Unveiling Biocompatibility: Comprehensive Study on Epoxy–Polyetheramine-Based Polymeric Nanogels in CHO-K1 Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
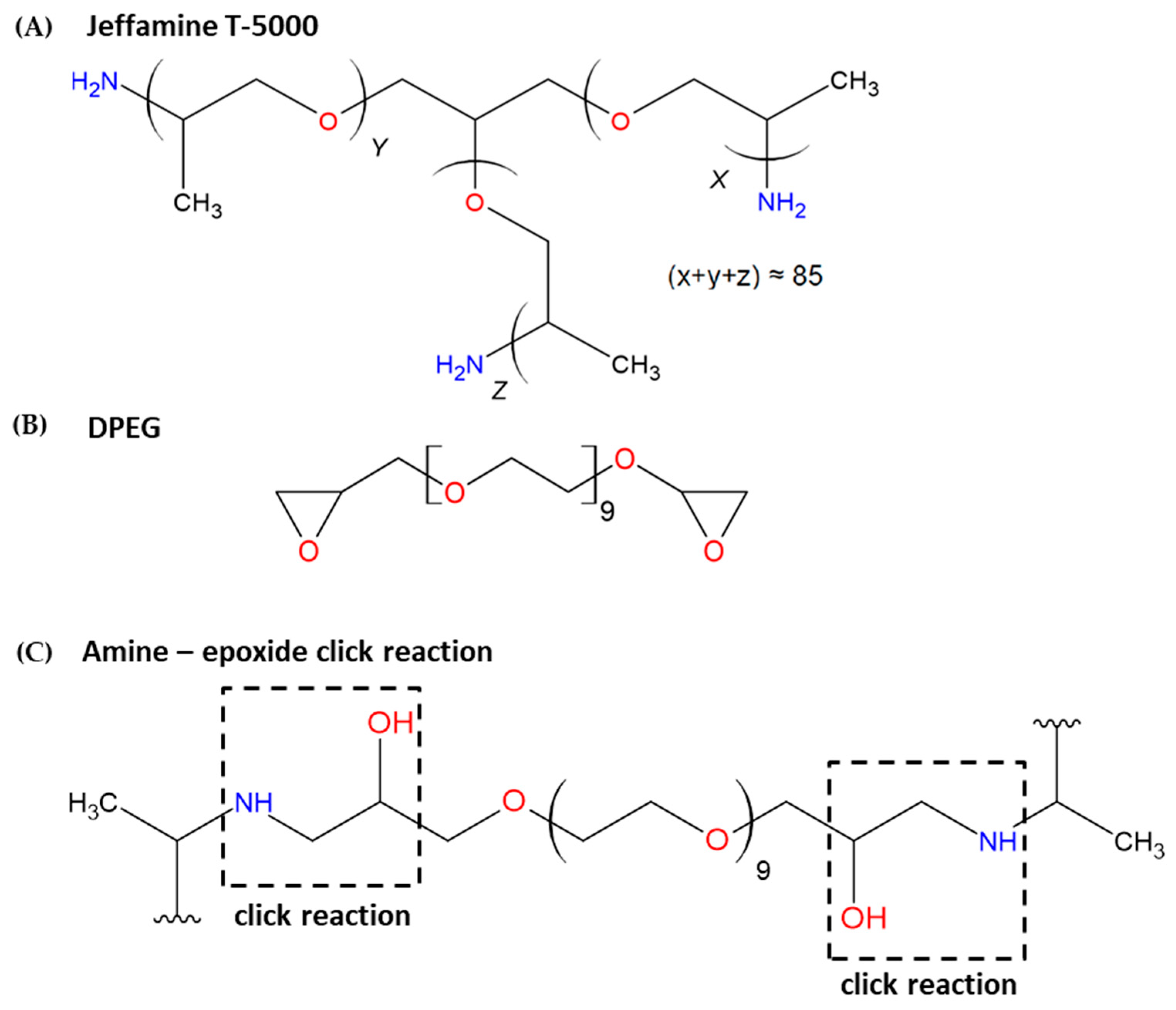
2.2. Synthesis of the Polyetheramine–Epoxide Nanogels (PPO–DPEG)
2.3. Characterization of the Polymeric Nanogels
2.4. Transmission Electron Microscopy (TEM)
2.5. Cell Cultures
2.6. Cell Viability—XTT
2.7. Clonogenic Assay
2.8. Trypan Blue Exclusion Test
2.9. Cytokinesis-Blocked Micronucleus Assay (CBMN)
2.10. Single Cell Gel Electrophoresis Assay
2.11. Statistical Analysis
3. Results
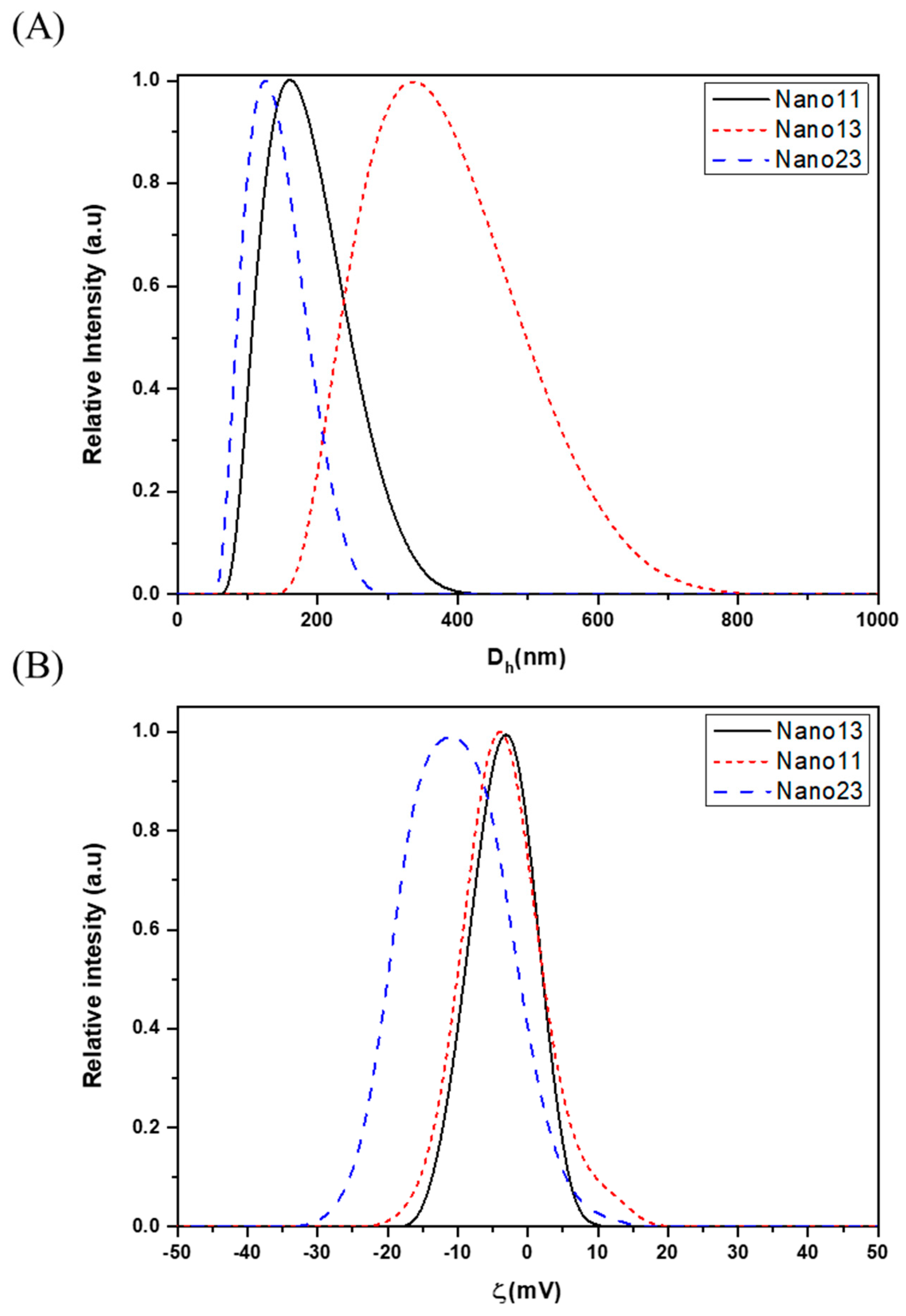
3.1. Characterization
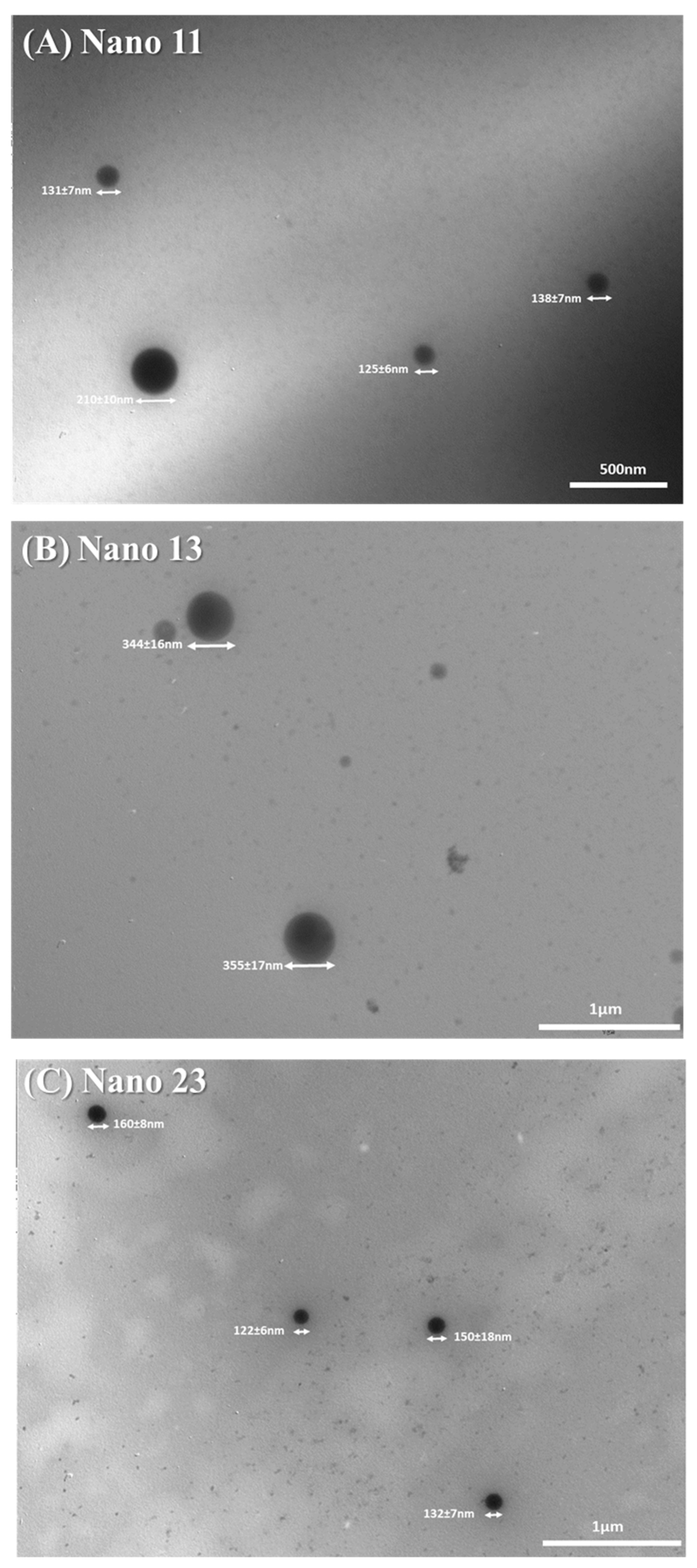
3.2. Morphological Analysis of Nanogels by TEM
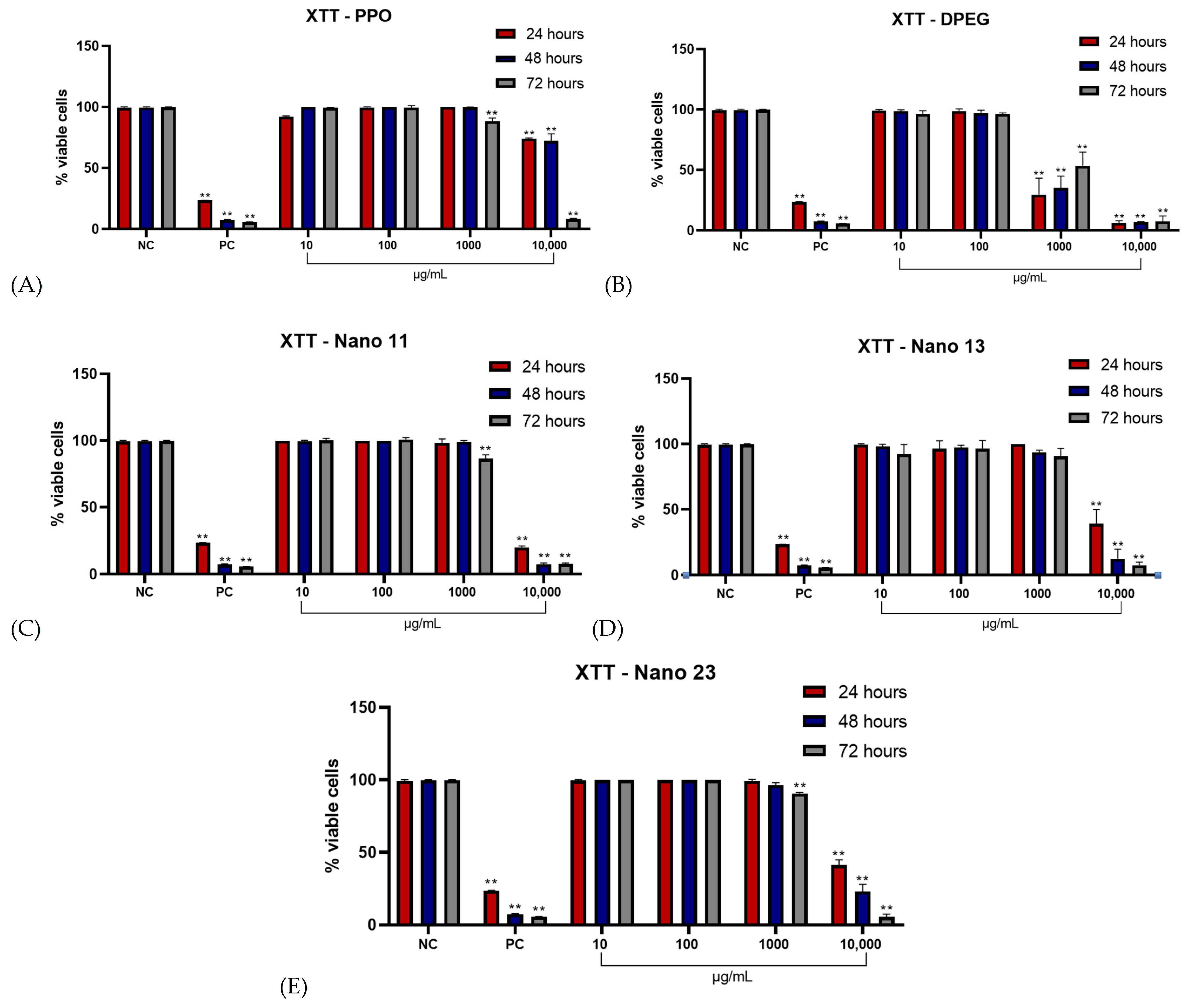
3.3. Effect of Nanogels on CHO-K1 Cell Viability (XTT Assay)
3.4. Long-Term Cell Survival and Proliferative Capacity
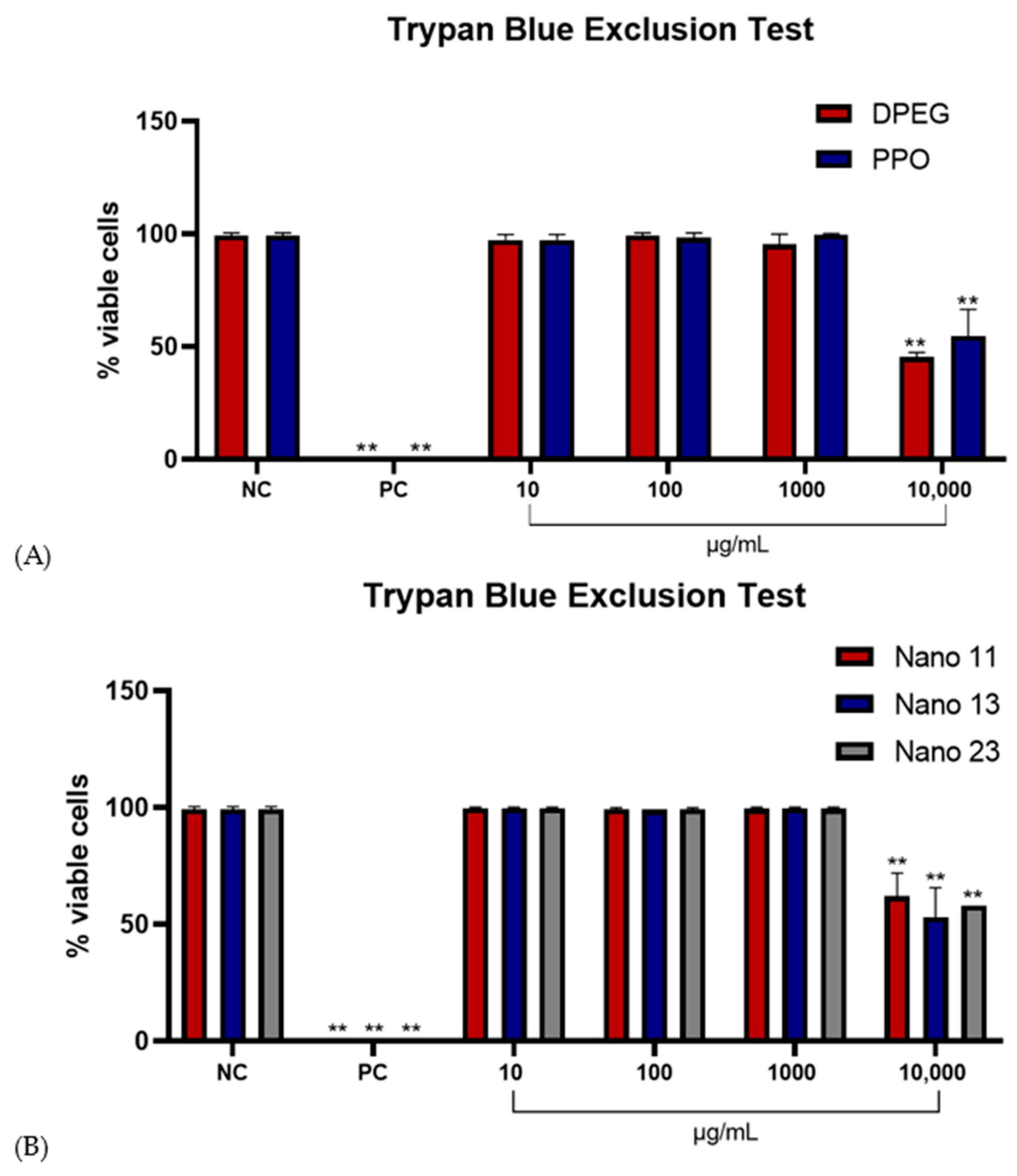
3.5. Effect of Nanogels on CHO-K1 Cell Viability (Trypan Blue Exclusion Test)
3.6. Evaluation of Genotoxic Effects by Micronucleus Formation
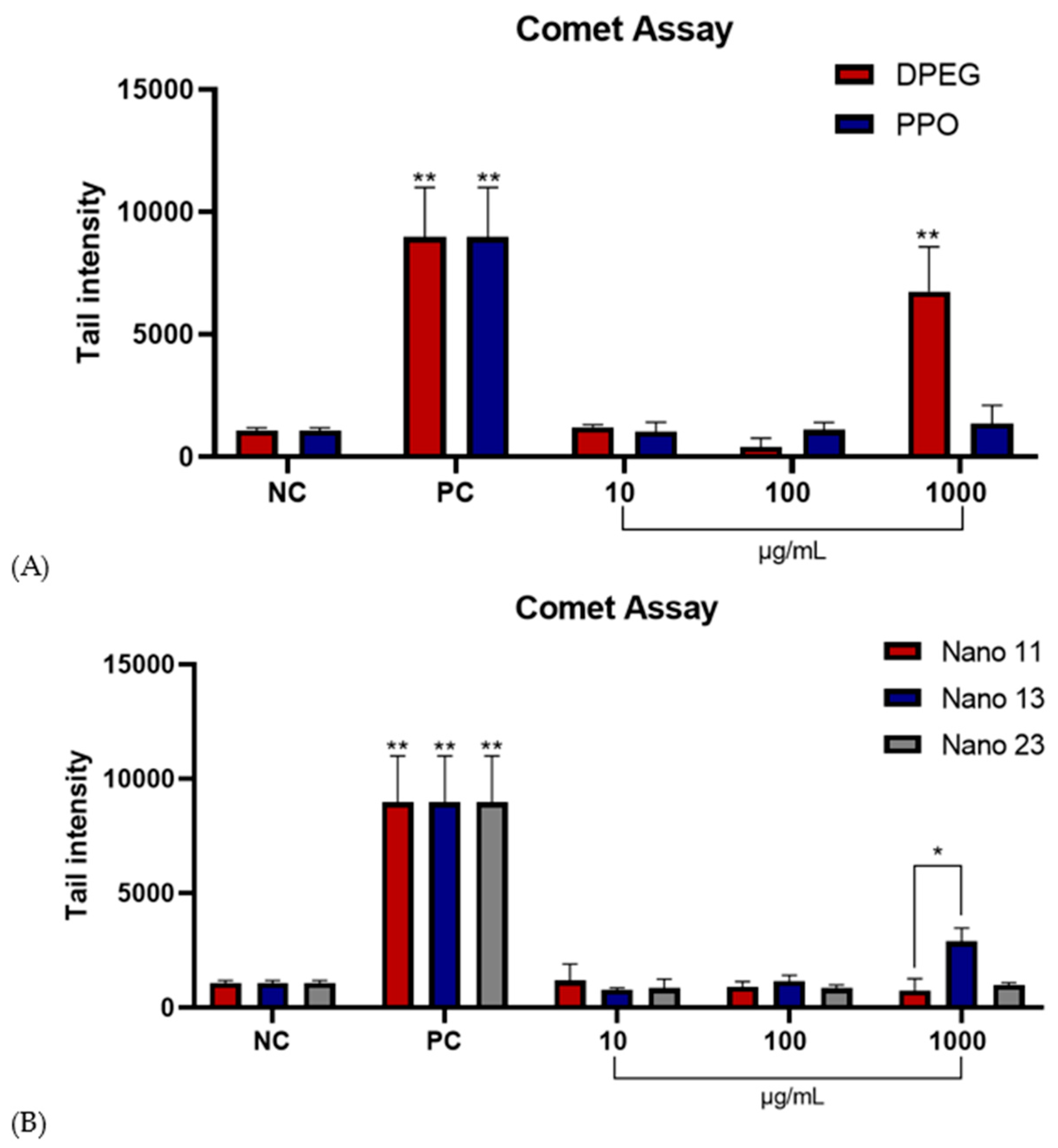
3.7. Comet Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIN | Binucleated Cells |
| CHO-K1 | Chinese Hamster Ovary Cells |
| Dh | Hydrodynamic Diameter |
| DLS | Dynamic Light Scattering |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| DOX | Doxorubicin |
| DPEG | Diepoxy Poly(ethylene glycol) |
| MN | Micronucleus |
| MON | Mononucleated Cells |
| NC | Negative Control |
| NDI | Nuclear Division Index |
| NHK | Normal Human Keratinocytes |
| OECD | Organisation for Economic Co-operation and Development |
| PC | Positive Control |
| PDI | Polydispersity Index |
| TEM | Transmission Electron Microscopy |
| TRN | Trinucleated Cells |
