The Effect of Co-Administration of Levetiracetam or Brivaracetam with Ethanol on the Associative Learning and Anxiety Level of Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Ethanol Administration
2.3. Behavioral Testing
2.3.1. The Passive Avoidance Test
2.3.2. Contextual Fear Conditioning
2.3.3. Cued Fear Conditioning
2.3.4. Elevated Plus Maze Test
2.4. Data Analysis
3. Results
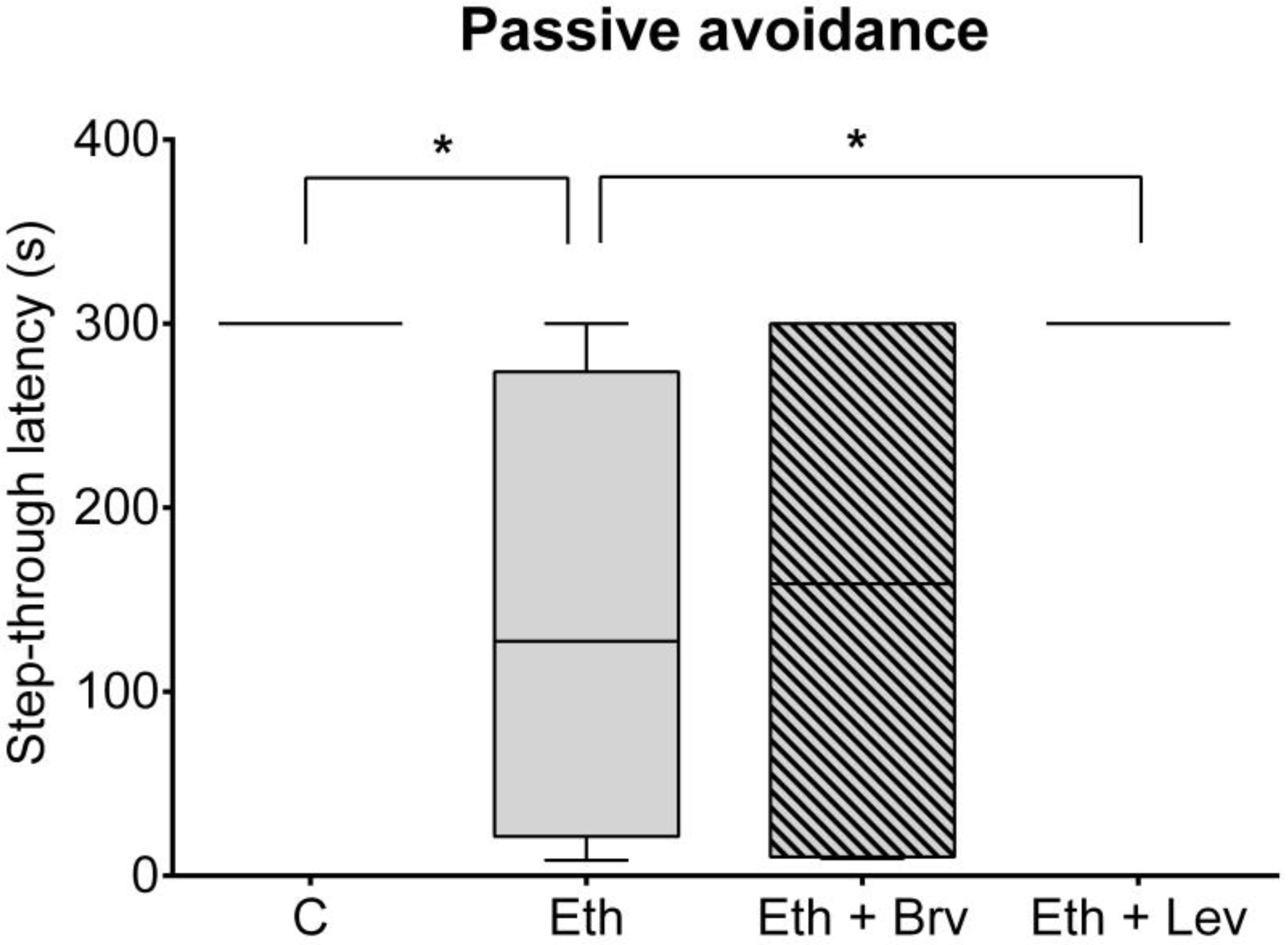
3.1. The Effect of Levetiracetam or Brivaracetam Co-Administered with Ethanol on Associative Memory in the Passive Avoidance Test
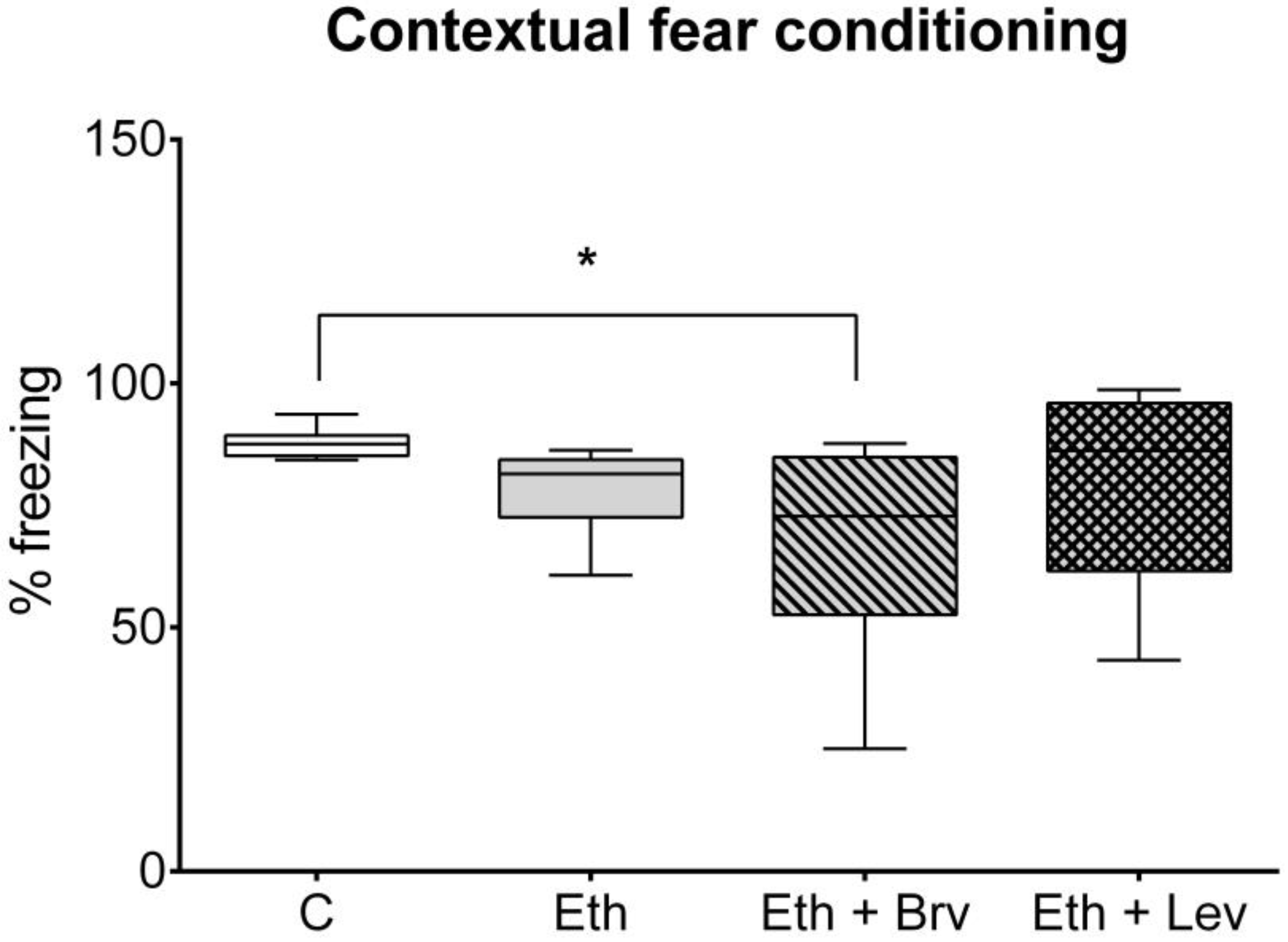
3.2. The Effect of Levetiracetam or Brivaracetam on Associative Memory in the Contextual Fear Conditioning Test 24 h After the Discontinuation of Ethanol Administration
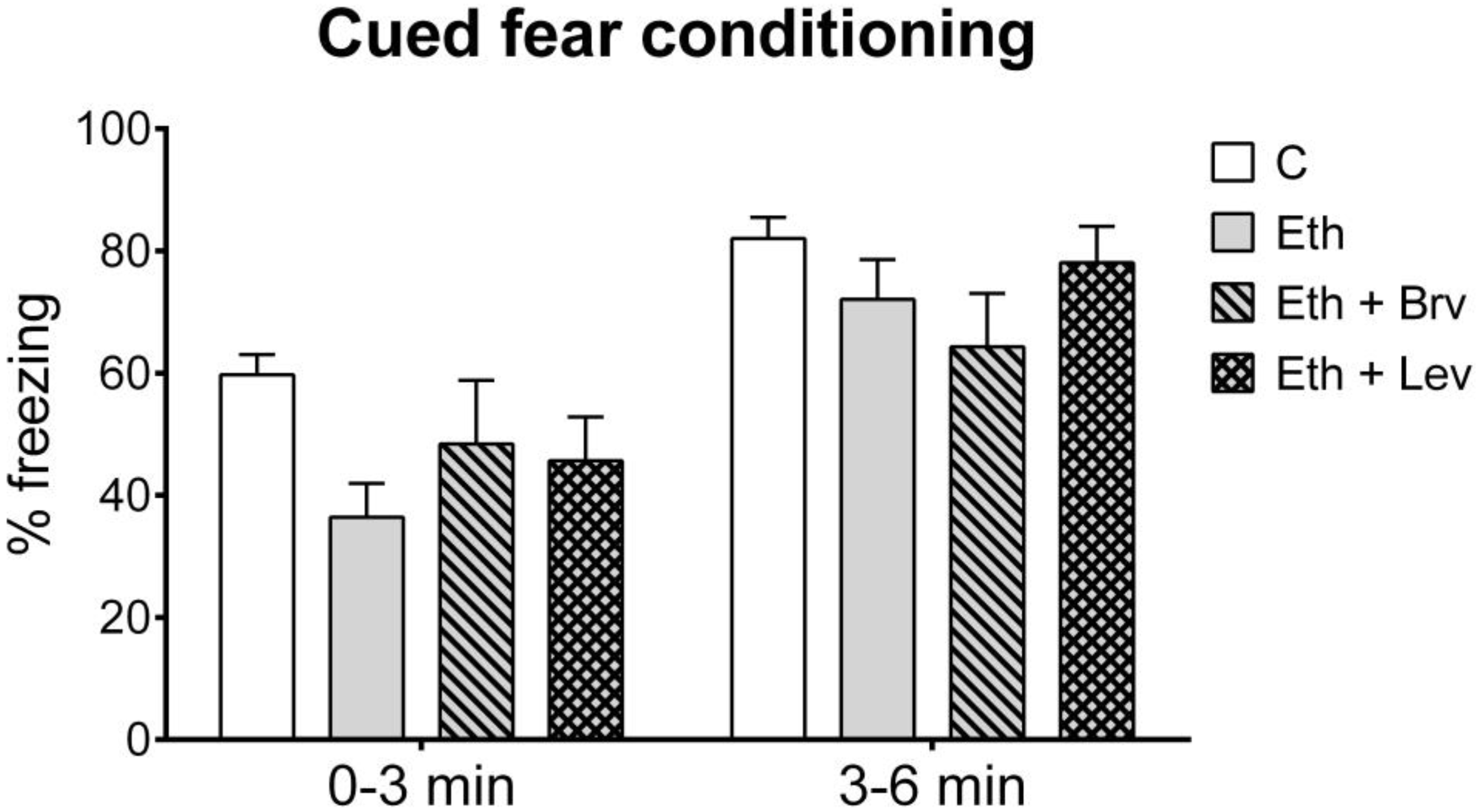
3.3. The Effect of Levetiracetam or Brivaracetam on Associative Memory in the Cued Fear Conditioning Test 24 h After the Discontinuation of Ethanol Administration
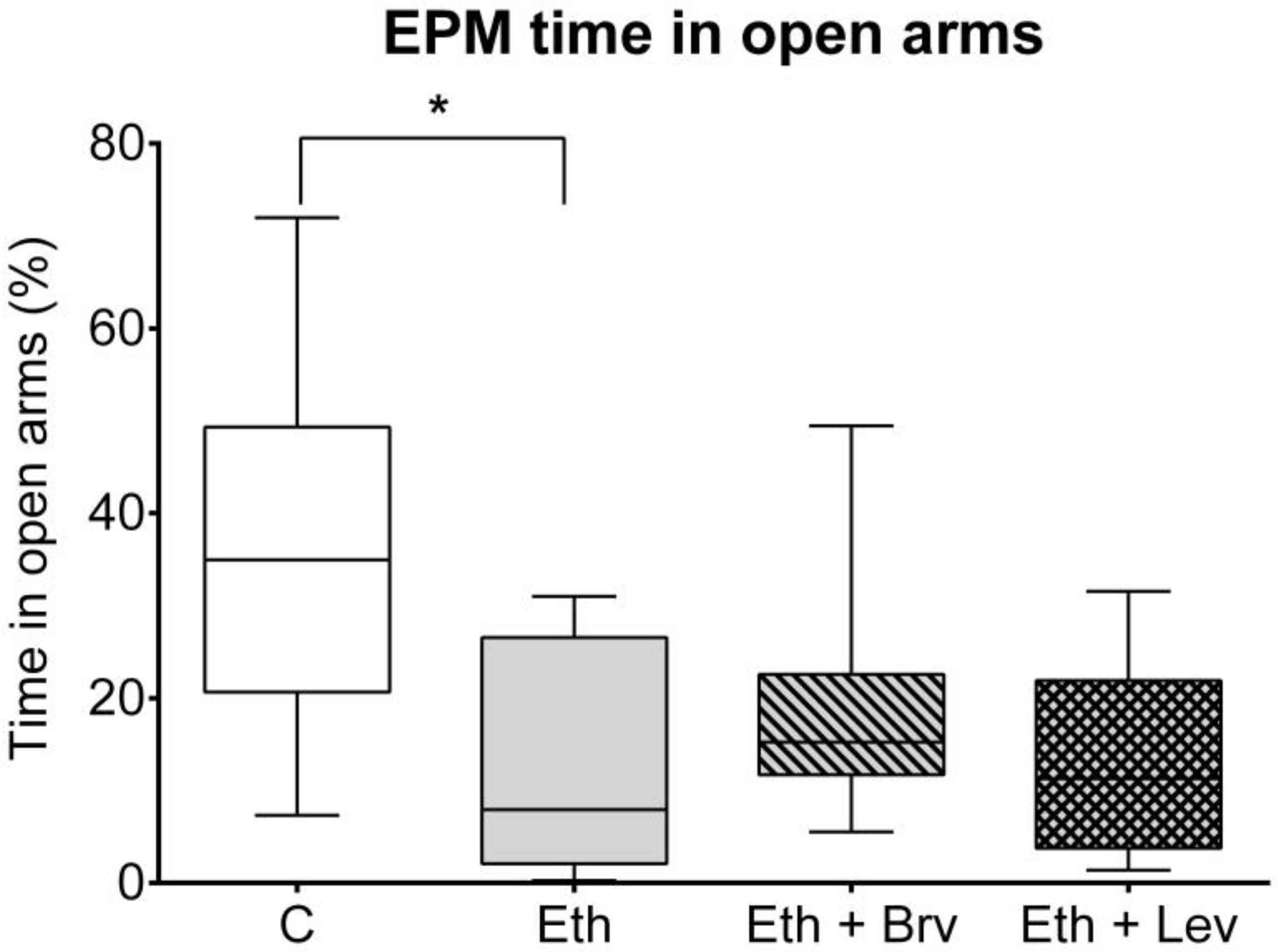
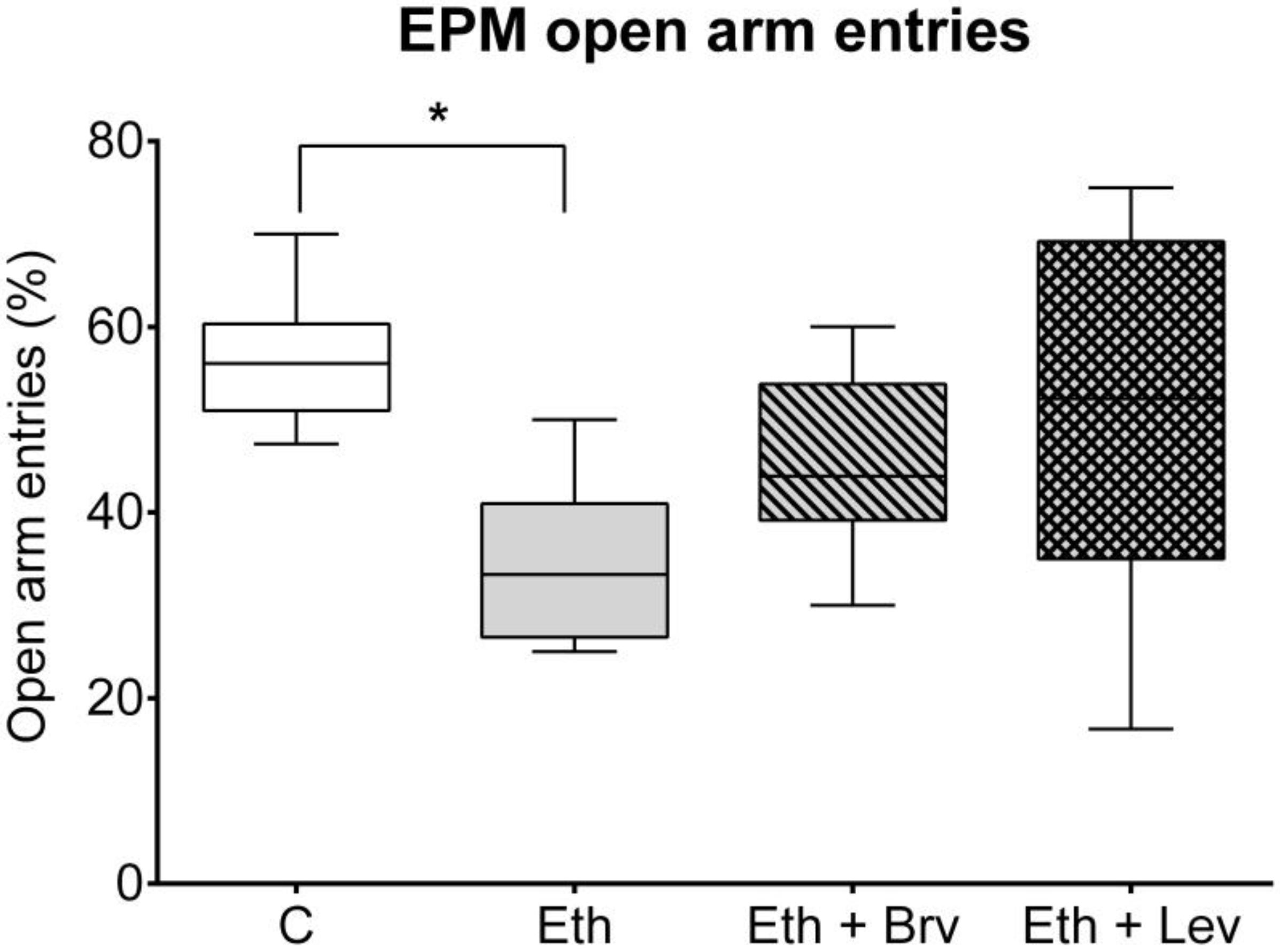
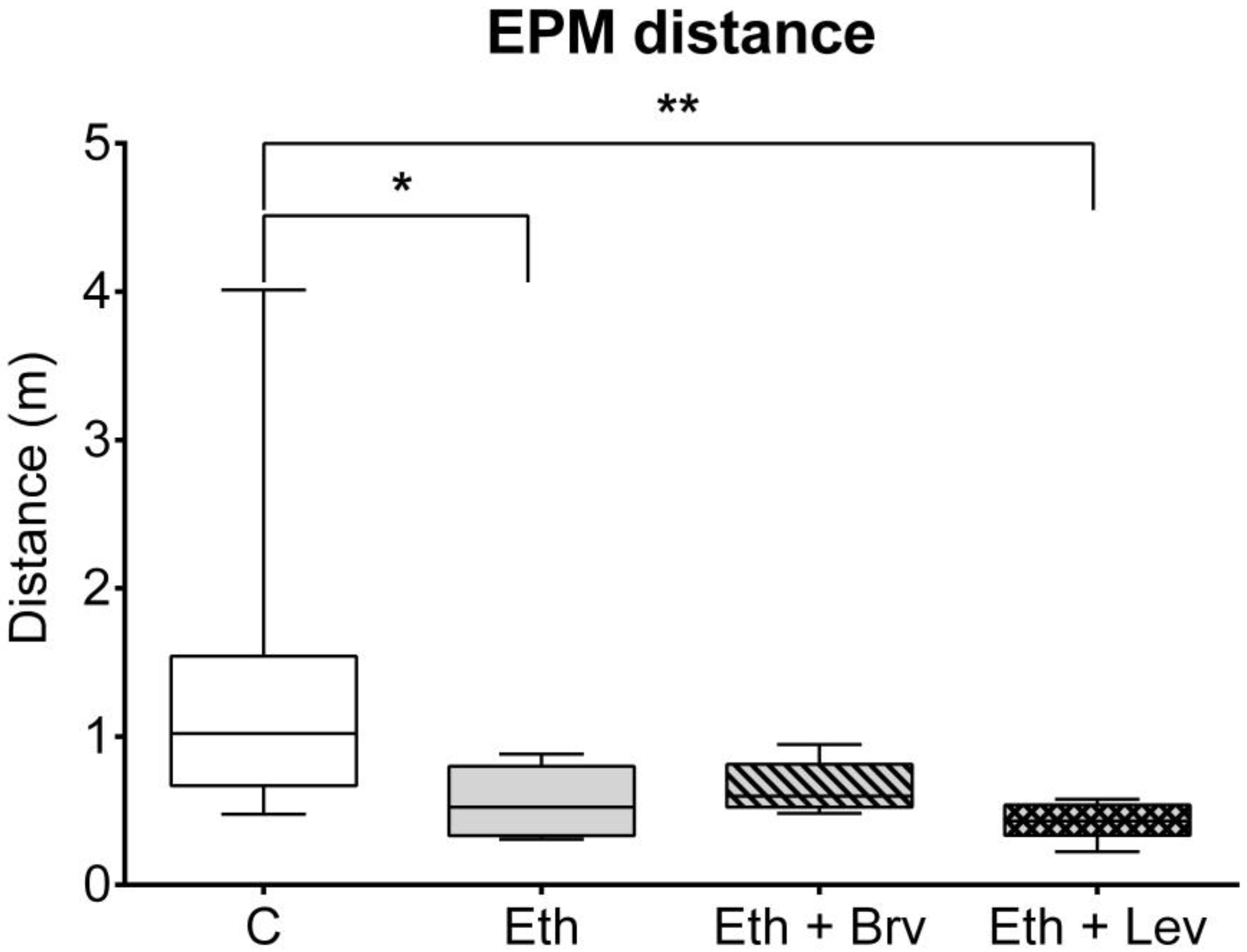
3.4. The Effect of Levetiracetam or Brivaracetam on Anxiety-like Behavior in the Elevated Plus Maze Test 24 h After the Discontinuation of Ethanol Administration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zorumski, C.F.; Mennerick, S.; Izumi, Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol 2014, 48, 1–17. [Google Scholar] [CrossRef]
- Hedayati-Moghadam, M.; Razazpour, F.; Pourfridoni, M.; Mirzaee, F.; Baghcheghi, Y. Ethanol’s impact on the brain: A neurobiological perspective on the mechanisms of memory impairment. Mol. Biol. Rep. 2024, 51, 782. [Google Scholar] [CrossRef]
