Abstract
Biofilm-associated infections caused by Gram-negative bacteria, especially multidrug-resistant strains, frequently occur in intensive care units and represent a major therapeutic challenge. The economic burden of biofilm-associated infections is considerable, making the search for new treatment approaches a focal point for policymakers and scientific funding bodies. Biofilm formation is regulated by quorum sensing (QS), a population density-dependent communication mechanism between cells mediated by small diffusible signaling molecules. QS modulates various intracellular processes, and some features of QS are common to all Gram-negative bacteria. While there are differences in the QS regulatory networks of different Gram-negative bacterial species, a common feature of most Gram-negative bacteria is the ability of N-acylhomoserine lactones (AHL) as inducers to diffuse across the bacterial membrane and interact with receptors located either in the cytoplasm or on the inner membrane. Targeting QS by inhibiting the synthesis, transport, or perception of signaling molecules using small molecules, quorum quenching enzymes, antibodies, combinatorial therapies, or nanoparticles is a promising strategy to combat virulence. In-depth knowledge of biofilm biology, antibiotic susceptibility, and penetration mechanisms, as well as a deep understanding of anti-QS agents, will contribute to the development of antimicrobial therapies to combat biofilm infections. Advancing antimicrobial therapies against biofilm infections requires a deep understanding of biofilm biology, antibiotic susceptibility, penetration mechanisms, and anti-QS strategies. This can be achieved through in vivo and clinical studies, supported by state-of-the-art tools such as machine learning and artificial intelligence.
1. Introduction
Gram-negative bacteria are a major cause of a variety of infections in humans, particularly in healthcare settings, notably urinary tract infections (Escherichia coli), respiratory tract infections (Klebsiella pneumoniae and Pseudomonas aeruginosa), gastrointestinal infections (Salmonella, Shigella, and Campylobacter), healthcare-associated infections (E. coli, K. pneumoniae, and Acinetobacter baumannii), and sexually transmitted infections (Neisseria gonorrhoeae) [1]. Clinical data indicate that Gram-negative infections are associated with high morbidity and mortality due to their pathogenicity and increasing resistance to antibiotics. For example, Gram-negative infections account for approximately 15% of infections in neonatal intensive care units, with P. aeruginosa infections causing morbidity in 42% to 75% of infected neonates [2]. Gram-negative bacteria employ various strategies to overcome host defenses and evade the effects of therapeutics, resulting in high morbidity and mortality. Biofilm is one of the most important virulence factors that provides the bacteria protection against both host factors and therapeutics. Biofilms can form either on the patient’s tissues or on the surface of medical devices. According to the National Institutes of Health, biofilms may be responsible for up to 80% of chronic microbial infections in humans. Understanding the role of biofilms in infectious diseases, including associated tolerance and resistance to clinically relevant antibiotics is critical for public health. The antibiotic resistance crisis, fueled by the development and spread of genetic determinants of resistance to antibiotics and biofilm-associated infections, is exacerbated by the inadequate number of antibiotics in clinical development [3]. Although much is being conducted by policymakers and funding agencies, pharmaceutical companies are reluctant to continue or even abandon antibiotic development programs [4]. Due to increasing funding, many researchers have started to explore alternatives to antibiotics, focusing on therapies that target virulence and thus prevent selection pressure and the development of resistance [5]. Considering that increased antibiotic resistance in biofilm-associated bacteria impairs the efficacy of current treatments, the development of innovative therapeutic strategies for managing biofilm-associated infections is urgently needed. These innovative strategies focus on alternating QS, antibodies, combinatorial therapies, and nanotechnology [6,7,8,9], which can dissolve biofilms and improve antibiotic penetration even at low doses [10].
This review aims to summarize, analyze, and critically examine the current state of knowledge on biofilms formed by clinically relevant Gram-negative bacteria, the molecular mechanisms underlying this phenomenon, and innovative approaches for the treatment of biofilm-associated infections.
2. Bacterial Biofilm
Biofilms are a typical form of bacterial growth in natural and anthropogenic environments and play a crucial role in various ecosystems by influencing biogeochemical processes and nutrient cycling [11]. Commensal microorganisms that are part of a holobiont form biofilms that enable their persistence [12]. However, biofilms are often considered harmful due to their role in bacterial infections, and are considered difficult to treat due to their impermeability to antibiotics and resistance to the host’s immune system [13]. Considering the health and socioeconomic impact of biofilm-associated infections in humans, animals, and plants, research has often prioritized biofilms formed by pathogenic bacteria. However, there are numerous other processes where biofilms have adverse effects, e.g., spoilage in the food industry, clogging of oil and gas wells, water supply contamination, and in many other industrial areas [14,15,16]. The persistence of bacteria within the biofilm is mainly due to a self-produced matrix of proteins, polysaccharides, and extracellular DNA surrounding the bacterial community.
2.1. Mechanism of Biofilm Development
Biofilm formation is a rapid process that involves an initial reversible attachment of bacteria to abiotic or biotic surfaces through non-specific interactions, followed by irreversible attachment promoted by covalent interactions and hydrogen bonding [17]. During these attachment phases, an initial shift in gene expression enables irreversible attachment, while subsequent changes in gene expression and cellular phenotype enable extracellular matrix production, biofilm maturation, and finally, at the end of development, biofilm dispersion [18] (Figure 1).
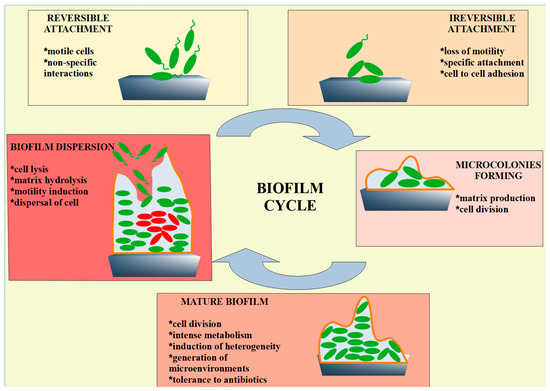
Figure 1.
Schematic representation of the most important phases of biofilm formation. During reversible attachment, bacteria transiently adhere to a surface through weak interactions such as van der Waals forces, electrostatic forces, and hydrophobic interactions. In the next step, stronger interactions occur when bacterial adhesins form stable covalent bonds with the surface, leading to irreversible attachment. The attached bacteria begin to divide and form clusters, creating microcolonies. The quorum-sensing system is activated, coordinating gene expression involved in biofilm development. The biofilm then becomes structurally complex (mature biofilm), consisting of layers of cells embedded in an extracellular matrix. Channels are formed that facilitate the exchange of nutrients and waste products, and the bacteria within the biofilm exhibit increased resistance to antibiotics and immune responses. Under certain conditions (e.g., nutrient depletion), some bacteria detach from the biofilm and return to a planktonic state (biofilm dispersion), allowing them to colonize new surfaces and initiate the formation of new biofilms.
Bacteria encounter solid surfaces through processes such as Brownian motion, sedimentation, or convection, and perceive chemical changes near the solid surface, initiating the attachment process, a process that is strongly influenced by the properties of the surface [19]. When the bacteria are close enough to the solid surface, van der Waals and electrostatic forces, together with hydrophobic interactions, enable initial attachment. The initial attachment process has been interpreted using DVLO theory (Derjaguin, Verwey, Landau, and Overbeek), the extended DVLO model, and thermodynamics as a balance between attractive interactions and repulsive forces based on electrostatic and hydration forces [20]. In transient, reversible attachment, bacteria use pili, flagella, proteins, non-protein polysaccharide capsules, or even exopolysaccharide—EPS (although it is crucial for biofilm development, it is also important in initial attachment) [21,22]. Due to the properties of these interactions, bacteria can be easily removed from the surface by fluid movement (e.g., body fluids or industrial rinsing). Stronger interactions between bacterial cells and the surface and a smaller distance between bacterial cells allow bacterial adhesins to form key covalent bonds with the solid surface. This mechano-sensor triggers signal transduction and a change in gene expression that allows a decrease in motility and increases the dwell time near the solid surface, leading to irreversible binding [23,24,25]. Flagella-mediated motility is important for the attachment steps during biofilm formation in several bacterial species such as P. aeruginosa, E. coli, and Vibrio cholerae, as flagella enable the overcoming of repulsive forces that may hinder cell–surface interactions [26,27,28,29].
