Oxygen-Generating Metal Peroxide Particles for Cancer Therapy, Diagnosis, and Theranostics
Abstract
1. Introduction
2. Synthesis of MePO Particles
3. Application of MePO Particles in Cancer Diagnosis and Therapy
3.1. Chemotherapy
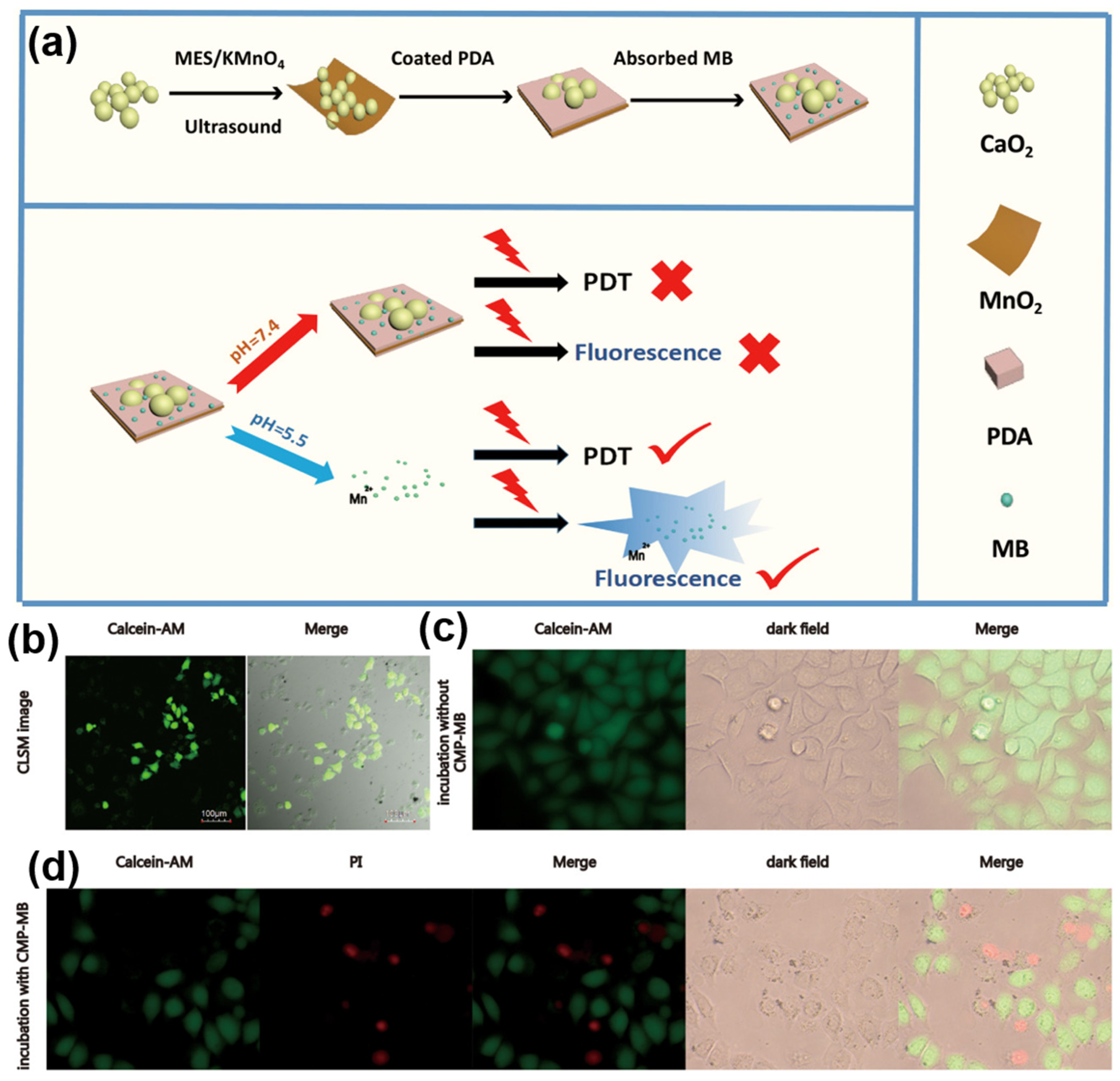
3.2. Photodynamic Therapy
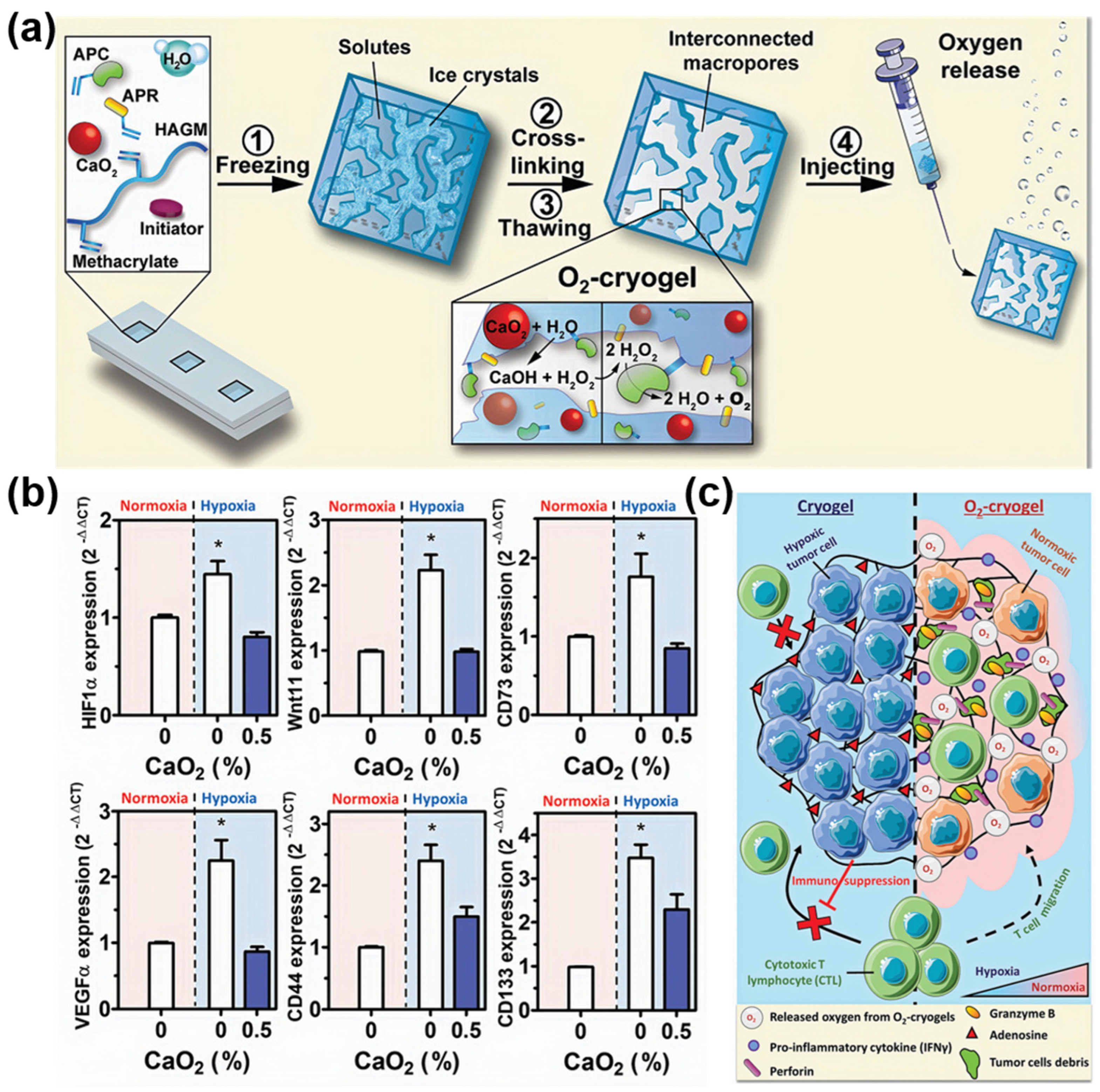
3.3. Immunotherapy and Radiation Therapy
4. Conclusions and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moorthy, H.; Govindaraju, T. Dendrimer Architectonics to Treat Cancer and Neurodegenerative Diseases with Implications in Theranostics and Personalized Medicine. ACS Appl. Bio Mater. 2021, 4, 1115–1139. [Google Scholar] [CrossRef] [PubMed]
- Kevadiya, B.D.; Ottemann, B.M.; Thomas, M.B.; Mukadam, I.; Nigam, S.; McMillan, J.E.; Gorantla, S.; Bronich, T.K.; Edagwa, B.; Gendelman, H.E. Neurotheranostics as Personalized Medicines. Adv. Drug Deliv. Rev. 2019, 148, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Cianni, R.; Bagni, O. Theranostic Approaches in Nuclear Medicine: Current Status and Future Prospects. Expert Rev. Med Devices 2020, 17, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Altinbasak, I.; Alp, Y.; Sanyal, R.; Sanyal, A. Theranostic Nanogels: Multifunctional Agents for Simultaneous Therapeutic Delivery and Diagnostic Imaging. Nanoscale 2024, 16, 14033–14056. [Google Scholar] [CrossRef] [PubMed]
- Bauri, S.; Tripathi, S.; Choudhury, A.M.; Mandal, S.S.; Raj, H.; Maiti, P. Nanomaterials as Theranostic Agents for Cancer Therapy. ACS Appl. Nano Mater. 2023, 6, 21462–21495. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Antoniou, A.C.; Fruk, L.; Rosenfeld, N. The Future of Early Cancer Detection. Nat. Med. 2022, 28, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Connal, S.; Cameron, J.M.; Sala, A.; Brennan, P.M.; Palmer, D.S.; Palmer, J.D.; Perlow, H.; Baker, M.J. Liquid Biopsies: The Future of Cancer Early Detection. J. Transl. Med. 2023, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- van de Looij, S.M.; Hebels, E.R.; Viola, M.; Hembury, M.; Oliveira, S.; Vermonden, T. Gold Nanoclusters: Imaging, Therapy, and Theranostic Roles in Biomedical Applications. Bioconjugate Chem. 2021, 33, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Dammes, N.; Peer, D. Monoclonal Antibody-Based Molecular Imaging Strategies and Theranostic Opportunities. Theranostics 2020, 10, 938–955. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Hajra, S.; Kaushik, A.; Rubahn, H.G.; Mishra, Y.K.; Kim, H.J. Smart Nanomaterials as the Foundation of a Combination Approach for Efficient Cancer Theranostics. Mater. Today Chem. 2022, 26, 101182. [Google Scholar] [CrossRef]
- Su, T.; Zhao, F.; Ying, Y.; Li, W.; Li, J.; Zheng, J.; Qiao, L.; Che, S.; Yu, J. Self-Monitoring Theranostic Nanomaterials: Emerging Visual Agents for Real-Time Monitoring of Tumor Treatment Processes. Small Methods 2023, 8, e2301470. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 587–775. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoçi, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef] [PubMed]