- Mira, R.G.; Tapia-Rojas, C.; Pérez, M.J.; Jara, C.; Vergara, E.H.; Quintanilla, R.A.; Cerpa, W. Alcohol impairs hippocampal function: From NMDA receptor synaptic transmission to mitochondrial function. Drug Alcohol Depend. 2019, 205, 107628. [Google Scholar] [CrossRef]
- Ehrlich, I.; Humeau, Y.; Grenier, F.; Ciocchi, S.; Herry, C.; Lüthi, A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009, 62, 757–771. [Google Scholar] [CrossRef]
- Ngui, H.H.L.; Kow, A.S.F.; Lai, S.; Tham, C.L.; Ho, Y.C.; Lee, M.T. Alcohol Withdrawal and the Associated Mood Disorders—A Review. Int. J. Mol. Sci. 2022, 23, 14912. [Google Scholar] [CrossRef]
- Rossi, R.; Arjmand, S.; Bærentzen, S.L.; Gjedde, A.; Landau, A.M. Synaptic Vesicle Glycoprotein 2A: Features and Functions. Front. Neurosci. 2022, 16, 864514. [Google Scholar] [CrossRef]
- Vanoye-Carlo, A.; Gómez-Lira, G. Differential expression of SV2A in hippocampal glutamatergic and GABAergic terminals during postnatal development. Brain Res. 2019, 1715, 73–83. [Google Scholar] [CrossRef]
- Wood, M.D.; Sands, Z.A.; Vandenplas, C.; Gillard, M. Further evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia 2018, 59, e147–e151. [Google Scholar] [CrossRef]
- Niespodziany, I.; Klitgaard, H.; Margineanu, D.G. Levetiracetam inhibits the high-voltage-activated Ca(2+) current in pyramidal neurones of rat hippocampal slices. Neurosci. Lett. 2001, 306, 5–8. [Google Scholar] [CrossRef]
- Ueda, Y.; Doi, T.; Nagatomo, K.; Tokumaru, J.; Takaki, M.; Willmore, L.J. Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection. Brain Res. 2007, 1151, 55–61. [Google Scholar] [CrossRef]