When the cells irreversibly adhere to the surface, there is a significant increase in aggregation and proliferation of cells, resulting in microcolonies that are crucial for the subsequent phases of biofilm formation. During this phase, the bacteria effectively coordinate group actions by sensing their density through QS. Once attached, the bacteria produce extracellular polymeric substances (EPS; polysaccharides, nucleic acids, proteins, lipids, and lipoproteins) that form a matrix that engulfs cells in close proximity and enables intracellular interactions in a mechanically stable microenvironment. In addition, the EPS matrix is a cornerstone of biofilm tolerance to antibiotics, biocides, and elements of the host immune system [30]. As architectural scaffolds, biofilm polysaccharides provide structural support and stability for biofilms while protecting bacterial cells from stress factors. In addition, the polysaccharides in the biofilm matrix prevent desiccation and penetration of antimicrobial agents into the biofilm. Due to their high water storage capacity, polysaccharides maintain hydration in the biofilm [31]. The proteins in the biofilm matrix play various roles from biofilm formation to biofilm dispersion, including involvement in cell attachment, scaffold formation, stabilisation, and enzymatic activity. Curli fibres, for example, are important protein components of the E. coli biofilm matrix and contribute to the stability of the biofilm. Curli consists of the CsgB protein, which initiates polymerization, and the CsgA protein, a major component that forms the curli fibre. The resulting fibres are considered amyloid-like because they are rich in β-sheets and are stable under denaturing conditions [32]. Lipid-based components of the matrix are important for adhesion to hydrophobic surfaces as well as for binding of heavy metals and resistance to detergents, solvents, and disinfectants [33]. Extracellular nucleic acids (eDNA and eRNA) are considered important biomolecules for biofilm adhesion and viscoelasticity [34,35].
Although growth in a biofilm offers many advantages, there are, as mentioned above, many setbacks in the natural biofilm cycle that lead from the formation to the dispersion of the biofilm. Since the biofilm is a 3D structure, different microconditions can exist at different points in the biofilm. These gradients enable the spatial organization of different bacterial species within the biofilm. Cells located farthest from the biofilm surface experience a lack of oxygen and nutrients as well as accumulation of toxic metabolic waste products, which trigger a series of stress responses [36]. These cells initiate native dispersion of mature biofilms, a process that begins with evacuation through openings in the matrix, resulting in hollowing structures. Furthermore, this process has been linked with the diameter and thickness of the biofilm [37]. It is generally believed that the transport of specific inducers (such as cis-2-decenoic acid, cis-11-methyl-2-dodecenoic acid, cis-2-dodecenoic acid, pyruvate, etc.) is an important factor in the biofilm spreading response, as these inducers accumulate with the growth of the biofilm [38].
A general, comprehensive model of the biofilm cycle would include three main phases: Aggregation and Attachment (AA); Growth and Accumulation (GA); and Disaggregation and Detachment (DD). This model considers that bacteria can aggregate with each other or attach to biotic or abiotic surfaces (AA phase). Aggregated and/or attached cells grow and recruit other cells (GA phase). Finally, the bacteria leave the biofilm as aggregates or single cells (DD phase) [39].
2.2. Why Is Biofilm the Most Important Virulence Factor?
Biofilm-associated infections are notorious for their persistence once the biofilm is established and has reached maturity. General estimates suggest that approximately 65% of all bacterial infections and 80% of chronic bacterial infections are associated with biofilms, both device-related and non-device-related [40]. The biofilm is considered an important virulence factor, as it not only enables the protection of pathogen cells but also provides a microenvironment that promotes virulence. The protection of the cells is reflected in a lower sensitivity to antimicrobial agents and resistance to the host’s immune system. The proximity of cells within the biofilm creates a microenvironment that promotes cell-to-cell signaling, leading to changes in gene expression that result in higher virulence, as well as horizontal gene transfer, particularly plasmid transfer, many of which carry genetic determinants of resistance to various antimicrobials. Thus, bacterial biofilms are important virulence factors as they are highly resistant to antibiotics and immune responses. The elimination of biofilm-associated infections is difficult, uncertain, and depends on whether they are associated with a device or not. If the infection is not device-associated, prolonged treatment with high doses of antibiotics targeting multiple bacterial pathways is usually administered. If a medical device is present, it usually needs to be removed to eliminate the infection.
2.3. Biofilm and Resistance to Antibiotics
Resistance to antibiotics is a critical global health and socio-economic problem. Bacterial pathogens evade the effects of antibiotics through various mechanisms, including biofilm formation. As antibiotic therapies become increasingly ineffective due to resistance, this threatens medical procedures and could have a devastating impact on infection-related mortality caused by infections in the future. The socio-economic impact of resistance to antibiotics is also enormous, with predicted losses amounting to trillions of dollars.
The growth of biofilms is considered to be one of the main causes of resistance to antibiotics due to their distinctive structure and the associated adaptation mechanisms. The specific structure of the biofilm offers the bacteria protection from antibiotics through EPS and allows clustering of cells, increasing cell density and resulting in QS communication. Adaptive mechanisms within the biofilm that promote antibiotic resistance include horizontal gene transfer, activation of efflux pumps, and mutations (e.g., within antibiotic targets) [41]. Rapid adaptation to antibiotics is one of the capabilities of biofilms and can be observed when biofilms are exposed to both low and high concentrations of antibiotics. It is known that bacteria in biofilms, including clinically relevant pathogens such as A. baumannii and P. aeruginosa, are up to 1000 times more resistant compared to their free-living forms [13,42].
Biofilm resistance to antibiotics is based on many mechanisms, including EPS as a physical barrier, slow bacterial growth and low metabolism, genetic variations, adaptive stress response, and persister cells. The physical characteristics of the biofilm (e.g., thickness, density, mechanical properties) have a significant impact on the antibiotic susceptibility of biofilms. The chemical composition of the EPS matrix also influences the sorptive properties of the biofilm and changes the susceptibility to antibiotics. The EPS matrix could serve as a molecular sieve that retains the antibiotics due to their size and 3D structure. Furthermore, it has been shown that functional groups in EPS can interact with antibiotics and bind them based on their chemical properties and reactivity [43]. In addition, EPS could contain antibiotic-modifying or antibiotic-degrading enzymes, which is of particular importance in mixed biofilms. Considering the nutrient and oxygen fluctuations in microenvironments of biofilms, the metabolic states of cells within those microenvironments may vary and lead to antibiotic tolerance and persistence [42]. Cells located in the centre of the biofilm, where oxygen and nutrient rates are low, have reduced growth rates, making them tolerant to antibiotics that target cell division or other active cellular functions [44]. Genetic variations are one of the drivers of resistance to antibiotics in biofilms and are promoted by horizontal gene transfer of mobile genetic elements, an increase in mutation rate, and stress responses. The resulting genetic plasticity drives the evolution of antibiotic resistance in bacterial biofilms [45].
3. Clinical Relevance of Gram-Negative Biofilm-Associated Infections
Gram-negative bacteria, especially multidrug-resistant bacteria (MDR-GNB), have become one of the most common causes of infections in humans, both in the general population and in healthcare settings [46]. MDR-GNB pose a particular threat to patients in intensive care units as they are associated with high morbidity and mortality [1]. Among MDR-GNB, Enterobacteriaceae and non-fermenting Gram-negative bacilli (BNF) are considered the most important pathogens causing infections in humans. Isolates belonging to the Enterobacteriaceae are responsible for about 80% of infections caused by GNB, mainly, but not only, urinary tract infections, pneumonia, diarrhea, meningitis, sepsis, and endotoxic shock [47]. On the other hand, BNFs as infectious agents are not as common as Enterobacteriaceae, but cause severe, often fatal infections, especially in clinical settings [47]. Common risk factors for various MDR-GNB are comorbidities, antibiotic use, previous colonization, ICU stay, dialysis, mechanical ventilation, travel, etc. [1]. Data show that E. coli, K. pneumoniae, and P. aeruginosa were each associated with more than 500,000 deaths worldwide in 2019 [48]. In addition, the burden of years of life lost was reported to be 31.4 million (23.2–41.5) for K. pneumoniae, 30.4 million (22.7–40.2) for E. coli, and 18.9 million (13.6–25.7) for P. aeruginosa [48]. The study by Ikuta and coauthors found that the age-standardized mortality rates for the five major GNB in 11 infectious syndromes studied were as follows: E. coli 12.6 (9.1–16.9), K. pneumoniae 10.6 (7.7–14.2), P. aeruginosa 7.4 (5.2–10.2), A. baumannii 5.8 (3.5–8.9), Enterobacter spp. 4.2 (2.8–6.1) [48].
Biofilms worsen the prognosis for MDR-GNB infections due to increasing resistance to antibiotics and the host’s immune system, leading to chronic infections, prolonged hospitalization, and higher mortality. Biofilms have been detected on medical devices such as catheters, contact lenses, heart valves, ventricular shunts, various implants, and joint prostheses, but also in human tissue independent of medical devices [1].
3.1. Biofilm-Mediated Infections in Human Tissues
Biofilms occur in most environments, including the human body, where biofilms are found in/on the tissues of healthy individuals, and are formed by commensal, non-pathogenic bacteria. In this review, however, we focus on infection-associated biofilms. The most common biofilm-associated infections in human tissues are endocarditis, osteomyelitis, periodontitis, cystic fibrosis, chronic tonsillitis, ulcers, skin wounds, and urinary tract infections [49].