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Silva, P.; Bonifácio, B.; Ramos, M.; Negri, K.; Maria Bauab, T.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems and Herbal Medicines: A Review. Int. J. Nanomed. 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Brown, S.; Kumar, S.; Sharma, B. Intra-Articular Targeting of Nanomaterials for the Treatment of Osteoarthritis. Acta Biomater. 2019, 93, 239–257. [Google Scholar] [CrossRef]
- Manivong, S.; Garcia Ac, A.; Patten, S.A.; Fernandes, J.C.; Benderdour, M.; Banquy, X.; Roullin, V.G. Chitosan-Based Nanogels: Synthesis and Toxicity Profile for Drug Delivery to Articular Joints. Nanomaterials 2022, 12, 1337. [Google Scholar] [CrossRef]
- Hussain AL-Mayahy, M.; Imad Hameed, H. Hydrogels and Nanogels as a Promising Carrier for Drug Delivery. In Hydrogels and Nanogels-Applications in Medicine; Umeyor, C., Uronnachi, E., Kakade, P., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Ludwig, J.D.C.; Grigoletto, D.F.; Renzi, D.F.; Abraham, W.-R.; De Paula, D.; Khalil, N.M. Hydrogels and Nanogels: Effectiveness in Dermal Applications. Beilstein J. Nanotechnol. 2025, 16, 1216–1233. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Blagojevic, L.; Kamaly, N. Nanogels: A Chemically Versatile Drug Delivery Platform. Nano Today 2025, 61, 102645. [Google Scholar] [CrossRef]
- Kubeil, M.; Suzuki, Y.; Casulli, M.A.; Kamal, R.; Hashimoto, T.; Bachmann, M.; Hayashita, T.; Stephan, H. Exploring the Potential of Nanogels: From Drug Carriers to Radiopharmaceutical Agents. Adv. Healthc. Mater. 2024, 13, 2301404. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as Potential Drug Nanocarriers for CNS Drug Delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Castan, L.; José da Silva, C.; Ferreira Molina, E.; Alves dos Santos, R. Comparative Study of Cytotoxicity and Genotoxicity of Commercial Jeffamines® and Polyethylenimine in CHO-K1 Cells. J. Biomed. Mater. Res. Part B 2018, 106, 742–750. [Google Scholar] [CrossRef]