- Wakita, M.; Kotani, N.; Kogure, K.; Akaike, N. Inhibition of excitatory synaptic transmission in hippocampal neurons by levetiracetam involves Zn2+-dependent GABA type A receptor-mediated presynaptic modulation. J. Pharmacol. Exp. Ther. 2014, 348, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Okada, M. Brivaracetam and levetiracetam suppress astroglial l-glutamate release through hemichannel via inhibition of synaptic vesicle protein. Int. J. Mol. Sci. 2022, 23, 4473. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Chen, C.C.; Liou, H.H. Levetiracetam inhibits glutamate transmission through presynaptic P/Q-type calcium channels on the granule cells of the dentate gyrus. Br. J. Pharmacol. 2009, 158, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.E.; Bartholomé, O.; Van den Ackerveken, P.; Ferrara, A.; Rogister, B.; Plenevaux, A.; Tirelli, E. Anxiety-like features and spatial memory problems as a consequence of hippocampal SV2A expression. PLoS ONE 2019, 14, e0217882. [Google Scholar] [CrossRef]
- Mecca, A.P.; Chen, M.K.; O’Dell, R.S.; Naganawa, M.; Toyonaga, T.; Godek, T.A.; Harris, J.E.; Bartlett, H.H.; Zhao, W.; Nabulsi, N.B.; et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 2020, 16, 974–982. [Google Scholar] [CrossRef]
- Nygaard, H.B.; Kaufman, A.C.; Sekine-Konno, T.; Huh, L.L.; Going, H.; Feldman, S.J.; Kostylev, M.A.; Strittmatter, S.M. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer’s disease mouse model. Alzheimers Res. Ther. 2015, 7, 25. [Google Scholar] [CrossRef]
- Thompson, J.C.; Levis Rabi, M.; Novoa, M.; Nash, K.R.; Joly-Amado, A. Evaluating the Efficacy of Levetiracetam on Non-Cognitive Symptoms and Pathology in a Tau Mouse Model. Biomedicines 2024, 12, 2891. [Google Scholar] [CrossRef]