- Onzi, G.; Guterres, S.S.; Pohlmann, A.R.; Frank, L.A. Passive Targeting and the Enhanced Permeability and Retention (EPR) Effect. In The ADME Encyclopedia; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (EPR): Nanoparticle-Based Precision Tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, D.; Peppas, N.A. Advanced Engineered Nanoparticulate Platforms to Address Key Biological Barriers for Delivering Chemotherapeutic Agents to Target Sites. Adv. Drug Deliv. Rev. 2020, 167, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zhang, M. Ligand Chemistry in Antitumor Theranostic Nanoparticles. Acc. Chem. Res. 2023, 56, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.K.; Singh, V.V.; Solanki, M.K.; Kumar, A.; Ruokolainen, J.; Kesari, K.K. Smart nanomaterials in cancer theranostics: Challenges and opportunities. ACS Omega 2023, 8, 14290–14320. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Saeed, A.; Elshaer, A.; Melaibari, A.A.; Memić, A.; Hassanin, H.; Essa, K. Fabrication and Characterization of Oxygen-Generating Polylactic Acid/Calcium Peroxide Composite Filaments for Bone Scaffolds. Pharmaceuticals 2023, 16, 627. [Google Scholar] [CrossRef] [PubMed]
- Colombani, T.; Eggermont, L.J.; Hatfield, S.M.; Rogers, Z.J.; Rezaeeyazdi, M.; Memic, A.; Sitkovsky, M.V.; Bencherif, S.A. Oxygen-Generating Cryogels Restore T Cell Mediated Cytotoxicity in Hypoxic Tumors. Adv. Funct. Mater. 2021, 31, 2102234. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.; Gauthaman, K.; Hammad, A.H.; Joshi Navare, K.; Alshahrie, A.A.; Bencherif, S.A.; Tamayol, A.; Memic, A. Oxygen-Releasing Antibacterial Nanofibrous Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 1233. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, S.; Liu, X.; Wang, J.; Huang, Y.; Zhang, A.; Zhang, X. CaO2 Nanomedicines: A Review of Their Emerging Roles in Cancer Therapy. Nanotechnology 2023, 34, 482002. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.H.; Kovacev, N.; Elshaer, A.; Melaibari, A.A.; Iqbal, J.; Hassanin, H.; Essa, K.; Memić, A. Preparation of Polylactic Acid/Calcium Peroxide Composite Filaments for Fused Deposition Modelling. Polymers 2023, 15, 2229. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fu, Z.; Wang, H.; Liu, Z.; Gao, M.; Luo, Y.; Zhang, M.; Wang, J.; Ni, D. Calcium Peroxide-Based Hydrogels Enable Biphasic Release of Hydrogen Peroxide for Infected Wound Healing. Adv. Sci. 2024, 11, 2404813. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, S.; Younesi, S.; Karkhaneh, A. Peroxide Mediated Oxygen Delivery in Cancer Therapy. Colloids Surf. B Biointerfaces 2022, 219, 112832. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, X.; Zhao, P.; Wang, H.; Gu, W.; Ye, L. Nanozyme-Catalyzed Oxygen Release from Calcium Peroxide Nanoparticles for Accelerated Hypoxia Relief and Image-Guided Super-Efficient Photodynamic Therapy. Biomater. Sci. 2020, 8, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, L.; Li, P.; Ma, L.; Na, W.; Cheng, C.; Gu, Y.; Deng, D. Inorganic Nanozyme with Combined Self-Oxygenation/Degradable Capabilities for Sensitized Cancer Immunochemotherapy. Nano-Micro Lett. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y. Manganese Oxide Nanoparticles as Mri Contrast Agents in Tumor Multimodal Imaging and Therapy. Int. J. Nanomed. 2019, 14, 8321–8344. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duan, J.; Liu, Y.; Kuang, Y.; Duan, J.; Liao, T.; Xu, Z.; Jiang, B.; Li, C. Multi-Stimuli Responsive Hollow MnO2-Based Drug Delivery System for Magnetic Resonance Imaging and Combined Chemo-Chemodynamic Cancer Therapy. Acta Biomater. 2021, 126, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Bu, W.; Shen, B.; He, Q.; Cui, Z.; Liu, Y.; Zheng, X.; Zhao, K.; Shi, J. Intelligent MnO2 Nanosheets Anchored with Upconversion Nanoprobes for Concurrent PH-/H2O2-Responsive UCL Imaging and Oxygen-Elevated Synergetic Therapy. Adv. Mater. 2015, 27, 4155–4161. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, Z.Q.; Tang, H.X.; Shi, Z.E.; Kang, J.; Liu, Q.; Qi, J. Efficacy-Shaping Nanomedicine by Loading Calcium Peroxide into Tumor Microenvironment-Responsive Nanoparticles for the Antitumor Therapy of Prostate Cancer. Theranostics 2020, 10, 9808–9829. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fu, L.H.; Qi, C.; Lin, J.; Huang, P. Metal Peroxides for Cancer Treatment. Bioact. Mater. 2021, 6, 2698–2710. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, X.; Yan, R.; Zhao, P.; Chen, Y.; Li, M.; Chen, C.; Fan, T.; Lu, Y.; Wang, C.; et al. Enhancement of Cisplatin Efficacy by Lipid-CaO2 Nanocarrier-Mediated Comprehensive Modulation of the Tumor Microenvironment. Biomater. Sci. 2019, 7, 4260–4272. [Google Scholar] [CrossRef] [PubMed]
- Heo, W.; Shin, H.; Ansari, J.R.; Park, K.; Seo, J. Preparation and Properties of Calcium Oxide and Calcium Peroxide from Eggshell Waste for Enhanced Antimicrobial Activity. Mater. Today Commun. 2024, 41, 110531. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, B.; Song, R.; Wang, H.; Lv, B.; Meng, X.; Liu, Y.; Liu, Y.; Zheng, X.; Su, W.; et al. Radiation-Assisted Metal Ion Interference Tumor Therapy by Barium Peroxide-Based Nanoparticles. Mater. Horiz. 2019, 6, 1034–1040. [Google Scholar] [CrossRef]
- Shen, S.; Mamat, M.; Zhang, S.; Cao, J.; Hood, Z.D.; Figueroa-Cosme, L.; Xia, Y. Synthesis of CaO2 Nanocrystals and Their Spherical Aggregates with Uniform Sizes for Use as a Biodegradable Bacteriostatic Agent. Small 2019, 15, e1902118. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-M.; Liu, Y.-Y.; Ni, D.-L.; Zhou, J.-J.; Zhang, M.; Zhao, P.-R.; Lv, B.; Wang, H.; Jin, D.-Y.; Bu, W.-B. Biodegradable Nanoprodrugs: “Delivering” ROS to Cancer Cells for Molecular Dynamic Therapy. Adv. Mater. 2020, 32, e1904011. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, M.; Meshkini, A.; Sistanipour, E. Self-Sustained H2O2 and O2 Generation by Calcium Carbonate/Zinc Peroxide Nanocomposite, Enhancing Osteosarcoma Cell Differentiation and Antibacterial Activity. Surf. Interfaces 2025, 56, 105575. [Google Scholar] [CrossRef]