- Pinelli, F.; Pizzetti, F.; Ortolà, Ó.F.; Marchetti, A.; Rossetti, A.; Sacchetti, A.; Rossi, F. Influence of the Core Formulation on Features and Drug Delivery Ability of Carbamate-Based Nanogels. Int. J. Mol. Sci. 2020, 21, 6621. [Google Scholar] [CrossRef]
- Van Le, H.; Dulong, V.; Picton, L.; Le Cerf, D. Thermoresponsive Nanogels Based on Polyelectrolyte Complexes between Polycations and Functionalized Hyaluronic Acid. Carbohydr. Polym. 2022, 292, 119711. [Google Scholar] [CrossRef]
- Andrada, H.E.; Fico, B.A.; Alves, F.B.; Paulino, J.M.; Silveira, N.N.; Dos Santos, R.A.; Molina, E.F.; José da Silva, C.; Ferreira Molina, E. Multifunctional Polyetheramine—Epoxide Gels and Their Prospective Applications in Health and Agriculture. New J. Chem. 2024, 48, 703–711. [Google Scholar] [CrossRef]
- Stropoli, S.J.; Elrod, M.J. Assessing the Potential for the Reactions of Epoxides with Amines on Secondary Organic Aerosol Particles. J. Phys. Chem. A 2015, 119, 10181–10189. [Google Scholar] [CrossRef]
- Hobson, D.W. Nanotoxicology: The Toxicology of Nanomaterials and Nanostructures. Int. J. Toxicol. 2016, 35, 3–4. [Google Scholar] [CrossRef]
- Alves, F.B.; Andrada, H.E.; Fico, B.A.; Reinaldi, J.S.; Tavares, D.C.; Squarisi, I.S.; Montanha, G.S.; Nuevo, L.G.; De Carvalho, H.W.P.; Pérez, C.A.; et al. Facilitating Seed Iron Uptake through Amine-Epoxide Microgels: A Novel Approach to Enhance Cucumber (Cucumis Sativus) Germination. J. Agric. Food Chem. 2024, 72, 14570–14580. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Boyd, M.R. Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture Using Human and Other Tumor Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic Assay of Cells In Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development. Test No. 487: In Vitro Mammalian Cell Micronucleus Test; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Mejía-Jaramillo, A.M.; Gómez-Hoyos, C.; Cañas Gutierrez, A.I.; Correa-Hincapié, N.; Zuluaga Gallego, R.; Triana-Chávez, O. Tackling the Cytotoxicity and Genotoxicity of Cellulose Nanofibers from the Banana Rachis: A New Food Packaging Alternative. Heliyon 2023, 9, e21560. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Bhattacharjee, S. In Relation to the Following Article “DLS and Zeta Potential—What They Are and What They Are Not?”. J. Control. Release 2016, 238, 311–312. [Google Scholar] [CrossRef]
- Roehm, N.W.; Rodgers, G.H.; Hatfield, S.M.; Glasebrook, A.L. An Improved Colorimetric Assay for Cell Proliferation and Viability Utilizing the Tetrazolium Salt XTT. J. Immunol. Methods 1991, 142, 257–265. [Google Scholar] [CrossRef]
- Rojas, E.; Lopez, M.C.; Valverde, M. Single Cell Gel Electrophoresis Assay: Methodology and Applications. J. Chromatogr. B 1999, 722, 225–254. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New Progress and Prospects: The Application of Nanogel in Drug Delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef]
- Sharma, A.; Garg, T.; Aman, A.; Panchal, K.; Sharma, R.; Kumar, S.; Markandeywar, T. Nanogel—An Advanced Drug Delivery Tool: Current and Future. Artif. Cells Nanomed. Biotechnol. 2016, 44, 165–177. [Google Scholar] [CrossRef]
- Guerrero-Ramírez, L.G.; Nuno-Donlucas, S.M.; Cesteros, L.C.; Katime, I. Smart Copolymeric Nanohydrogels: Synthesis, Characterization and Properties. Mater. Chem. Phys. 2008, 112, 1088–1092. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The Development of Microgels/Nanogels for Drug Delivery Applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Samah, N.A.; Williams, N.; Heard, C.M. Nanogel Particulates Located within Diffusion Cell Receptor Phases Following Topical Application Demonstrates Uptake into and Migration across Skin. Int. J. Pharm. 2010, 401, 72–78. [Google Scholar] [CrossRef]
- Sawada, S.I.; Sasaki, Y.; Nomura, Y.; Akiyoshi, K. Cyclodextrin-Responsive Nanogel as an Artificial Chaperone for Horseradish Peroxidase. Colloid Polym. Sci. 2011, 289, 685–691. [Google Scholar] [CrossRef]