- Rao, N.R.; DeGulis, O.; Nomura, T.; Lee, S.; Hark, T.J.; Dynes, J.C.; Dexter, E.X.; Dulewicz, M.; Ge, J.; Upadhyay, A.; et al. Levetiracetam prevents Aβ42 production through SV2a-dependent modulation of App processing in Alzheimer’s disease models. bioRxiv 2024. [Google Scholar] [CrossRef]
- Sen, A.; Toniolo, S.; Tai, X.Y.; Akinola, M.; Symmonds, M.; Mura, S.; Galloway, J.; Hallam, A.; Chan, J.Y.C.; Koychev, I.; et al. Safety, tolerability, and efficacy outcomes of the Investigation of Levetiracetam in Alzheimer’s disease (ILiAD) study: A pilot, double-blind placebo-controlled crossover trial. Epilepsia Open 2024, 9, 2353–2364. [Google Scholar] [CrossRef]
- Sanon, N.T.; Gagné, J.; Wolf, D.C.; Aboulamer, S.; Bosoi, C.M.; Simard, A.; Messiet, E.; Desgent, S.; Carmant, L. Favorable adverse effect profile of brivaracetam vs levetiracetam in a preclinical model. Epilepsy Behav. 2018, 79, 117–125. [Google Scholar] [CrossRef]
- Zwierzyńska, E.; Pietrzak, B. The differential effect of levetiracetam on memory and anxiety in rats. Epilepsy Behav. 2022, 136, 108917. [Google Scholar] [CrossRef]
- Zwierzyńska, E.; Pietrzak, B. The impact of brivaracetam on cognitive processes and anxiety in various experimental models. Pharmacol. Rep. 2024, 76, 86–97. [Google Scholar] [CrossRef]
- Fish, E.W.; Agoglia, A.E.; Krouse, M.C.; Muller, R.G.; Robinsonm, J.E.; Malanga, C.J. Levetiracetam results in increased and decreased alcohol drinking with different access procedures in C57BL/6J mice. Behav. Pharmacol. 2014, 25, 61–70. [Google Scholar] [CrossRef]