The first biofilm-associated infections were found in the lungs of cystic fibrosis patients [50] and are considered a hallmark of chronic infections, although more recent evidence shows that biofilms are also associated with acute respiratory infections [51]. It is estimated that more than 70,000 people worldwide live with cystic fibrosis, with an economic impact of more than USD 7 billion annually, most of which is due to complications associated with biofilms [52]. Biofilms have also been found in the middle ear and upper respiratory tract. Biofilms in the respiratory tract are predominantly monospecies biofilms, where multiple species are not involved in the infection [53]. The GNB that form biofilms in the human respiratory tract mainly include P. aeruginosa, Burkholderia cepacia complex, H. influenzae, Moraxella catarrhalis, and K. pneumoniae [52]. Thick biofilms of monospecies or multispecies were found in wounds, with P. aeruginosa being the most common GNB. It is reported that more than 70% of chronic wounds are associated with biofilms. It is estimated that global expenditure on wounds associated with biofilms exceeds USD 250 billion [53]. Osteomyelitis-associated biofilms are predominantly multispecies with P. aeruginosa, Bacterioides spp., and Serratia marcescens as the most common GNB [54,55]. In the urogenital tract, GNB-associated biofilms formed by P. aeruginosa, E. coli, K. pneumoniae or Proteus mirabilis have been found in chronic prostatitis, bladder infections, and on/in kidney stones [56,57]. Biofilms are associated with infections of the circulatory system, such as endocarditis (10,000–15,000 cases/year in the USA) and with atherosclerosis, but these biofilms are mainly formed by Gram-positive bacteria such as S. aureus, Streptococcus spp., and Enterococcus spp. [54].
In the models of biofilm-associated pathogenesis, it is still controversial whether the biofilm itself is the cause of the disease or whether it forms due to favorable conditions and exploits these conditions of the underlying disease.
3.2. Biofilm-Related Infections on Medical Implants and Devices
Healthcare-associated infections caused by non-sterile medical devices, particularly urinary catheters, orthopedic implants, ophthalmic devices, and intravascular catheters, lead to an increase in mortality and morbidity and pose a serious threat in both underdeveloped and highly developed countries worldwide [58]. The incidence of biofilm-associated infections in medical devices is exacerbated by the increasing length of stay of patients in hospitals and inadequate epidemiologic measures in some countries. The specific surface characteristics of the device, its chemical composition, and localization in the patient’s body determine the preference and ability of bacteria to form a biofilm. Biofilm-forming bacteria originate from the skin of patients, healthcare workers, or the environment. Biofilm formation on medical devices makes it difficult to eliminate the infection-causing bacteria and is very costly due to the persistence of the infection, inflammation, failure of antibiotic therapies, and recurrence of the infection. Gram-negative bacteria are known to be prevalent or present in biofilms on certain devices: Gram-negative bacilli (orthopedic devices, intravascular catheters), E. coli (catheters), P. aeruginosa (endotracheal tubes, contact lenses), A. baumannii (ventilators and indwelling catheters), K. pneumoniae (bronchoscopy), Enterobacter spp. (urinary tract infections and intravascular devices), and Prevotella intermedia (dental implants) [59,60].
Biofilms are the main cause of the recurrence of catheter-associated infections. The route of infection, e.g., in central venous catheters, is mainly through the migration of bacteria along the part of the catheter placed outside the body, under the skin, and in the tissue, and colonization of the intravascular part of the catheter, where a biofilm forms. The annual cost of medical device-related infections, including those caused by biofilms, is substantial and is estimated at USD 11.6 billion worldwide for central venous catheters and USD 1 billion for urinary tract infections [61]. Although the insertion of pacemakers and defibrillators is considered a low-risk procedure, up to 3% of patients develop a biofilm-associated infection within the first year [62]. Treatment of these infections usually requires replacement of the devices in addition to antibiotic therapy, at an estimated cost of 220 million dollars annually [62,63]. Endotracheal tubes are considered high-risk for the development of biofilm-associated infections, which have sometimes been detected within the first 24 h after intubation. These infections can lead to pneumonia, and given the number of patients receiving mechanical ventilation in ICUs, the burden of these infections is significant and is estimated to be close to 1 billion in the US alone [62,64]. In some cases, biofilm-associated infections associated with medical devices can occur years after implantation, as has been reported in the case of prosthetic joints. These infections are usually resolved by surgical removal of the joint prosthesis and therefore result in high annual average costs, estimated at approximately USD 8 billion worldwide [65,66]. Ophthalmic complications due to biofilm infections from contact and artificial lenses are also significant, with the estimated risk of infection being almost nine times higher when wearing contact lenses [67].
4. Quorum Sensing Mechanisms in Major Gram-Negative Pathogens
QS is a sophisticated regulatory system that enables bacteria to communicate either within the same species or between different species in a shared community. The communication process is based on the production, secretion, recognition, and response to extracellular signaling molecules called autoinducers (AI) [68]. When the concentration of signalling molecules exceeds a critical threshold, bacteria recognize these signals, leading to changes in the expression of target genes involved in numerous processes. Functionally, these processes can be divided into four categories: (i) maintenance and proliferation of the bacterial population—production of exoenzymes, sporulation, siderophore synthesis, acid resistance; (ii) behavioral responses—biofilm formation, maturation and dispersal, adhesion, motility; (iii) horizontal gene transfer—competence development, plasmid conjugation; (iv) interactions with other microorganisms and the host—production of virulence factors and EPSs, antibiotic synthesis, bioluminescence and factors involved in host colonization [69]. Gram-negative bacteria utilize two main types of QS systems. Type 1 QS is predominantly used for intra-species signaling and is based on AHLs classified as autoinducers-1 (AI-1). These AHL molecules interact with their specific receptor, the transcriptional regulator LuxR, to influence the expression of target genes. Type 2 QS facilitates both intraspecies and interspecies communication and utilizes cyclic furanone compounds known as autoinducers-2 (AI-2). The enzyme LuxS plays a central role in the synthesis of AI-2 molecules [70]. This review provides a detailed overview of the QS network in Gram-negative bacteria, P aeruginosa, A. baumannii, K. pneumoniae, E. coli, and Burkholderia spp. Most of these bacteria belong to the ESKAPE pathogen list (E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, Enterobacter spp.) and have been recognized as emerging public health threats for which effective therapies are urgently needed [71]. The elucidation of QS mechanisms in Gram-negative pathogenic bacteria can therefore contribute to the development of innovative strategies for infection control.
4.1. Pseudomonas aeruginosa QS Systems
P. aeruginosa is one of the most important opportunistic pathogens frequently associated with chronic infections in immunocompromised individuals, such as those with cystic fibrosis, burns, and hospitalized individuals. Its ability to form biofilms, produce an arsenal of virulence factors, and exhibit a high degree of antibiotic resistance poses a challenge for clinical management. In particular, some strains of P. aeruginosa are resistant even to carbapenems and third-generation cephalosporins, which are often considered a last resort for the treatment of MDR Gram-negative bacterial infections [72].
QS is of fundamental importance for the regulation of pathogenicity and virulence of P. aeruginosa. This bacterium has one of the most complex and well-studied QS networks, consisting of three QS systems—las, rhl, and the quinolone-based pqs system (Figure 2). The las and rhl systems use AHLs (oxo-C12-HSL and C4-HSL, respectively), while the pqs system uses 2-heptyl-4-quinolone (PQS) as signaling molecules. The auto-inducer 3-oxo-C12-HSL forms a complex with the transcription activator LasR, which leads to the activation of its transcription. This mechanism creates a positive feedback loop in which 3-oxo-C12-HSL induces its synthesis. LasR was originally identified as a key regulator of lasB gene expression, which encodes the metalloprotease elastase. In addition to elastase, the las system also controls the production of exotoxin A, alkaline protease, pyocyanin, hydrogen cyanide, and lectin, and controls biofilm formation [73]. The second QS system, rhl, was initially identified as the regulatory mechanism responsible for rhamnolipid biosynthesis. Later studies showed that it also controls the production of other exoproducts, including hemolysin, chitinase, hydrocyanic acid, and pyocyanin. Its signaling molecule C4-HSL, which is synthesized under the control of the rhlI gene, forms a complex with the RhlR receptor that activates the transcription of target genes. This creates a positive feedback loop, similar to the mechanism described for the Las system [74]. Furthermore, these two systems play an important role in the adaptation and response of P. aeruginosa to various environmental stressors, including heavy metals, high salinity, heat, and oxidative stress [75]. The synthesis of the third autoinducer, PQS, is under the control of the pqsABCDE operon. Upon reaching a threshold concentration, PQS binds to its receptor, PqsR (also known as MvfR), leading to the activation of target gene expression, which in turn results in the synthesis of pyocyanin and PQS itself. This QS system is crucial for biofilm formation, motility, and the production of virulence factors such as pyocyanin and lectins [73]. In addition to this role, the pqs system plays an important role in bacterial iron metabolism. It has iron chelating activity and forms complexes with ferric iron at physiological pH, which is important for iron acquisition in iron-deficient environments [76]. All three QS systems of P. aeruginosa are organized in a complex hierarchical network. At the top of this network is the las system, which regulates the other two systems, while each QS system can autoregulate through positive feedback loops. The rhl system occupies the lowest node in this hierarchy and integrates signals from the las and pqs systems [73].