- Garg, T.; Singh, S.; Goyal, A. Stimuli-Sensitive Hydrogels: An Excellent Carrier for Drug and Cell Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 429–464. [Google Scholar] [CrossRef]
- Garg, T.; Goyal, A.K. Biomaterial-Based Scaffolds—Current Status and Future Directions. Expert Opin. Drug Deliv. 2014, 11, 767–789. [Google Scholar] [CrossRef]
- Soni, S.; Ruhela, R.K.; Medhi, B. Nanomedicine in Central Nervous System (CNS) Disorders: A Present and Future Prospective. Adv. Pharm. Bull. 2016, 6, 319–330. [Google Scholar] [CrossRef]
- He, L.; Liang, H.; Lin, L.; Shah, B.R.; Li, Y.; Chen, Y.; Li, B. Green-Step Assembly of Low Density Lipoprotein/Sodium Carboxymethyl Cellulose Nanogels for Facile Loading and pH-Dependent Release of Doxorubicin. Colloids Surf. B 2015, 126, 288–296. [Google Scholar] [CrossRef]
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.; Neumann, F.; Meinke, M.C.; et al. Biocompatibility and Characterization of Polyglycerol-Based Thermoresponsive Nanogels Designed as Novel Drug-Delivery Systems and Their Intracellular Localization in Keratinocytes. Nanotoxicology 2017, 11, 267–277. [Google Scholar] [CrossRef]
- Ansari, M.M.; Ahmad, A.; Mishra, R.K.; Raza, S.S.; Khan, R. Zinc Gluconate-Loaded Chitosan Nanoparticles Reduce Severity of Collagen-Induced Arthritis in Wistar Rats. ACS Biomater. Sci. Eng. 2019, 5, 3380–3397. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer Nanogels: A Versatile Nanoscopic Drug Delivery Platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The Shape of Things to Come: Importance of Design in Nanotechnology for Drug Delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Sharma, N. Precision Nanotoxicology in Drug Development: Current Trends and Challenges in Safety and Toxicity Implications of Customized Multifunctional Nanocarriers for Drug-Delivery Applications. Pharmaceutics 2022, 14, 2463. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, S.; Kumar, V. Challenges for Assessing Toxicity of Nanomaterials. In Biochemical Toxicology-Heavy Metals and Nanomaterials; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Vyas, B.; Pillai, S.A.; Bahadur, P. Influence of Surfactant’s Polar Head Group Charge on the Self-Assembly of Three PEO–PPO–PEO Triblock Copolymers of Widely Varying Hydrophobicity. J. Mol. Liq. 2020, 316, 113858. [Google Scholar] [CrossRef]
- Bar, H.; Bianco-Peled, H. Modification of Shellac Coating Using Jeffamine® for Enhanced Mechanical Properties and Stability. Prog. Org. Coat. 2020, 141, 105559. [Google Scholar] [CrossRef]
- Pashirova, T.; Afonso, A.; Terekhova, N.; Kamalov, M.; Masson, P.; Souto, E.B. Nanogels for Drug Delivery: Physicochemical Properties, Biological Behavior, and In Vivo Applications; Academic Press: Cambridge, MA, USA, 2023; pp. 95–131. [Google Scholar] [CrossRef]
- Zalipsky, S. Chemistry of Polyethylene Glycol Conjugates with Biologically Active Molecules. Adv. Drug Deliv. Rev. 1995, 16, 157–182. [Google Scholar] [CrossRef]
- Zalba, S.; Ten Hagen, T.L.M.; Burgui, C.; Garrido, M.J. Stealth Nanoparticles in Oncology: Facing the PEG Dilemma. J. Control. Release 2022, 351, 22–36. [Google Scholar] [CrossRef]
- Gaballa, S.; Naguib, Y.; Mady, F.; Khaled, K. Polyethylene Glycol: Properties, Applications, and Challenges. J. Adv. Biomed. Pharm. Sci. 2023, 7, 26–36. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Shin, C.Y.; Kim, K.-B. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef]
- Paderes, M.C.; James, C.; Jamieson, S.A.; Mai, A.H.; Limon, J.H.; Dolatkhani, M.; Fernandez-Prieto, S.; De Borggraeve, W.M.; Fratini, E. Tuning the Properties of Polyether Alkyl Urea Derivatives as Rheology Modifiers in Cosmetic Solvents. ACS Appl. Polym. Mater. 2020, 2, 2902–2909. [Google Scholar] [CrossRef]




| PPO | DPEG | |
|---|---|---|
| Nano 11 | 0.90 g | 0.10 g |
| Nano 13 | 0.77 g | 0.23 g |
| Nano 23 | 0.87 g | 0.23 g |