- Zalewska-Kaszubska, J.; Bajer, B.; Czarnecka, E.; Dyr, W.; Gorska, D. Voluntary alcohol consumption and plasma beta-endorphin levels in alcohol preferring rats chronically treated with levetiracetam: A preliminary study. Physiol. Behav. 2011, 102, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.M.; Ciraulo, D.A.; Sarid-Segal, O.; Richardson, M.A.; Devine, E.; Streeter, C.C.; Oscar-Berman, M.; Surprise, C.; Colaneri, L.; Putnam, M.; et al. Zonisamide, topiramate, and levetiracetam: Efficacy and neuropsychological effects in alcohol use disorders. J. Clin. Psychopharmacol. 2015, 35, 34–42. [Google Scholar] [CrossRef]
- Sarid-Segal, O.; Piechniczek-Buczek, J.; Knapp, C.; Afshar, M.; Devine, E.; Sickles, L.; Uwodukunda, E.; Richambault, C.; Koplow, J.; Ciraulo, D. The effects of levetiracetam on alcohol consumption in alcohol-dependent subjects: An open label study. Am. J. Drug Alcohol Abuse 2008, 34, 441–447. [Google Scholar] [CrossRef]
- Fertig, J.B.; Ryan, M.L.; Falk, D.E.; Litten, R.Z.; Mattson, M.E.; Ransom, J.; Rickman, W.J.; Scott, C.; Ciraulo, D.; Green, A.I.; et al. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcohol Clin. Exp. Res. 2012, 36, 1421–1430. [Google Scholar] [CrossRef]
- Youland, K.M.; Miller, R.F.; Mahoney, L.J.; Borgert, A.J.; Gundrum, J.D. Levetiracetam as adjunctive therapy for acute alcohol withdrawal syndrome in hospitalized patients. J. Clin. Psychopharmacol. 2014, 34, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.J.; Levin, F.R. Levetiracetam for the treatment of co-occurring alcohol dependence and anxiety: Case series and review. Am. J. Drug Alcohol Abuse 2008, 34, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, A.C.; Watanabe, S.; Peeters, P.A.; de Kam, M.L.; Zuiker, R.G.; Stevens, J.; van Gerven, J.M.; Stockis, A. Pharmacological interactions between brivaracetam and ethanol in healthy males. J. Psychopharmacol. 2017, 31, 915–926. [Google Scholar] [CrossRef]