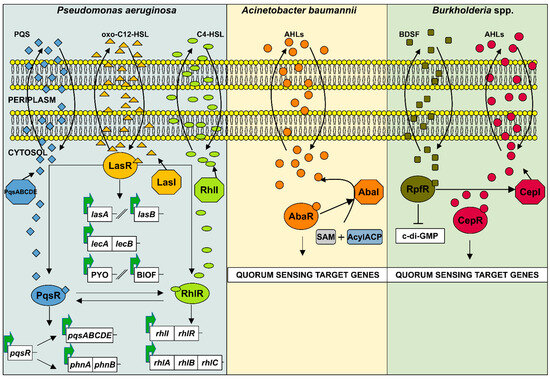
Figure 2.
Schematic representation of AHL-based quorum sensing systems in Gram-negative bacteria Pseudomonas aeruginosa, Acinetobacter baumannii, and Burkholderia spp. (1) The QS network of P. aeruginosa consists of three interconnected systems las, rhl, and pqs. The las and rhl systems produce the AHLs 3-oxo-C12-HSL and C4-HSL, respectively, while pqs produces the Pseudomonas quinolone signal (PQS). These signaling molecules form complexes with their cognate transcriptional regulators (LasR, RhlR, and PqsR) to activate both their own synthesis and the expression of target genes associated with virulence and biofilm formation. (2) In A. baumannii, the LuxI-type autoinducer synthase AbaI catalyzes the synthesis of AHLs from S-adenosyl-L-methionine (SAM) and acyl-acyl carrier protein (acyl-ACP). Once a threshold level of AHL concentration is reached, the molecules bind to the receptor AbaR, which then regulates the expression of QS target genes. (3) Burkholderia spp. utilize a two-component QS system, CepI/R. The autoinducer synthase CepI produces AHLs, which bind to the receptor CepR. Activated CepR then binds specific promoter regions to regulate QS target gene expression. In addition, B. cenocepacia utilizes the BDSF system to regulate virulence. BDSF binds to the receptor RpfR, leading to a decrease in intracellular c-di-GMP levels and increased production of virulence factors and biofilm formation.
Finally, there has been much controversy about the fourth QS system of P. aeruginosa, iqs, but the latest observation by Cornelis [77] suggests that the IQS molecule is aeruginaldehyde, a by-product of the siderophore pyochelin, and not the autoinducer of the fourth QS system as previously thought. Furthermore, the ambABCDE gene cluster, once thought to be responsible for IQS synthesis, is not involved in its production.
4.2. Acinetobacter baumannii QS Systems
A. baumannii is another notorious Gram-negative opportunistic pathogen widely distributed in natural environments. Due to its remarkable ability to adhere, colonize, and persist on biotic and abiotic surfaces, it can survive in hospital environments, especially in intensive care units. A. baumannii has been associated with a number of infections, including ventilator-associated pneumonia, bloodstream infections, secondary meningitis, and surgical wound infections [78]. These infections mainly affect immunocompromised individuals and are often difficult to treat due to the multidrug resistance of A. baumannii strains and their exceptional ability to form a robust biofilm.
The QS system of A. baumannii consists of a two-component system, AbaI/AbaR, which is homologous to LuxI/LuxR typically found in Gram-negative bacteria (Figure 2). The AbaI encodes several AHLs with an acyl chain length of 10–16 C atoms, with 3-hydroxy-C12-HSL being the most important AHL [79]. As in P. aeruginosa, the AHLs interact with the receptor protein AbaR and subsequently regulate the expression of genes involved in resistance to antibiotics, biofilm formation, surface motility, and EPS production. The aba locus also contains a third gene, abaM, which is located between abaI and abaR, and encodes a protein homologous to the RsaM family that regulates AHL-based QS in other bacteria. The authors López-Martín and colleagues [80] postulate that AbaM plays a central role in the regulation of surface motility and biofilm formation in A. baumannii.
The crucial role of the aba-QS system for virulence and resistance to antibiotics was also confirmed in two further studies. An AHL-deficient mutant of A. baumannii strain S (AbS-M) showed increased susceptibility to meropenem and piperacillin compared to the wild-type strain. Resistance was restored by exogenous addition of 3-hydroxy-C12-HSL. In addition, AbS-M showed decreased expression of important antibiotic resistance genes (Oxa-51, ampC, adeA, and adeB) in the presence of meropenem, while the addition of 3-hydroxy-C12-HSL also restored the expression of these genes [81]. In another study, an isogenic mutant of abaR was constructed. The results showed that the abaR-deficient mutant is characterized by a significant reduction in the expression of abaI and significant defects in motility, pellicle, and biofilm formation of this pathogen [82].
Recent studies by Cui and colleagues [83] have reported that A. baumannii may use indole as a signaling molecule to regulate its physiology and virulence. It is already known that some bacteria produce indole, a molecule that regulates intraspecies signalling and interspecies or interorganism communication. Its biosynthesis is mediated by AbiS, while the response regulator AbiR binds indole to modulate gene expression. This indole-based AbiS/AbiR signaling system may represent a new QS mechanism in A. baumannii but needs to be confirmed and further investigated.
4.3. Burkholderia spp. QS System
The species of the Burkholderia cepacia complex (BCC) are ubiquitous in the environment. Their ecological versatility is attributed to their unusually large and complex genomes, typically comprising two to four replicons, including chromosomes and megaplasmids, and their ability to utilize a wide range of compounds as sole sources of carbon and energy [84]. In addition, BCC species can successfully colonize hospital environments, mainly due to their intrinsic resistance to antibiotics and disinfectants, as well as their successful patient-to-patient transmission. This is of particular concern for people with cystic fibrosis, as person-to-person transmission can lead to the development of the fatal necrotizing pneumonia known as cepacia syndrome [85].
BCC uses an AHL-based two-component QS system, CepI/R, to regulate virulence and various phenotypic traits, including colonization and niche adaptation (Figure 2). The CepI synthesizes mainly C8-HSL and, as a by-product, C6-HSL. In addition, certain strains of B. vietnamiensis produce the signaling molecule C10-HSL. In its activated form, CepR binds to the specific DNA sequence known as Cep boxes located upstream of target genes, resulting in the induction or repression of their expression. This QS system controls biofilm formation, motility, proteolytic and antifungal activities, as well as the production of siderophores and proteases [86].
Burkholderia has also been reported to utilize another QS mechanism to regulate various physiological processes. BDSF—Burkholderia diffusible signal factor is a QS system based on cis-2-dodecenoic acid and was first identified in B. cenocepacia [87]. BDSF is synthesized by RpfF and recognized by RpfR. This signaling molecule accumulates in a cell density-dependent manner, but has no positive feedback regulation like the CepI/R system [88]. BDSF and RpfR negatively control intracellular c-di-GMP levels while positively controlling bacterial motility, biofilm formation, proteolytic activity, and EPS production [88]. In addition, DSF family molecules, including BDSF, are involved in interspecies and interkingdom communication [86].
4.4. Klebsiella pneumoniae QS Systems
K. pneumoniae is considered one of the most worrying opportunistic pathogens and is often associated with nosocomial infections, accounting for about one-third of all Gram-negative infections. This bacterium can cause urinary tract infections, cystitis, surgical wound infections, pneumonia, septicemia, and endocarditis [89]. The plasticity of the K. pneumoniae genome facilitates the rapid acquisition of antibiotic resistance genes, such as those encoding extended-spectrum β-lactamases and carbapenemases [90]. In addition, several virulence factors contribute to its pathogenicity, including a protective polysaccharide capsule, robust biofilm formation, and efficient iron acquisition systems (siderophores).
In addition to several Gram-positive and Gram-negative bacteria, K. pneumoniae also utilizes a type 2 QS system to control virulence factor production and biofilm formation [91] (Figure 3). As previously mentioned, AI-2 is synthesized by LuxS synthase, a crucial enzyme in the activated methyl cycle that catalyzes the conversion of SAM to homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD), the latter of which is spontaneously rearranged to generate AI-2 molecules [92]. Like in E. coli, the export of AI-2 molecules is regulated by TqsA transmembrane proteins. The AI-2 metabolism is regulated by the lsrACDBFG and lsrRK operons, which control the uptake, processing, and regulation of AI-2 in K. pneumoniae. The lsrACDB genes encode an ABC transporter, while lsrFG are involved in the processing of AI-2. Internalized AI-2 is phosphorylated by LsrK, and phospho-AI-2 inactivates the repressor LsrR, thereby promoting transcription of the lsrACDBFG operon. The lsrACDBFG and lsrRK operons are upregulated in mature biofilm [93]. In this bacterium, QS regulates biofilm development by modulating the expression of genes involved in key structural components such as fimbriae and EPSs, such as lipopolysaccharides (LPSs). Balestrino and colleagues [91] reported that a luxS-deficient mutant strain of K. pneumoniae exhibited a reduced ability to form microcolonies, especially in the early stages of biofilm formation, although the strain was still able to form a mature biofilm. In another study, K. pneumoniae strains lacking both AI-2 synthesis and transport were reported to have increased expression of LPS-associated genes. This led to the formation of biofilms with larger biomass but altered architecture [94]. The authors proposed that AI-2 plays a regulatory role in both biofilm formation and LPS biosynthesis in sessile K. pneumoniae cells.