- Pirouzmand, M.; Sani, P.S.; Ghasemi, Z.; Azizi, S. Citric Acid-Crosslinked β-Cyclodextrin Supported Zinc Peroxide as a Biocompatible H2O2 Scavenger. J. Biol. Inorg. Chem. 2020, 25, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, Y.; Yu, L.; Lin, K.; Wang, X. Magnetic Hyperthermia–Synergistic H2O2 Self-Sufficient Catalytic Suppression of Osteosarcoma with Enhanced Bone-Regeneration Bioactivity by 3D-Printing Composite Scaffolds. Adv. Funct. Mater. 2020, 30, 1907071. [Google Scholar] [CrossRef]
- Han, Y.; Ouyang, J.; Li, Y.; Wang, F.; Jiang, J.H. Engineering H2O2 Self-Supplying Nanotheranostic Platform for Targeted and Imaging-Guided Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, R.; Liu, Y.; Yi, Z.; Meng, X.; Zhang, J.; Tang, Z.; Yao, Z.; Liu, Y.; Liu, X.; et al. Calcium-Overload-Mediated Tumor Therapy by Calcium Peroxide Nanoparticles. Chem 2019, 5, 2171–2182. [Google Scholar] [CrossRef]
- Mbugua, S.N. Targeting Tumor Microenvironment by Metal Peroxide Nanoparticles in Cancer Therapy. Bioinorg. Chem. Appl. 2022, 2022, 5041399. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Arenas, A.; Bailón-García, E.; Lozano-Castelló, D.; Da Costa, P.; Bueno-López, A. Stable NiO–CeO2 Nanoparticles with Improved Carbon Resistance for Methane Dry Reforming. J. Rare Earths 2022, 40, 57–62. [Google Scholar] [CrossRef]
- Gedanken, A.; Perelshtein, I.; Perkas, N. Power Ultrasound for the Production of Nanomaterials. In Power Ultrasonics: Applications of High-Intensity Ultrasound, 2nd ed.; Elsevier Ltd.: London, UK, 2023. [Google Scholar]
- Nobre, F.X.; Mendes, O.C.; da Silva, A.P.J.; Junior, J.L.S.; do Nascimento, M.V.B.; Pessoa Junior, W.A.G.; Manzato, L.; Brandim, A.S.; Matos, J.M.E.; Brito, W.R.; et al. Fast and Efficient Green Synthesis of CaWO4 NPs Using Eggshells as a Biogenic Calcium Source: Structure, Optical Property, and Morphology. J. Photochem. Photobiol. A Chem. 2023, 439, 114589. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, B.; Kaushik, A.; Mostafavi, E. Nanobiotechnological Prospects of Probiotic Microflora: Synthesis, Mechanism, and Applications. Sci. Total Environ. 2022, 838, 156212. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.J.; Suslick, K.S. Nanostructured Materials Synthesis Using Ultrasound. Top. Curr. Chem. 2017, 375, 12. [Google Scholar] [CrossRef] [PubMed]
- Perdana, M.Y.; Hassan, M.; Ramelan, A.H.; Gondal, M.A. Synthesis and Characterizations of Zinc Peroxide by Pulsed Laser Ablation in Liquid (PLAL) and Zinc Oxide Nanoparticles by Simple and Low-Temperature Heating Treatment. J. Phys. Conf. Ser. 2023, 2556, 012004. [Google Scholar] [CrossRef]
- Yang, D.; Gondal, M.A.; Yamani, Z.H.; Baig, U.; Qiao, X.; Liu, G.; Xu, Q.; Xiang, D.; Mao, J.; Shen, K. 532 Nm Nanosecond Pulse Laser Triggered Synthesis of ZnO2 Nanoparticles via a Fast Ablation Technique in Liquid and Their Photocatalytic Performance. Mater. Sci. Semicond. Process. 2017, 57, 124–131. [Google Scholar] [CrossRef]