- Majchrowicz, E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia 1975, 43, 245–254. [Google Scholar] [CrossRef]
- Szmigielski, A.; Szmigielska, H.; Wejman, I. The effect of prolonged ethanol administration on central alpha 2-adrenoceptors sensitivity. Pol. J. Pharmacol. Pharm. 1989, 41, 263–272. [Google Scholar]
- Ogren, S.O.; Stiedl, O. Passive avoidance. In Encyclopedia of Psychopharmacology, 2nd ed.; Stolerman, I.P., Price, L.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1220–1228. [Google Scholar]
- Stolerman, I.P.; Price, L.H. Context and Cued Conditioning. In Encyclopedia of Psychopharmacology, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 1392. [Google Scholar]
- Alavi, M.S.; Fanoudi, S.; Hosseini, M.; Sadeghnia, H.R. Beneficial effects of levetiracetam in streptozotocin-induced rat model of Alzheimer’s disease. Metab. Brain Dis. 2022, 37, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, S.C.; Kakkar, A.K.; Kumar, R.; Gupta, Y.K. Effect of lamotrigine, levetiracetam & topiramate on neurobehavioural parameters & oxidative stress in comparison with valproate in rats. Indian J. Med. Res. 2016, 144, 104–111. [Google Scholar] [PubMed]
- Devi, L.; Ohno, M. Effects of levetiracetam, an antiepileptic drug, on memory impairments associated with aging and Alzheimer’s disease in mice. Neurobiol. Learn. Mem. 2013, 102, 7–11. [Google Scholar] [CrossRef]