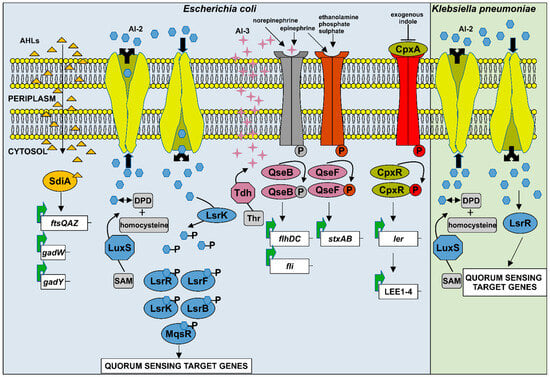
Figure 3.
Schematic representation of the quorum sensing systems in Escherichia coli and Klebsiella pneumoniae. (1) E. coli has four QS systems: SdiA, AI-2, AI-3, and indole-based QS. The SdiA regulator senses AHLs produced by other bacteria in the environment and modulates the expression of numerous genes involved in virulence and biofilm formation. The AI-2 system is considered the most important QS system in E. coli. AI-2, which is synthesized from the precursors SAM and DPD, is phosphorylated by the kinase LsrK and binds to the repressor LsrR, inactivating it. The enzymes LsrF and LsrG further convert the phosphorylated AI-2 into compounds dihydroxyacetone phosphate and acetyl-CoA. The global regulators LsrK and LsrF influence numerous pathogenicity-related processes, while LsrB promotes aggregation and biofilm formation, and MqsR modulates bacterial motility. The third signaling molecule, AI-3, is synthesized from threonine (Thr) by the enzyme threonine dehydrogenase (Tdh). AI-3, together with the host-derived catecholamines epinephrine and norepinephrine, and other metabolites such as ethanolamine, phosphate, and sulphate, is recognized by the two-component systems QseC/QseB and QseE/QseF. These systems activate QS-regulated genes via phosphorylated response regulators QseB and QseF. Indole, another QS signal, is sensed by the CpxA/CpxR two-component system. In this pathway, CpxA autophosphorylates and transfers the phosphate to CpxR, leading to activation of LEE genes expression and regulation of various virulence-associated phenotypes. (2) Similar to E. coli, K. pneumoniae also utilizes the AI-2 QS system. After synthesis, AI-2 is phosphorylated by LsrK, and phosphorylated AI-2 inactivates LsrR, promoting transcription of QS-regulated genes involved in virulence factor production and biofilm formation.
Although K. pneumoniae does not produce AI-1 molecules (AHLs), which are communication signals for type 1 QS, this bacterial species encodes SdiA, a LuxR-type receptor that can sense and respond to AHL molecules synthesized by other bacteria [95]. As a QS regulator, SdiA suppresses the expression of fimbriae and the adherence and aggregation of bacterial cells, thereby downregulating biofilm formation. K. pneumoniae frequently coexists in mixed biofilms with P. aeruginosa and increases resistance to antibiotics (tobramycin) and detergents [96], but interestingly, these interactions appear to be independent of the las and rhl QS systems of P. aeruginosa [97].
4.5. Escherichia coli QS Systems
E. coli is a Gram-negative bacterium that frequently occurs as a commensal in the gastrointestinal tract of humans and animals. While many strains are harmless, certain variants have evolved into opportunistic pathogens that can cause serious infections, including gastrointestinal diseases and urinary tract infections. The emergence of MDR serotypes has exacerbated the threat posed by E. coli, as these strains can lead to a variety of diseases and are increasingly difficult to treat. As a result, E. coli is considered one of the most challenging human pathogens worldwide [98]. The pathogenesis of E. coli is not only attributed to its MDR phenotype but also to its virulence potential, which is controlled by a complex QS network.
E. coli has four major QS systems: (i) the orphan SdiA regulator (LuxR homolog), (ii) the LuxS-based AI-2, (iii) the AI-3/epinephrine/norepinephrine system, and (iv) the indole-based QS system [99] (Figure 3). E. coli does not synthesize AHLs but can sense these molecules in the environment through the SdiA regulator. SdiA binds a broad spectrum of exogenous AHLs produced by other bacterial species and enables E. coli to detect the presence and density of neighboring bacteria. Upon AHL binding, SdiA modulates the expression of specific genes, leading to either activation or repression, thus facilitating interspecies communication. This ability provides E. coli with an adaptive advantage in responding to QS signals from both cooperating and competing bacteria, and allows it to adapt to complex environmental conditions [100]. The SdiA-mediated QS regulates multiple virulence-associated phenotypes, including acid and drug resistance, colonization and survival in the gastrointestinal tract, adherence, and biofilm formation [99].
The major QS system in E. coli is mediated by the LuxS/AI-2 pathway. As in the previously described K. pneumoniae, AI-2 in E. coli is derived from SAM via the enzyme LuxS synthase and then transported into the extracellular environment by the TqsA protein, where it accumulates. Once the extracellular concentration of AI-2 reaches a threshold level, it is internalized by the ABC type of Lsr transporter and phosphorylated by the LsrK kinase, resulting in phospho-AI-2. This phosphorylated form binds to the transcriptional repressor LsrR, which is thereby inactivated and induces expression of the lsr operon, which includes genes involved in the uptake and processing of AI-2. The enzymes LsrF and LsrG are involved in the processing of phospho-AI-2 and degrade it to products such as dihydroxyacetone phosphate and acetyl-CoA [101]. In addition to its role in AI-2 metabolism, the AI-2 QS system influences various physiological processes in E. coli via its global regulators LsrK and LsrF, including colonization, adherence, aggregation, and biofilm formation. In addition, AI-2 has been shown to influence chemotactic responses by interacting with the periplasmic binding protein LsrB to promote aggregation and biofilm formation. Via the motility QS regulator MqsR, AI-2 is also involved in the regulation of genes related to bacterial motility [99,102].
The third QS system in E. coli, known as AI-3, facilitates interkingdom communication [103]. AI-3 is synthesized from threonine (Thr) by the enzyme threonine dehydrogenase (Tdh). This autoinducer interacts with host-derived catecholamine hormones such as epinephrine and norepinephrine, as well as other metabolites such as ethanolamine, phosphate, and sulphate. These molecules are recognized by two-component systems, mainly QseBC and QseEF. When QseC senses these signals, it is autophosphorylated and transfers the phosphate to response regulators, including QseB and QseF, initiating a complex signalling cascade. This cascade regulates various virulence-associated phenotypes in E. coli, such as colonization, motility, and biofilm formation [99,103].
Indole, as a QS signaling molecule, is a product of tryptophan degradation through the activity of the enzyme tryptophanase. This signal molecule is recognized by the CpxA/CpxR two-component system. In this system, CpxA autophosphorylates and subsequently phosphorylates the response regulator CpxR, which leads to the activation of LEE gene expression. Indole influences numerous cellular functions, including antibiotic resistance, plasmid stability, sporulation, and biofilm formation [104]. However, the role of indole as a QS molecule is controversial. While some researchers consider it a QS signal involved in cell density-dependent communication processes, others argue that it functions primarily as a metabolic by-product that influences bacterial physiology [99].
5. Mechanisms of Gram-Negative QS Silencing (Quorum Quenching)
The worrying emergence and spread of bacterial resistance to antibiotics has prompted the search for new strategies to combat this problem. One of the new strategies to combat bacterial infections targets the cellular processes responsible for virulence and pathogenicity. This approach is known as antivirulence or antipathogenic therapy. In contrast to conventional antibiotics, antivirulence agents offer potential advantages in the fight against infection, as they specifically disarm the pathogens instead of inhibiting their growth. This reduces the selection pressure and therefore the likelihood of resistance developing. As these agents target specific pathogens, they are also likely to have minimal impact on commensal bacteria [105]. The primary target of antivirulence strategies is the QS system, which regulates the production of virulence factors and facilitates host tissue invasion, key mechanisms of bacterial pathogenicity. The discovery of bacterial cell-to-cell communication silencing has enabled the development of novel antivirulence therapies. This phenomenon, quorum quenching (QQ), was first described in 2000 when Dong and colleagues identified the enzyme AiiA lactonase from the strain Bacillus sp. 240B1, which showed remarkable efficacy in reducing the virulence of the plant pathogen Erwinia carotovora [106]. Quorum quenching interferes with bacterial communication by targeting one of the three main components of the QS system: the synthesis of signaling molecules, the signaling molecules themselves, or their receptors. Various QQ mechanisms have now been identified that differ in their chemical nature, modes of action, and molecular targets [69]. Based on their nature, QQ agents are categorized into quorum-sensing inhibitors (QSI), which are small non-protein molecules, and quorum quenching enzymes (QQE), which are protein macromolecules. The terms quorum quenching and quorum sensing inhibitors were initially used to describe the enzymatic degradation of AHL signaling and the inhibition of QS by small molecule antagonists. To avoid semantic confusion, the term quorum quenching is now used in a broader sense to refer to any disruption of the QS system, including QS inhibitors (QSIs) and quorum quenching enzymes (QQEs).
