A.; Ghigo, D.; Bosia, A. NO donor and biological properties of different benzofuroxans. Pharm Res. 1999, 16, 956–960. [Google Scholar] [CrossRef]
- Bussygina, O.G.; Pyatakova, N.V.; Khropov, Y.V.; Ovchinnikov, I.V.; Makhova, N.N.; Severina, I.S. Benzodifuroxan as an NO-dependent activator of soluble guanylate cyclase and a novel highly effective inhibitor of platelet aggregation. Biochemistry 2000, 65, 457–462. [Google Scholar] [PubMed]
- Macdonald, R.L. Clazosentan: An endothelin receptor antagonist for treatment of vasospasm after subarachnoid hemorrhage. Expert Opin. Investig. Drugs 2008, 17, 1761–1767. [Google Scholar] [CrossRef]
- Iglarz, M.; Binkert, C.; Morrison, K.; Fischli, W.; Gatfield, J.; Treiber, A.; Weller, T.; Bolli, M.H.; Boss, C.; Buchmann, S.; et al. Pharmacology of Macitentan, an Orally Active Tissue-Targeting Dual Endothelin Receptor Antagonist. J. Pharmacol. Exp. Ther. 2008, 327, 736–745. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Radhakrishnan, J.; Alpers, C.E.; Barratt, J.; Bieler, S.; Diva, U.; Inrig, J.; Komers, R.; Mercer, A.; Noronha, I.L.; et al. Sparsentan in patients with IgA nephropathy: A prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet 2023, 401, 1584–1594. [Google Scholar] [CrossRef]
- Ehlers, J.P. PER-001, a long-acting endothelin antagonist intravitreal implant, improved structure and visual function in patients with diabetic retinopathy. In Proceedings of the 43rd Annual Meeting of the American Society of Retina Specialists, Long Beach, CA, USA, 30 July–2 August 2025. [Google Scholar]
- Froogh, G.; Garcia, V.; Laniado Schwartzman, M. The CYP/20-HETE/GPR75 axis in hypertension. Adv. Pharmacol. 2022, 94, 1–25. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Morsing, P.; Pollock, D.M. Enalapril attenuates endothelin-1-induced hypertension via increased kinin survival. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, 1899–1903. [Google Scholar] [CrossRef]
- Meis, T.; Behr, J. Riociguat for the treatment of pulmonary hypertension. Expert Opin. Pharmacother. 2014, 15, 2419–2427. [Google Scholar] [CrossRef]
- Kehl, F.; Cambj-Sapunar, L.; Maier, K.G.; Miyata, N.; Kametani, S.; Okamoto, H.; Hudetz, A.G.; Schulte, M.L.; Zagorac, D.; Harder, D.R.; et al. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am. J. Physiol. Circ. Physiol. 2002, 282, H1556–H1565. [Google Scholar] [CrossRef]
- Cambj-Sapunar, L.; Yu, M.; Harder, D.R.; Roman, R.J. Contribution of 5-Hydroxytryptamine 1B Receptors and 20-Hydroxyeiscosatetraenoic Acid to Fall in Cerebral Blood Flow After Subarachnoid Hemorrhage. Stroke 2003, 34, 1269–1275. [Google Scholar] [CrossRef]