- Cavichioli, A.M.; Santos-Silva, T.; Grace, A.A.; Guimarães, F.S.; Gomes, F.V. Levetiracetam Attenuates Adolescent Stress-induced Behavioral and Electrophysiological Changes Associated with Schizophrenia in Adult Rats. Schizophr. Bull. 2023, 49, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.E.; Chen, M.; Stamatakis, A.M.; Krouse, M.C.; Howard, E.C.; Faccidomo, S.; Hodge, C.W.; Fish, E.W.; Malanga, C.J. Levetiracetam has opposite effects on alcohol- and cocaine-related behaviors in C57BL/6J mice. Neuropsychopharmacology 2013, 38, 1322–1333. [Google Scholar] [CrossRef]
- Wood, M.D.; Gillard, M. Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia 2017, 58, 255–262. [Google Scholar] [CrossRef]
- Pietrzak, B.; Czarnecka, E. Pharmaco-EEG-based assessment of interaction between ethanol and levetiracetam. Alcohol 2008, 42, 115–122. [Google Scholar] [CrossRef]
- Zwierzyńska, E.; Klimczak, M.; Nasiadek, M.; Stragierowicz, J.; Pietrzak, B. Impact of levetiracetam and ethanol on memory, selected neurotransmitter levels, oxidative stress parameters, and essential elements in rats. Pharmacol. Rep. 2024, 76, 1363–1376. [Google Scholar] [CrossRef]
- Santana-Gómez, C.E.; Valle-Dorado, M.G.; Domínguez-Valentín, A.E.; Hernández-Moreno, A.; Orozco-Suárez, S.; Rocha, L. Neuroprotective effects of levetiracetam, both alone and combined with propylparaben, in the long-term consequences induced by lithium-pilocarpine status epilepticus. Neurochem. Int. 2018, 120, 224–232. [Google Scholar] [CrossRef]
- Matsuo, T.; Komori, R.; Nakatani, M.; Ochi, S.; Yokota-Nakatsuma, A.; Matsumoto, J.; Takata, F.; Dohgu, S.; Ishihara, Y.; Itoh, K. Levetiracetam Suppresses the Infiltration of Neutrophils and Monocytes and Downregulates Many Inflammatory Cytokines during Epileptogenesis in Pilocarpine-Induced Status Epilepticus Mice. Int. J. Mol. Sci. 2022, 23, 7671. [Google Scholar] [CrossRef]