5.1. Quorum Sensing Inhibition by Small Molecules
QSIs represent an extremely diverse group of molecules that are able to inactivate AI synthases or disrupt the QS system through competitive binding or structural modification of AI receptors. A variety of QSI molecules have been identified in organisms from all domains of life, including microorganisms; fungi; plants; and animals from freshwater, marine, and terrestrial ecosystems. Plants are considered to be one of the main sources of QSI [69]. In addition to natural QSI, numerous synthetic compounds with QSI activity have also been developed [107,108]. However, despite the abundance of organisms and extracts with QSI activity, the active compounds have only been fully characterized in a few cases. The biochemical nature of QSIs is very diverse. With the exception of structural analogs of signaling molecules, which generally act as competitive inhibitors, there is no consistent relationship between the chemical structure or functional groups of a QSI and its specific QS target.
Table 1 provides a list of QSI and QQ molecules and extracts that silence QS systems of P. aeruginosa, A. baumannii, K. pneumoniae E. coli, and Burkholderia spp., including antivirulence and antibiofilm agents. There is also information on their mode of action and use, including in vitro or in vivo application, as well as on their origin—synthetic or natural, including bacteria, animals, and fungi. Additionally, QQ molecules of plant origin, including both extracts and molecules, are given in Table 2 with detailed information about the origin, application, and mode of action/effect.

Table 1.
The list of small molecules QSI inhibitors and QQ enzymes effective against the studied Gram-negative bacteria.

Table 2.
The list of plant extracts and molecules with QQ and antibifilm activity.
5.1.1. Inhibitors of QS Molecules Synthesis
To prevent the production of signaling molecules, either the synthesis of precursors or the activity of autoinducer synthases must be inhibited. AHLs are synthesized from two important precursors: S-adenosylmethionine (SAM) and acyl carrier protein (ACP). Since both SAM and ACP are essential bacterial metabolites, complete inhibition of their synthesis is lethal. However, triclosan has been shown to inhibit AHL synthesis at sublethal concentrations [69]. The activity of LuxI synthase and its homologs can be inhibited by end products, reaction intermediates, and substrate analogs. For example, butyryl-SAM and sinefungin, structural analogs of SAM, effectively block AHL synthesis [209]. Extensive data are also available on inhibitors of the pqs system of P. aeruginosa [209]. Storz et al. [210] identified a new class of PqsD inhibitors that significantly reduce the PQS precursor and block biofilm formation in P. aeruginosa. Chang and coauthors [168] identified salicylic acid, tannic acid, and trans-cinnamaldehyde as potential inhibitors of acyl-HSL synthases. Later, trans-cinnamaldehyde was shown to specifically inhibit RhlI without affecting the growth of P. aeruginosa. Molecular docking analyzes suggested that this inhibition might be due to the fact that trans-cinnamaldehyde occupies the substrate binding pocket of the synthase.
The flavonoids licoricon, glycyrin, and glycarin from the plant Glycyrrhiza glabra act as inhibitors of abaI synthesis as well as biofilm formation and motility of A. baumannii [190]. In addition, unsaturated fatty acids, nervonic acid and oleic acid, bind to the AbaI synthase and thus block AHL synthesis and biofilm formation of clinical and laboratory strains of A. baumannii [124]. Molecular docking analyses revealed that ketoprofen, piroxicam, and indomethacin have a strong binding affinity to AbaI through hydrogen and/or hydrophobic interactions, while functional tests showed their efficacy in inhibiting biofilm formation and surface motility [125]. Cadavid and Echeverri [132] described 3-methyl-2(5H)-furanone as a natural inhibitor of biofilm formation and the activity of the autoinducer C6-HSL in K. pneumoniae. The biflavonoid ginkgetin, isolated from Ginkgo biloba, has been shown to have QSI properties. Ginkgetin reduces biofilm formation, EPS production, and motility of E. coli without affecting its growth or metabolic activity. In addition, Bai et al. [201] demonstrated that ginkgetin inhibits the production of the AI-2 signaling molecule and downregulates the transcription of curli-related genes, genes responsible for flagella formation, and QS-related genes. Screening of chemical libraries identified competitive AHL synthase inhibitors such as J8-C8, which targets C8-HSL synthase, and indole-3-acetic acid (a plant hormone known as auxin), which inhibits Burkholderia mallei AHL synthase [211,212].
5.1.2. Inhibitors of QS Molecules Transport
Various mechanisms contribute to reducing or completely blocking the transport of QS signaling molecules. Some identified compounds act as autoinducer sequestrants. For example, antibodies have shown strong efficacy in sequestering QS signaling, such as RS2-1G9, which can inhibit 3-oxo-C12-AHL-based QS signaling in P. aeruginosa [213] (see Section 4.3. Antibodies as Quorum Quenching Molecules). Another example is vanillin and its analogous compounds, which can alter membrane permeability and thus block or reduce the transport of QS molecules. Aldehyde functional groups are thought to be involved in immobilization during membrane modification. Consequently, this activity reduces biofilm formation of P. aeruginosa [169]. In addition, cyclodextrin has been shown to scavenge QS in P. aeruginosa by trapping las and rhl QS signaling molecules, thus reducing pyocyanin production [117]. AHL molecules can also be scavenged by cyclodextrin after it has been immobilized on a cellulose ether gel.
In some bacteria, AIs can be sequestered and immobilized by the action of cytoplasmic enzymes that phosphorylate them, preventing their transport across the membrane. A classic example of this mechanism is found in E. coli, where the kinase LsrK phosphorylates autoinducers and ultimately inhibits bacterial QS [214].
5.1.3. Inhibitors of QS Molecules Perception
Most of the QSI identified so far act as antagonists of QS signaling. These compounds act by preventing the binding of the signaling molecule to its receptor and/or by altering the conformation of the signal-receptor complex, thereby blocking its dimerization or interaction with the target DNA region. Various natural and synthetic AHL analogs, such as quercetin, curvularin, thiolactone analogs, isothiocyanates (iberin), and furanone derivatives, have been shown to inhibit the las and rhl QS systems in P. aeruginosa [111,115,116,166,167]. In addition, farnesol, a compound produced by the fungus Candida albicans, inhibits the production of the PQS signaling molecule in P. aeruginosa by binding to the PqsR receptor. This interaction leads to a conformational change in the receptor that prevents its binding to the promoter region of the pqsA gene [110]. Ferulic acid and sinapinic acid, synthetic cinnamic acid derivatives, are antagonists of the las and pqs-QS systems and consequently disrupt biofilm architecture, impair swarm motility, and virulence factor production in P. aeruginosa [114]. Interestingly, long-chain AHL (C18-HSL) is found to block the las QS system and pyocyanin synthesis [109].
Inhibitors of QS molecule perception by other Gram-negative bacteria have also been identified. Non-native AHLs have been shown to act as potent antagonists of the AbaR receptor. Two unsaturated fatty acids, palmitoleic and myristoleic acids, significantly reduced abaR expression in A. baumannii, resulting in decreased AHL production. In addition, these compounds reduced biofilm formation, facilitated biofilm dispersion, disrupted biofilm architecture, and strongly inhibited surface motility [126]. Furthermore, it was suggested that streptomycin acts as an AbaR antagonist and can prevent positive feedback activation of abaI by functional 3-oxo-C12-HSL-AbaR complexes in A. baumannii [127,128]. In addition, the extracellular protein of Aspergillus oryzae and the aqueous extract of Warburgia salutari had antagonistic activity against the QS systems of K. pneumoniae and E. coli [131,134,202].
5.2. Quorum Quenching Enzymes
Although quorum quenching enzymes can originate from a variety of sources, most characterized QQEs have been isolated from bacteria. These enzymes are produced by both QS-emitting and non-QS-emitting microorganisms, although their exact physiological role often remains unclear. Phylogenetic analyses have shown that QQE-producing bacteria are clustered into three major clades, with the most abundant genera belonging to β- and γ-proteobacteria and Gram-positive bacteria such as Bacillus and Rhodococcus [215]. Based on the mechanism of AHL signaling degradation, QQEs are divided into three groups: (i) lactonases—catalyze the hydrolysis of the lactone ring; (ii) acylases (amidases)—cleave amide bonds and separate the acyl chain (fatty acid) from the lactone ring; (iii) oxidoreductases—modify the acyl chain by oxidation or reduction.
The enzymatic degradation of QS signals can effectively disrupt bacterial communication within a population. It is believed that some microorganisms use QQEs not only as a defense mechanism against competitors, but also to utilize AHL molecules and their degradation products as carbon and nitrogen sources. For example, P. aeruginosa PAO1 and closely related pseudomonads can degrade long-chain AHLs, which they subsequently use as energy sources [215]. Since Gram-positive bacteria are not dependent on AHL-based QS systems, the ecological role of QQEs produced by Bacillus species remains unclear. One hypothesis is that these enzymes serve as detoxifiers and not only degrade AHLs, as Gram-positive bacteria are often sensitive to AHLs. Consequently, AHLs could act as antibiotic-like compounds against Bacillus, and lactonases could act as resistance factors [69].