- Benter, I.; Yousif, M.; Canatan, H.; Akhtar, S. Inhibition of Ca/calmodulin-dependent protein kinase II, RAS-GTPase and 20-hydroxyeicosatetraenoic acid attenuates the development of diabetes-induced vascular dysfunction in the rat carotid artery. Pharmacol. Res. 2005, 52, 252–257. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Mao, S.; Wang, Y.; Cui, X.; Pu, Y. Management of SAH with traditional Chinese medicine in China. Neurol. Res. 2006, 28, 436–444. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, H.-G.; Jin, C.; Yang, S.-B.; Cho, S.-Y.; Park, S.-U.; Jung, W.-S.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; et al. Herbal medicines for the prevention and treatment of cerebral vasospasm after subarachnoid hemorrhage: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e23388. [Google Scholar] [CrossRef]
- Mouratoglou, S.A.; Arvanitaki, A.; Papadopoulos, G.; Souza, R.; Giannakoulas, G. Pulmonary arterial hypertension treatment. A new era. Int. J. Cardiol. Congenit. Heart Dis. 2025, 21, 100594. [Google Scholar] [CrossRef]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2013, 10, 44–58. [Google Scholar] [CrossRef]
- Cho, S.S.; Kim, S.-E.; Kim, H.C.; Kim, W.J.; Jeon, J.P. Clazosentan for Aneurysmal Subarachnoid Hemorrhage: An Updated Meta-Analysis with Trial Sequential Analysis. World Neurosurg. 2019, 123, 418–424.e3. [Google Scholar] [CrossRef]
- Ezra, M.; Franko, E.; Spronk, D.B.; Lamb, C.; Okell, T.W.; Pattinson, K.T. Trial of the cerebral perfusion response to sodium nitrite infusion in patients with acute subarachnoid haemorrhage using arterial spin labelling MRI. Nitric Oxide Biol. Chem. 2024, 153, 50–60. [Google Scholar] [CrossRef]
- Achrol, A.S.; Steinberg, G.K. Personalized Medicine in Cerebrovascular Neurosurgery: Precision Neurosurgical Management of Cerebral Aneurysms and Subarachnoid Hemorrhage. Front. Surg. 2016, 3, 34. [Google Scholar] [CrossRef]
- Durgin, B.G.; Hahn, S.A.; Schmidt, H.M.; Miller, M.P.; Hafeez, N.; Mathar, I.; Freitag, D.; Sandner, P.; Straub, A.C. Loss of smooth muscle CYB5R3 amplifies angiotensin II–induced hypertension by increasing sGC heme oxidation. J. Clin. Investig. 2019, 4, e129183. [Google Scholar] [CrossRef]
- Moustakas, D.; Mani, I.; Pouliakis, A.; Iacovidou, N.; Xanthos, T. The Effects of IRL-1620 in Post-ischemic Brain Injury: A Systematic Review and Meta-analysis of Experimental Studies. Neurocritical Care 2024, 41, 665–680. [Google Scholar] [CrossRef]
- Abu-Soud, H.M.; Presta, A.; Mayer, B.; Stuehr, D.J. Analysis of Neuronal NO Synthase under Single-Turnover Conditions: Conversion of Nω-Hydroxyarginine to Nitric Oxide and Citrulline. Biochemistry 1997, 36, 10811–10816. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef]
- van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef]
- Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 11522. [Google Scholar] [CrossRef]
- Fathi, A.R.; Pluta, R.M.; Bakhtian, K.D.; Qi, M.; Lonser, R.R. Reversal of cerebral vasospasm via intravenous sodium nitrite after subarachnoid hemorrhage in primates: Laboratory investigation. J. Neurosurg. 2011, 115, 1213–1220. [Google Scholar] [CrossRef]