- Christensen, K.V.; Leffers, H.; Watson, W.P.; Sánchez, C.; Kallunki, P.; Egebjerg, J. Levetiracetam attenuates hippocampal expression of synaptic plasticity-related immediate early and late response genes in amygdala-kindled rats. BMC Neurosci. 2010, 11, 9. [Google Scholar] [CrossRef]
- Ge, Y.X.; Lin, Y.Y.; Bi, Q.Q.; Chen, Y.J. Brivaracetam Prevents the Over-expression of Synaptic Vesicle Protein 2A and Rescues the Deficits of Hippocampal Long-term Potentiation In Vivo in Chronic Temporal Lobe Epilepsy Rats. Curr. Neurovasc. Res. 2020, 17, 354–360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwierzyńska, E.; Pietrzak, B. The Effect of Co-Administration of Levetiracetam or Brivaracetam with Ethanol on the Associative Learning and Anxiety Level of Rats. Future Pharmacol. 2025, 5, 45. https://doi.org/10.3390/futurepharmacol5030045
Zwierzyńska E, Pietrzak B. The Effect of Co-Administration of Levetiracetam or Brivaracetam with Ethanol on the Associative Learning and Anxiety Level of Rats. Future Pharmacology. 2025; 5(3):45. https://doi.org/10.3390/futurepharmacol5030045
Chicago/Turabian StyleZwierzyńska, Ewa, and Bogusława Pietrzak. 2025. "The Effect of Co-Administration of Levetiracetam or Brivaracetam with Ethanol on the Associative Learning and Anxiety Level of Rats" Future Pharmacology 5, no. 3: 45. https://doi.org/10.3390/futurepharmacol5030045
APA StyleZwierzyńska, E., & Pietrzak, B. (2025). The Effect of Co-Administration of Levetiracetam or Brivaracetam with Ethanol on the Associative Learning and Anxiety Level of Rats. Future Pharmacology, 5(3), 45. https://doi.org/10.3390/futurepharmacol5030045