Naturally occurring QQEs exhibit remarkable properties and hold significant biotechnological potential; however, protein engineering has improved their stability, catalytic efficiency, and substrate specificity for broader applications. Through directed evolution and rational design, a growing number of QQEs, particularly lactonases, have been successfully developed. Prominent examples are the hyperthermostable SsoPox paraoxonase from Sulfolobus solfataricus and the thermoacidophilic lactonase from Geobacillus caustophilus [216]. In addition, the human-derived PON2 lactonase has been engineered to have significant QQ potential against P. aeruginosa [217]. The QQ activity of these enzymes has been validated both in vitro, mainly using P. aeruginosa as a model, and in vivo in Caenorhabditis elegans, Galleria mellonella, and mouse models.
5.2.1. Lactonases
Lactonases are metalloenzymes (except the α/β-hydrolase superfamily) that hydrolyze the ester bond within the lactone ring of AHL molecules. Their mechanism of action is reversible and mirrors the process of pH-mediated lactonolysis; under acidic conditions, the lactone ring can recycle. These enzymes have been shown to exhibit broad substrate specificity, as their main target is the conserved lactone ring, which is common to all AHLs, while the variable acyl chain interacts non-specifically with the enzyme’s active site [218]. To date, more than 30 types of AHL lactonases have been identified. The amino acid sequence and structure of lactonases are very diverse, and therefore these enzymes are categorized into four protein superfamilies: metallo-β-lactamases, phosphotriesterases, paraoxonases, and α/β-hydrolases. Despite very low sequence identity between the different subgroups, all AHL lactonases from the metallo-β-lactamase superfamily possess a highly conserved HXHXDH zinc-binding motif, which is essential for the degradation of signaling molecules. Phosphotriesterases are characterized by a dinuclear metal centre embedded in a (β/α)8-barrel structural scaffold, while the paraoxonase (PON) family consists mainly of the mammalian lactonases PON1, PON2, and PON3. PON enzymes exhibit a six-bladed β-propeller fold and contain both a structural and a catalytic Ca2+ ion, which contributes to their role in the detoxification of organophosphates. In contrast, enzymes from the α/β-hydrolase family do not require metal cofactors for their catalytic activity [219].
AHL lactonases, such as AidB (from Bosea sp.), RmmL (Ruegeria mobilis) and MomL (Muricauda olearia), YtnP, Y2aiiA (B. cepacia), and YtnP (Stenotrophomonas maltophilia), show very broad substrate specificity with respect to the length of the acyl chain in AHL molecules [137,138,139,140,141] (Table 1), but differ in their physicochemical properties. Numerous studies have shown that these enzymes are able to degrade AHL signaling molecules and thus prevent biofilm formation [140,141,143,144,146,147,148] and the production of virulence factors in P. aeruginosa [137,138,139,140,141,144,146,147,148]. The efficacy of several enzymes has been confirmed in vivo [139,141,145,148]. P. aeruginosa is one of the most frequently used model systems in studies investigating the antivirulence potential of QSI and QQ molecules. Most previous research has been based on laboratory strains such as P. aeruginosa PAO1 and PA14 [137,138,143,144,145,146,148], while silencing of the QS system in clinical isolates of P. aeruginosa has only been investigated in a limited number of studies [140,141,147].
AHL lactonases have also shown promising antivirulence and biofilm activities against other Gram-negative bacteria addressed in this study, including A. baumannii, K. pneumoniae, E. coli, and B. cepacia (Table 1).
5.2.2. Acylases
In contrast to lactonases, acylases carry out an irreversible hydrolysis of the amide bond between the acyl chain and the lactone ring of AHL, producing fatty acids and homoserine lactone. The irreversible hydrolysis is considered an advantageous property from a biotechnological point of view, as it prevents the regeneration of functional AHL. The substrate specificity of acylases is based on the length of the acyl chain of AHL as well as the presence of substitutions at the C3 atom, which is due to structural constraints at the active site of the enzyme for ligand interactions [218]. Most acylases show a preference for long-chain AHLs (with or without substitutions at the C3 atom). The first identified AHL acylase originated from the β-proteobacterium Variovorax paradoxus, which utilizes the degradation products of AHLs as energy and nitrogen sources for metabolic processes [220]. Since it can recognize the acyl chain, the acylase enzyme has a higher substrate selectivity than lactonases, such as the acylase PvdQ from Pseudomonas [159]. So far, acylases have been found in species of Pseudomonas, Ralstonia, Bacillus, Streptomyces, and Psychrobacter (Table 1). Like lactonases, this group of enzymes also exhibits significant QQ potential by reducing the production of virulence factors such as elastase, protease, pyocyanin, pyoverdins, and biofilm formation of P. aeruginosa [221] (Table 1). Promising results have been obtained in vivo using different infection model systems, C. elegans, G. mellonella, Artemia salina, and mouse model [157,158,159,161,163].
5.2.3. Oxidoreductases
Enzymes belonging to the class of oxidoreductases do not degrade AHL molecules but convert them into inactive forms by oxidation or reduction in the acyl chain. The synthesis of oxidoreductases is considered a protective mechanism in certain bacterial species [221]. It has been shown that the oxidoreductase BpiB09, derived from a metagenomic library, inactivates 3-oxo-C12-HSL and thereby inhibits pyocyanin production, motility, and biofilm formation in P. aeruginosa PAO1, which reduces the pathogenicity of the bacterium towards C. elegans [164]. In addition, immobilization of oxidoreductases on a glass surface can prevent biofilm formation by K. pneumoniae [222]. The QQ-2 protein, an oxidoreductase from a metagenome library, most likely converts the signaling molecules AHL and AI-2 to QS-inactive hydroxy derivatives and inhibits biofilm formation of K. pneumoniae clinical isolate [165].
5.3. Antibodies as Quorum Quenching Molecules
Antibodies have proven to be very promising QQ strategies due to their high specificity and binding affinity to QS signaling molecules. The most studied antibodies are those that inhibit the QS system of P. aeruginosa. The RS2-1G9, 3-oxo-C12-HSL monoclonal antibody, downregulates the QS system and protects mouse macrophages from the detrimental effects of P. aeruginosa and prevents the activation of the mitogen-activated protein kinase p38 [223]. In another study, highly sensitive monoclonal antibodies against 3-oxo-C12-HSL were developed using sheep immunization and phage display techniques. They showed more than 100-fold improved sensitivity in detecting AI compared to previous antibodies and could be used both in therapy and diagnostics of infections, particularly in cystic fibrosis patients. Monoclonal antibodies significantly increased the survival rate of C. elegans infected with antibiotic-resistant P. aeruginosa. The therapeutic efficacy of these antibodies was also investigated in a mouse model for Pseudomonas infections, in which groups treated with HSL-2 and HSL-4 antibodies had significantly higher survival rates compared to control groups seven days post-infection [224]. The third QS system—pqs—was also the target for the development of antibody therapy. Anti-PQS antibodies showed promising results in combination with 3-oxo-C12-HSL antibodies [225]. In addition, antibodies targeting other AHL molecules have been developed. Chen and colleagues [226] used the supernatant of a B. cepacia culture to identify antibodies that recognize OC10-HSL and C8-HSL molecules.
The antibody-based QQ strategy also has the potential to target biofilm-specific components and facilitate host cell-mediated elimination of bacteria. A human monoclonal antibody targeting the Psl biofilm exopolysaccharide of P. aeruginosa (anti-Psl antibody) reduced mature biofilm biomass in the presence of neutrophils [227]. The human antibody HuTipMab destroyed biofilms formed by various respiratory pathogens, including Gram-negative P. aeruginosa, A. baumannii, and B. cenocepacia [228]. Biofilms were significantly and rapidly disrupted in a dose and time-dependent manner. Bacteria released from a disrupted biofilm were as sensitive or more sensitive than planktonic bacteria to killing by the antibiotics tobramycin, levofloxacin, and linezolid, which are often ineffective against MDR biofilm-forming pathogens.
Another interesting example is TRL1068, a monoclonal antibody with high affinity against the DNABII protein family, whose conformational epitope is located in the DNA-binding region, which explains its high conservation. A BLAST (Bethesda, MA, USA) search revealed hundreds of DNABII homologs in which this epitope is highly conserved in numerous bacterial species, including most CDC-listed antibiotic-resistant pathogens and all ESKAPE organisms, suggesting that TRL1068 may provide broad-spectrum antibiofilm efficacy [229]. In this study, the efficacy of TRL1068 in disrupting biofilms was first demonstrated in vitro, followed by an in vivo evaluation using a mouse model of catheter-related biofilm infection of the skin and soft tissue caused by an MDR clinical isolate of A. baumannii. The combination of TRL1068 and imipenem significantly reduced catheter-adherent bacteria compared to antibiotic monotherapy, suggesting that TRL1068 has great potential as an adjunct to standard antibiotic treatment for difficult bacterial infections.