| Sample | Dh (nm) | PDI | ζ (mV) |
|---|---|---|---|
| Nano11 | 170 ± 8 | 0.20 | −3 ± 1 |
| Nano13 | 350 ± 17 | 0.07 | −5 ± 2 |
| Nano23 | 130 ± 6 | 0.25 | −13 ± 5 |
| Treatments | Micronucleus Test—CHO-K1 | ||
|---|---|---|---|
| NDI/1000 Cells | Binucleated Cells with MN | MN/1000 Cells | |
| NC | 1.88 ± 0.01 | 1.7 ± 0.17 | 1.63 ± 0.21 |
| PC | 1.16 ± 0.04 ** | 14 ± 0.28 ** | 12.5 ± 0.32 ** |
| PPO 10 | 1.86 ± 0.006 | 2.30 ± 0.45 | 2.10 ± 0.30 |
| PPO 100 | 1.82 ± 0.01 | 2.53 ± 0.31 | 2.33 ± 0.27 |
| DPEG 10 | 1.73 ± 0.005 | 2.13 ± 0.03 | 1.93 ± 0.12 |
| DPEG 100 | 1.72 ± 0.03 | 5.86 ± 0.61 ** | 5.4 ± 0.58 ** |
| Nano11 10 | 1.90 ± 0.05 | 2.20 ± 0.45 | 2.10 ± 0.50 |
| Nano11 100 | 1.81 ± 0.06 | 2.86 ± 1.41 | 2.56 ± 1.16 |
| Nano13 10 | 1.83 ± 0.03 | 1.63 ± 0.14 | 1.46 ± 0.88 |
| Nano13 100 | 1.78 ± 0.04 | 2.23 ± 0.63 | 2.13 ± 0.53 |
| Nano23 10 | 1.89 ± 0.01 | 1.30 ± 0.20 | 1.2 ± 0.15 |
| Nano23 100 | 1.77 ± 0.03 | 1.53 ± 0.17 | 1.43 ± 0.17 |
| Treatments | Micronucleus Test—CHO-K1 | |
|---|---|---|
| Binucleated Cells with MN | MN/1000 Cells | |
| PPO 100 | 2.53 ± 0.31 * | 2.33 ± 0.27 * |
| DPEG 100 | 5.86 ± 0.61 | 5.40 ± 0.58 |
| Nano11 100 | 2.86 ± 1.41 | 2.56 ± 1.16 * |
| Nano13 100 | 2.23 ± 0.63 * | 2.13 ± 0.53 * |
| Nano23 100 | 1.53 ± 0.17 * | 1.43 ± 0.17 ** |
| Nanogel | Experimental Model | Assays | Results | Reference |
|---|---|---|---|---|
| Lipoprotein/carboxymethylcellulose sodium nanogels | HeLa and HepG2 cell lines (in vitro) | MTT assay | No damage at the highest tested concentration (8 μg/mL) | [42] |
| Thermoresponsive polyglycerol-based nanogels (tNG_dPG_tP and tNG_dPG_pNIPAM) | NHK cell line (in vitro) | MTT assay, comet assay, and Carboxy-H2DCFDA test | No damage at the highest tested concentration (500 μg/mL) | [43] |
| Chitosan-based nanogels | rTERT-BJ and L-929 cell lines (in vitro) | MTT assay | No damage at the highest tested concentration (400 μg/mL) | [45] |