- Elbahri, M.; Abdelaziz, R.; Disci-Zayed, D.; Homaeigohar, S.; Sosna, J.; Adam, D.; Kienle, L.; Dankwort, T.; Abdelaziz, M. Underwater Leidenfrost Nanochemistry for Creation of Size-Tailored Zinc Peroxide Cancer Nanotherapeutics. Nat. Commun. 2017, 8, 15319. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, R.; Bai, W.; Ye, T.; Wang, S. Oxygen-Based Nanocarriers to Modulate Tumor Hypoxia for Ameliorated Anti-Tumor Therapy: Fabrications, Properties, and Future Directions. Front. Mol. Biosci. 2021, 8, 683519. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Pan, M.H.; Wang, L.; Li, W.; Jiang, C.; He, J.; Abouzid, K.; Liu, L.Z.; Shi, Z.; Jiang, B.H. Hypoxia-Mediated Mitochondria Apoptosis Inhibition Induces Temozolomide Treatment Resistance through MiR-26a/Bad/Bax Axis. Cell Death Dis. 2018, 9, 1128. [Google Scholar] [CrossRef] [PubMed]
- Newland, B.; Baeger, M.; Eigel, D.; Newland, H.; Werner, C. Oxygen-Producing Gellan Gum Hydrogels for Dual Delivery of Either Oxygen or Peroxide with Doxorubicin. ACS Biomater. Sci. Eng. 2017, 3, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Chan, C.H.; Wang, B.J.; Yeh, Y.L.; Wang, Y.J.; Chiu, H.W. The Oxygen-Generating Calcium Peroxide-Modified Magnetic Nanoparticles Attenuate Hypoxia-Induced Chemoresistance in Triple-Negative Breast Cancer. Cancers 2021, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.E.; Lin, S.J.; Chen, L.C.; Chen, C.C.; Lai, P.L.; Huang, C.C. Optimizing an Injectable Composite Oxygen-Generating System for Relieving Tissue Hypoxia. Front. Bioeng. Biotechnol. 2020, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.M.; Dias, L.M.; Surur, A.K.; de Moraes, D.A.; Pavarina, A.C.; Fontana, C.R.; Correa, D.S. Electrospun Composite Bead-on-String Nanofibers Containing CaO2 Nanoparticles and MnO2 Nanosheets as Oxygen-Release Systems for Biomedical Applications. ACS Appl. Nano Mater. 2022, 5, 14425–14436. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Sun, Y.; Liu, X.; Chen, Y.; Chen, C.; Yan, R.; Fan, T.; Yang, T.; Lu, Y.; et al. A Smart O2-Generating Nanocarrier Optimizes Drug Transportation Comprehensively for Chemotherapy Improving. Acta Pharm. Sin. B 2021, 11, 3608–3621. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Li, T.; Chen, Z.; Wang, Y.; Qin, C. Properties of Calcium Peroxide for Release of Hydrogen Peroxide and Oxygen: A Kinetics Study. Chem. Eng. J. 2016, 303, 450–457. [Google Scholar] [CrossRef]