- Oldfield, E.H.; Loomba, J.J.; Monteith, S.J.; Crowley, R.W.; Medel, R.; Gress, D.R.; Kassell, N.F.; Dumont, A.S.; Sherman, C. Safety and pharmacokinetics of sodium nitrite in patients with subarachnoid hemorrhage: A Phase IIA study. J. Neurosurg. 2013, 119, 634–641. [Google Scholar] [CrossRef]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary Inorganic Nitrate Improves Mitochondrial Efficiency in Humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef]
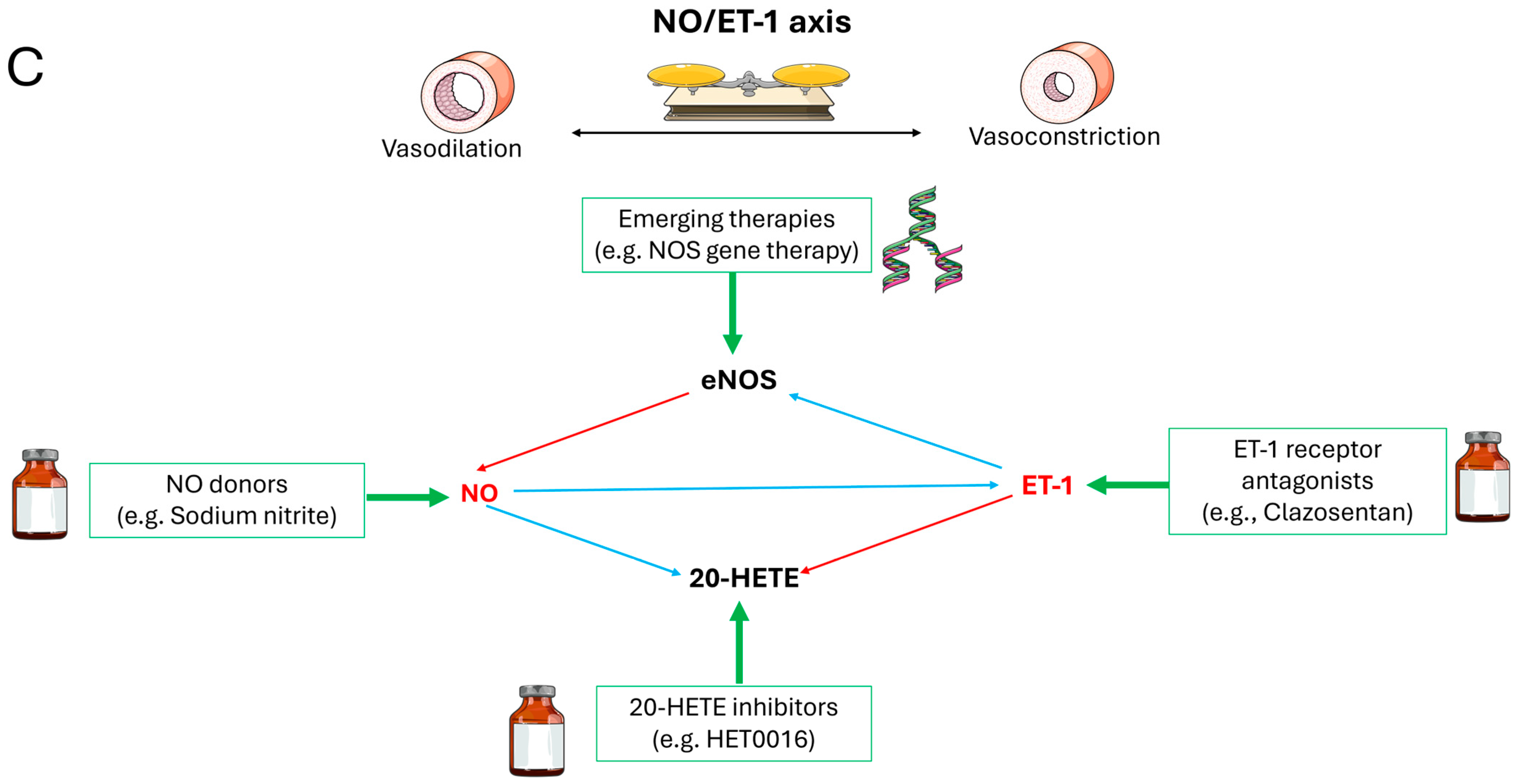
| Strategy | Agents | Mechanism | Ref. |
|---|---|---|---|
| NO Donors | Sodium nitrite, L-Arginine | Hypoxia-triggered NO release; sGC activation | Østergaard L et al., 2013 [14]; Lilla N et al., 2016 [57] |
| ET-1 Antagonists | Clazosentan, Bosentan | Selective ETA blockade → ROS/eNOS uncoupling reversal | Macdonald RL et al., 2008 [91]; Galiè N et al., 2008 [92] |
| NO Bioavailability Enhancers | NAC, Glutathione | Scavenge ROS → reduce NO degradation, potentially reversing eNOS uncoupling | Kim M et al., 2023 [93] |
| Multi-Target Agents | HET0016, TS-011 | Multi-component synergy → restore NO-cGMP signaling, NO synthesis↑ | Tsai IJ et al., 2011 [94]; Miyata N et al., 2005 [95] |
| Adjuvant Therapies | Statins, Erythropoietin | Akt-eNOS phosphorylation → NO synthesis ↑ | Vergouwen MD et al., 2008 [96]; Vergouwen MD et al., 2010 [97] |
| Emerging Approaches | NOS gene therapy, Nanocarriers | Targeted eNOS delivery/activation | Khurana VG et al., 2002 [98]; Zhao YD et al., 2005 [99]; Taneja G et al., 2019 [100] |
| Drug Class | Representatives | Mechanism | Ref. |
|---|---|---|---|
| ACEi/ARBs | Enalapril | Inhibit Ang II → ↓ET-1 synthesis + ↑NO release | Elmarakby AA et al., 2003 [111] |
| sGC Stimulators | Riociguat | Directly activate sGC (NO-independent) → ↑cGMP | Meis T et al., 2014 [112] |
| 20-HETE Inhibitors | HET0016, TS-011 | Inhibit CYP4A → restore NO-cGMP signaling, reverse pericyte constriction | Kehl F et al., 2002 [113]; Cambj-Sapunar L et al., 2003 [114]; Tsai IJ et al., 2011 [94]; Benter IF et al., 2005 [115]; Takeuchi K et al., 2005 [44] |
| Herbal Formulations | Ligusticum chuanxiong | Multi-component synergy: ↓ET-1/IL-5 + ↑NO | Wang C et al., 2006 [116]; Seo Y et al., 2020 [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, K.; Lu, K. Bidirectional Regulation of Nitric Oxide and Endothelin-1 in Cerebral Vasospasm: Mechanisms and Therapeutic Perspectives. Future Pharmacol. 2025, 5, 59. https://doi.org/10.3390/futurepharmacol5040059
Becker K, Lu K. Bidirectional Regulation of Nitric Oxide and Endothelin-1 in Cerebral Vasospasm: Mechanisms and Therapeutic Perspectives. Future Pharmacology. 2025; 5(4):59. https://doi.org/10.3390/futurepharmacol5040059
Chicago/Turabian StyleBecker, Katrin, and Kaihui Lu. 2025. "Bidirectional Regulation of Nitric Oxide and Endothelin-1 in Cerebral Vasospasm: Mechanisms and Therapeutic Perspectives" Future Pharmacology 5, no. 4: 59. https://doi.org/10.3390/futurepharmacol5040059
APA StyleBecker, K., & Lu, K. (2025). Bidirectional Regulation of Nitric Oxide and Endothelin-1 in Cerebral Vasospasm: Mechanisms and Therapeutic Perspectives. Future Pharmacology, 5(4), 59. https://doi.org/10.3390/futurepharmacol5040059