| PEG-PEI, PE-PEI, and Plu-PEI nanogels | Microglia culture (in vitro) | MTS and LDH assays | No damage at the highest tested concentration (0.05 w/v) | [15] |
| Chitosan-based nanogels | Chondrocytes, synoviocytes, and osteoblasts (in vitro) Zebrafish (in vivo) | MTS, LDH, nitric oxide quantification, DNA drag test, and zebrafish embryo assay | Did not affect cell proliferation or nitric oxide production in vitro but exhibited moderate genotoxicity in a dose-dependent manner, without inducing embryotoxicity | [6] |
| Polyetheramine–epoxide-based nanogels | GM07492-A (in vitro) | XTT assay | No damage up to a concentration of 1000 μg/mL | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, N.N.; Andrada, H.E.; Paulino, J.M.; Boaretto, N.C.d.S.; Molina, E.F.; dos Santos, R.A. Unveiling Biocompatibility: Comprehensive Study on Epoxy–Polyetheramine-Based Polymeric Nanogels in CHO-K1 Cell Line. Future Pharmacol. 2025, 5, 54. https://doi.org/10.3390/futurepharmacol5030054
Silveira NN, Andrada HE, Paulino JM, Boaretto NCdS, Molina EF, dos Santos RA. Unveiling Biocompatibility: Comprehensive Study on Epoxy–Polyetheramine-Based Polymeric Nanogels in CHO-K1 Cell Line. Future Pharmacology. 2025; 5(3):54. https://doi.org/10.3390/futurepharmacol5030054
Chicago/Turabian StyleSilveira, Natalia Nascimento, Heber Eduardo Andrada, Julia Mirian Paulino, Naiara Cristina da Silva Boaretto, Eduardo Ferreira Molina, and Raquel Alves dos Santos. 2025. "Unveiling Biocompatibility: Comprehensive Study on Epoxy–Polyetheramine-Based Polymeric Nanogels in CHO-K1 Cell Line" Future Pharmacology 5, no. 3: 54. https://doi.org/10.3390/futurepharmacol5030054
APA StyleSilveira, N. N., Andrada, H. E., Paulino, J. M., Boaretto, N. C. d. S., Molina, E. F., & dos Santos, R. A. (2025). Unveiling Biocompatibility: Comprehensive Study on Epoxy–Polyetheramine-Based Polymeric Nanogels in CHO-K1 Cell Line. Future Pharmacology, 5(3), 54. https://doi.org/10.3390/futurepharmacol5030054