- He, C.; Zhang, X.; Chen, C.; Liu, X.; Chen, Y.; Yan, R.; Fan, T.; Gai, Y.; Lee, R.J.; Ma, X.; et al. A Solid Lipid Coated Calcium Peroxide Nanocarrier Enables Combined Cancer Chemo/Chemodynamic Therapy with O2/H2O2 Self-Sufficiency. Acta Biomater. 2021, 122, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. Photodynamic Therapy—An up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Sobhani, N.; Samadani, A.A. Implications of Photodynamic Cancer Therapy: An Overview of PDT Mechanisms Basically and Practically. J. Egypt. Natl. Canc. Inst. 2021, 33, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, L.; Zhang, M.; Liu, Z.; Wu, C.; Pan, X.; Huang, Z.; Lu, C.; Quan, G. Photodynamic Therapy for Cancer: Mechanisms, Photosensitizers, Nanocarriers, and Clinical Studies. MedComm 2024, 5, e603. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Huang, T.; Liu, C.; Zhao, M.; Xie, M.; Li, G.; Liu, S.; Huang, W.; Zhao, Q. Oxygen Self-Sufficient NIR-Activatable Liposomes for Tumor Hypoxia Regulation and Photodynamic Therapy. Chem. Sci. 2019, 10, 9091–9098. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Nesbitt, H.; Callan, B.; Taylor, M.A.; Love, M.; McHale, A.P.; Callan, J.F. Oxygen Generating Nanoparticles for Improved Photodynamic Therapy of Hypoxic Tumours. J. Control. Release 2017, 264, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lai, H.; Xing, F.; Xiao, P. Polymer-Coated Calcium Peroxide Nanoparticles as an Oxygen Self-Supplying Platform for Enhanced Photodynamic Therapy. Eur. Polym. J. 2022, 177, 111458. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Yuan, G.; Guo, X.; Cen, J.; Gong, Y.; Liu, J.; Gang, Y. In Situ Fabrication of MS@MnO2hybrid as Nanozymes for Enhancing ROS-Mediated Breast Cancer Therapy. Nanoscale 2020, 12, 22317–22329. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ji, J.; Liu, Z. Multifunctional MnO2 Nanoparticles for Tumor Microenvironment Modulation and Cancer Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1720. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.T.; Cao, P.P.; Zhang, H.; Li, Y.H.; Yin, X.B. GSH-Activated MRI-Guided Enhanced Photodynamic- and Chemo-Combination Therapy with a MnO2-Coated Porphyrin Metal Organic Framework. Chem. Commun. 2019, 55, 6241–6244. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Zhang, H.; Deng, Y.; Jiang, A.; Bao, X.; Guo, M.; Li, Z.; Wu, M.; Ji, X.; Zeng, X.; et al. Dual-Response Oxygen-Generating MnO2 Nanoparticles with Polydopamine Modification for Combined Photothermal-Photodynamic Therapy. Chem. Eng. J. 2020, 389, 124494. [Google Scholar] [CrossRef]
- Ji, C.; Lu, Z.; Xu, Y.; Shen, B.; Yu, S.; Shi, D. Self-Production of Oxygen System CaO2/MnO2@PDA-MB for the Photodynamic Therapy Research and Switch-Control Tumor Cell Imaging. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; He, F.; Gulzar, A.; Kuang, Y.; Zhang, F.; Gai, S.; Yang, P.; Wang, C. In Situ Oxygenating and 808 Nm Light-Sensitized Nanocomposite for Multimodal Imaging and Mitochondria-Assisted Cancer Therapy. J. Mater. Chem. B 2021, 9, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, P.; Chen, X.; Zhang, M.; Han, Q.; Yuan, Q. Dual-Responsive Nanoplatform for Integrated Cancer Diagnosis and Therapy: Unleashing the Power of Tumor Microenvironment. Front. Chem. 2024, 12, 1475131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, D.; Liu, X.; Deng, Z.; Li, J.; Zhu, S.; Ma, B.; Liu, R.; Zhu, H. High Photocytotoxicity Iridium (III) Complex Photosensitizer for Photodynamic Therapy Induces Antitumor Effect Through GPX4-Dependent Ferroptosis. Small 2025, 21, 2403165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Qiao, B.; Lin, X.; Cao, J.; Zhang, N.; Guo, H.; Liu, W.; Zhu, L.; Xie, X.; Wan, L.; et al. A Hydrogen Peroxide Economizer for On-Demand Oxygen Production-Assisted Robust Sonodynamic Immunotherapy. Theranostics 2022, 12, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Pilard, C.; Ancion, M.; Delvenne, P.; Jerusalem, G.; Hubert, P.; Herfs, M. Cancer Immunotherapy: It’s Time to Better Predict Patients’ Response. Br. J. Cancer 2021, 125, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.; Suryawanshi, H. Immunotherapy: The Future of Cancer Treatment. J. Oral Maxillofac. Pathol. 2021, 25, 371. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Figarella, K.; Kim, J.; Ruan, W.; Mills, T.; Eltzschig, H.K.; Yuan, X. Hypoxia-Adenosine Axis as Therapeutic Targets for Acute Respiratory Distress Syndrome. Front. Immunol. 2024, 15, 1328565. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, Y.; Wang, C.; Jiang, X.; Liu, H.; Yuan, A.; Yan, J.; Hu, Y.; Wu, J. Light-Controlled Oxygen Production and Collection for Sustainable Photodynamic Therapy in Tumor Hypoxia. Biomaterials 2021, 269, 120621. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Y.; Yu, Y.; Yang, S.; Feng, J.; Zhu, Y.; Huang, W.; Qin, B.; Guan, X.; He, Z.; et al. Micro-to-Nano Oncolytic Microbial System Shifts from Tumor Killing to Tumor Draining Lymph Nodes Remolding for Enhanced Immunotherapy. Adv. Mater. 2024, 36, e2306488. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Chong, G.; Dong, H.; Gu, J.; Zang, J.; He, R.; Sun, J.; Zhang, T.; Zhao, Y.; Zheng, X.; et al. Nanovaccine Biomineralization for Cancer Immunotherapy: A NADPH Oxidase-Inspired Strategy for Improving Antigen Cross-Presentation via Lipid Peroxidation. Biomaterials 2021, 277, 121089. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Nakayama, M.; Salah, M.; Akasaka, H.; Kubota, H.; Nakahana, M.; Tagawa, T.; Morita, K.; Nakaoka, A.; Ishihara, T.; et al. A Comparative Assessment of Mechanisms and Effectiveness of Radiosensitization by Titanium Peroxide and Gold Nanoparticles. Nanomaterials 2020, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Sasaki, R.; Ogino, C.; Tanaka, T.; Morita, K.; Umetsu, M.; Ohara, S.; Tan, Z.; Nishimura, Y.; Akasaka, H.; et al. Titanium Peroxide Nanoparticles Enhanced Cytotoxic Effects of X-Ray Irradiation against Pancreatic Cancer Model through Reactive Oxygen Species Generation in Vitro and in Vivo. Radiat. Oncol. 2016, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.; Akasaka, H.; Shimizu, Y.; Morita, K.; Nishimura, Y.; Kubota, H.; Kawaguchi, H.; Sogawa, T.; Mukumoto, N.; Ogino, C.; et al. Reactive Oxygen Species-Inducing Titanium Peroxide Nanoparticles as Promising Radiosensitizers for Eliminating Pancreatic Cancer Stem Cells. J. Exp. Clin. Cancer Res. 2022, 41, 146. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.Z.; Zhu, X.H.; Wang, B.; Liu, M.; Li, S.K.; Yang, Y.S.; An, H.; Zhu, H.L. A Versatile Nanoplatform Based on Multivariate Porphyrinic Metal-Organic Frameworks for Catalytic Cascade-Enhanced Photodynamic Therapy. J. Mater. Chem. B 2021, 9, 4678–4689. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yu, L.; Qian, X.; Chen, Y.; Chen, B.; Li, Y. Chemoreactive Nanotherapeutics by Metal Peroxide Based Nanomedicine. Adv. Sci. 2021, 8, 2000494. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and Polymer Polyethylene Glycol (PEG) Coating Reduce Both 5 and 30 Nm Iron Oxide Nanoparticle Cytotoxicity in 2D and 3D Cell Culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kim, Y.J.; Im, G.B.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic Nanoparticles Applied as Functional Therapeutics. Adv. Funct. Mater. 2021, 31, 2008171. [Google Scholar] [CrossRef]
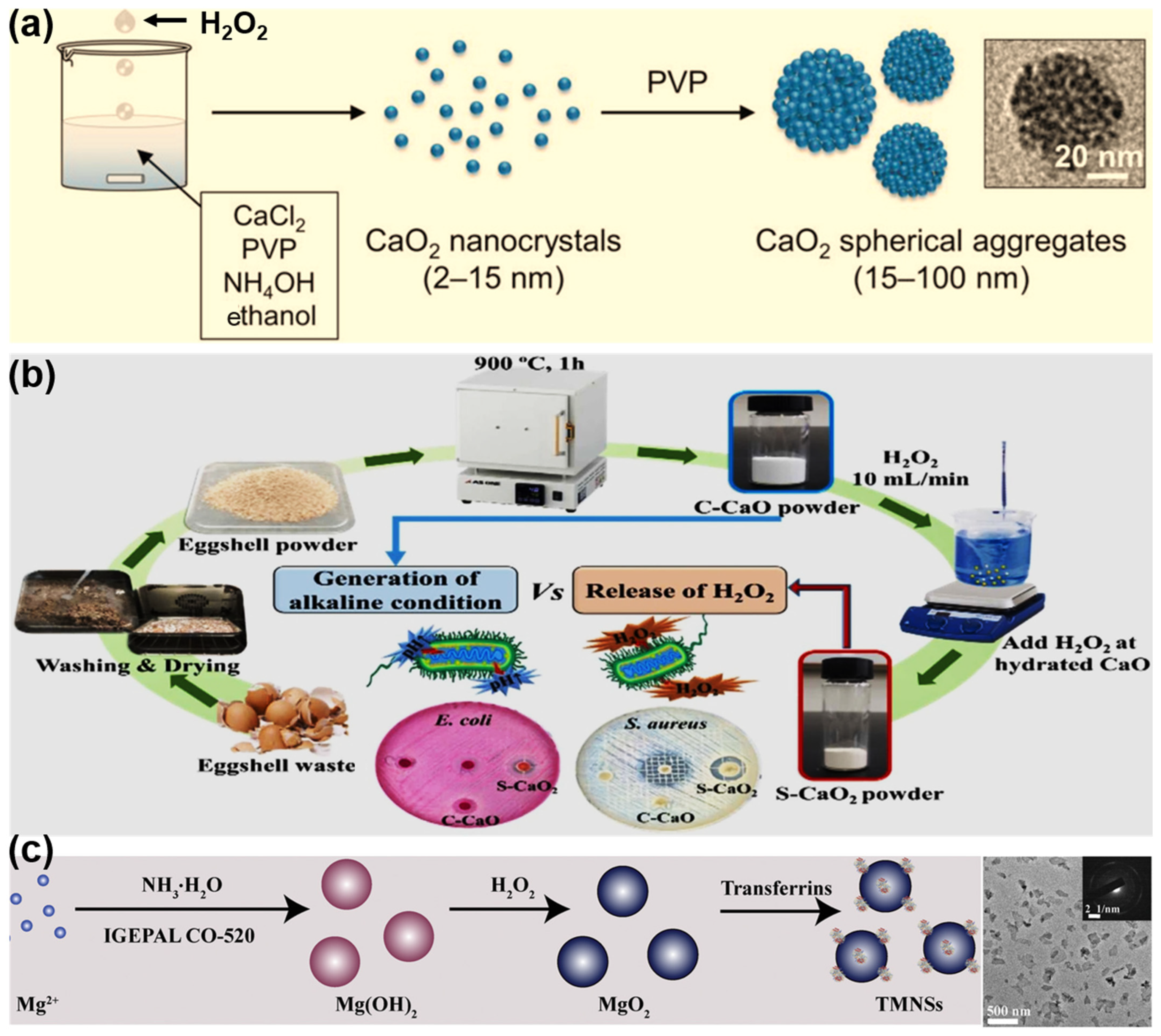
| Method | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Hydrolyzation–Precipitation | Metal ions form hydroxo–peroxo complexes → nucleation → growth and precipitation. | Simple, low cost, low temperature, scalability, good component homogeneity | Limited control over size/morphology; wide size distribution; possible incomplete precipitation; biocompatibility requires surface functionalization |
| Reversed-Phase Microemulsion | Nanodroplets act as microreactors—controlled nucleation/growth inside micelles. | Produces narrow size distribution, controlled morphology, versatile shapes (core–shell, nanowires) | Surfactant residues may lead to cytotoxicity and are difficult to remove; complex formulation; scale-up challenging due to emulsion stability |
| Sonochemical | Acoustic cavitation produces radicals → peroxo complexes → rapid nucleation inside bubbles. | Green (no toxic reagents), quick, mild conditions, yields clean, small (<30 nm), porous particles, easy core–shell formation, moderate scalability | Requires specialized ultrasound equipment; potential agglomeration if not controlled |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memić, A.; Abdullah, T. Oxygen-Generating Metal Peroxide Particles for Cancer Therapy, Diagnosis, and Theranostics. Future Pharmacol. 2025, 5, 41. https://doi.org/10.3390/futurepharmacol5030041
Memić A, Abdullah T. Oxygen-Generating Metal Peroxide Particles for Cancer Therapy, Diagnosis, and Theranostics. Future Pharmacology. 2025; 5(3):41. https://doi.org/10.3390/futurepharmacol5030041
Chicago/Turabian StyleMemić, Adnan, and Turdimuhammad Abdullah. 2025. "Oxygen-Generating Metal Peroxide Particles for Cancer Therapy, Diagnosis, and Theranostics" Future Pharmacology 5, no. 3: 41. https://doi.org/10.3390/futurepharmacol5030041
APA StyleMemić, A., & Abdullah, T. (2025). Oxygen-Generating Metal Peroxide Particles for Cancer Therapy, Diagnosis, and Theranostics. Future Pharmacology, 5(3), 41. https://doi.org/10.3390/futurepharmacol5030041