Outer membrane protein A (OmpA) regulates adhesion and biofilm formation. It is one of the earliest identified and most extensively studied antigens. Passive immunization with antibodies targeting OmpA has been investigated as a potential therapeutic strategy against MDR and extensively drug-resistant (XDR) A. baumannii infections [230]. In addition, the biofilm-associated protein (Bap) plays a key role in the adhesion and biofilm formation of A. baumannii and contributes to its persistence during infection. Conserved and antigenic regions of Bap have been identified, with surface epitopes being 43% identical in isolates associated with outbreaks. Bap has shown strong immunoreactivity, with studies finding high IgG titers and improved survival in immunized mice in an acute systemic infection model [231]. Antibodies targeting CsuE have been shown to inhibit biofilm formation in A. baumannii ATCC 19696 as well as in five clinical isolates. Similarly, anti-CsuA/B antibodies interrupted biofilm development at higher concentrations, supporting the potential of Csu pili-targeting agents as promising prophylactic agents to prevent A. baumannii biofilm formation [232].
Since biofilms play an important role in the pathogenesis of Klebsiella, the ability of antibodies to inhibit biofilm formation could be a protective mechanism against infection with this bacterium. Diago-Navarro and colleagues [233] showed that monoclonal antibodies (MAbs) generated in mice immunized with carbapenem-resistant K. pneumoniae capsular polysaccharides fused to the anthrax protective antigen impaired biofilm formation, increased complement deposition and phagocytic killing, and decreased serum resistance. In vivo, pre-opsonized bacteria resulted in reduced bacterial proliferation in mice. Similarly, another research group identified anti-MrkA antibodies targeting the major type III fimbrial protein through phage display and hybridoma techniques. These antibodies inhibited biofilm formation and bacterial adhesion to lung epithelial cells in vitro, while immunization with MrkA provided protection in a mouse model of lung inflammation [234].
The aforementioned OmpA protein, a highly conserved and immunogenic antigen, plays an important role in E. coli adhesion and bacterial aggregation. Therefore, OmpA has been identified as a promising antigen for the development of vaccines targeting intestinal pathogenic E. coli strains [235]. In another study, antibodies against PNAG (anti-PIA/PNAG), an EPS involved in E. coli biofilm formation, showed a significant reduction in biofilm development in vitro compared to serum from non-immunized controls. In addition, mice immunized with PIA showed significantly increased antibody responses and protective IgG titers compared to the control group [236]. A parasteric monoclonal antibody was developed against the fimbrial adhesin FimH of uropathogenic E. coli [237]. Compared to an orthosteric antibody, the parasteric antibody showed superior efficacy in inhibiting bacterial adhesion, surface-associated biofilm formation, and in vivo colonization. These results suggest that the development of parasteric inhibitors holds great potential for the development of preventive and therapeutic anti-adhesion strategies.
5.4. Combinatorial Therapy
Combinatorial therapy is a promising approach and represents one of the most effective strategies for combating bacterial infections. Although QQ molecules can be used individually, they have shown the strongest effect when used in combination therapies to treat infections caused by clinical pathogens. Such therapies may include the combined use of antibiotics with non-antibiotic agents, multiple QQ molecules, or QQ agents in combination with bacteriophages. The most studied combinatorial strategy involves plant-derived QSI, including plant extracts or isolated compounds, in combination with antibiotics against Gram-negative pathogens.
Since antibiotics are largely ineffective against bacteria embedded in biofilms, recent research has focused on QQ agents to prevent biofilm formation or to disrupt established biofilm structures, thereby exposing the bacteria to direct antibiotic action. The synergistic effect of antibiotics and QQ agents aims to reduce the required antibiotic dose and increase therapeutic efficacy [221]. In addition, disruption of the QS system by combination therapy can significantly reduce the survival of P. aeruginosa under stress conditions [238]. A study by Rezzoagli and colleagues [239] showed that the use of the QSI furanone C30 and gallium in combination with clinically relevant antibiotics—ciprofloxacin, meropenem, colistin, and tobramycin—can inhibit the growth of most antibiotic-resistant P. aeruginosa clones. The study also showed that such a combination therapy can completely reverse the resistant phenotype, underlining the potential of this approach for treating infections and limiting the spread of antibiotic resistance. The importance of QS inhibitors in reducing the resistance of P. aeruginosa PAO1 by synergizing with clinically important antibiotics has been confirmed in several studies [122,240,241]. In addition, this approach is effective in the treatment of MDR clinical isolate P. aeruginosa MMA83 when gentamicin and meropenem were used in combination with D. tsuruhatensis QSI extract [109] and YtnP lactonase from S. maltophilia [141]. In addition, ajoene, furanone-30, and horseradish extract were shown to decrease the expression of virulence factors in P. aeruginosa and significantly increase susceptibility to tobramycin. Similarly, a synergistic effect was observed between curcumin and the antibiotics gentamicin and azithromycin; at a significantly reduced antibiotic dose, curcumin exerted a significant QQ effect against P. aeruginosa. Brackaman and coauthors [122] reported the promising results of the QSI molecules baicalin hydrate and cinnamaldehyde in combination with tobramycin against P. aeruginosa and the B. cepacia complex in vivo using G. mellonella, C. elegans, and mouse lung infection model systems. Finally, the combination of AiiA (QQE) and G1 (QSI) almost completely blocked the P. aeruginosa las and rhl QS systems [238], while the SsoPox-W263I lactonase in combination with ciprofloxacin and bacteriophage ΦIntesti-PA14 showed a synergistic effect both in vitro and in vivo against P. aeruginosa strain PA14R1, which was resistant to the phage cocktail alone [242].
Chrysin, a natural flavonoid found in honey and known for its anti-inflammatory and antioxidant properties, showed a synergistic effect together with colistin against A. baumannii. This combination disrupted the outer bacterial membrane and altered the membrane potential, resulting in an inhibitory effect on biofilm formation. The synergy enables the use of lower colistin concentrations for the treatment of A. baumannii infections, which could minimize dose-dependent side effects [243]. Myrtenol showed potent biofilm activity without affecting the growth or metabolic viability of A. baumannii strains and improved the susceptibility of these strains to conventional antibiotics such as amikacin, ciprofloxacin, gentamicin, and trimethoprim [244]. Then, approved QSI with known safety, and pharmacokinetic profiles curcumin, piroxicam, indomethacin, and ketoprofen in combination with piperacillin showed synergistic effects on both P. aeruginosa and A. baumannii strains [245]. Baicalein, a trihydroxyflavone derived from the root extracts of Scutellaria baicalensis, showed synergistic or additive antibacterial activity in combination with meropenem against all tested XDR/PDR strains of A. baumannii. The baicalein-meropenem combination showed significantly improved biofilm activity compared to either agent alone. In silico studies suggest that these effects may be due to the inhibition of β-lactamases and/or penicillin-binding proteins of A. baumannii by baicalein [191].
Indole, identified as QSI, in combination with ciprofloxacin, destroys persister cells and inhibits biofilm formation of K. pneumoniae [246]. Thymoquinone, an inhibitor of bacterial adhesion and biofilm formation, showed synergistic effects with gentamicin, ofloxacin, penicillin, and nalidixic acid against K. pneumoniae and an additive effect with tetracycline and chloramphenicol [247]. In addition, malvidin, an anthocyanin from Syzygium cumini, showed a pronounced inhibitory effect on QS-regulated violacein production, biofilm formation, and EPS production in K. pneumoniae. The synergistic activity of the conventional antibiotics ofloxacin, tetracycline, and chloramphenicol increased the susceptibility of K. pneumoniae by up to 58.45% [198].
Wojnicz and colleagues [248] found that the pentacyclic triterpenes ursolic acid and asiatic acid enhanced the activity of ciprofloxacin against E. coli. In their study, the combination of ciprofloxacin with ursolic acid led to a significant reduction in biofilm formation. In addition, ciprofloxacin in combination with both agents was able to penetrate the biofilm structure. Studies on the antibiofilm properties of nitroxides showed that these compounds mimic biofilm dispersion activity and inhibit biofilm formation in vitro [249]. In a later study by the same group, nitroxides were shown to enhance the efficacy of ciprofloxacin against biofilms formed by both P. aeruginosa and E. coli [250]. The phytochemicals carvacrol, cinnamaldehyde, and eugenol were identified as effective QSI agents against uropathogenic E. coli. These compounds also showed a synergistic effect in combination with antibiotics such as ceftriaxone, colistin, and tigecycline [251]. Finally, ginkgetin, a natural, non-toxic biflavone with remarkable antibiofilm and antivirulence properties against E. coli, has shown synergistic effects with clinically used antibiotics such as gentamicin, colistin B, and colistin E to overcome antibiotic resistance, showing promising potential for the treatment of E. coli infections [201].
5.5. Quorum Quenching Activity of Antibiotics
Numerous studies indicate that antibiotics in subinhibitory concentrations can effectively interrupt bacterial QS and prevent the formation of biofilms. Applied within subinhibitory concentrations, antibiotics can modulate bacterial communication systems without exerting a strong selective pressure to develop resistance. Several studies have shown that cephalosporins (cefepime, ceftazidime, and ceftriaxone), azithromycin, and tobramycin have anti-QS activity by reducing AHL synthesis, bacterial motility, pyocyanin production, and biofilm formation of P. aeruginosa [252,253,254]. Similarly, ciprofloxacin and streptomycin inhibited QS and biofilm formation of A. baumannii [128,255], while levofloxacin reduced the virulence of hypervirulent K. pneumoniae [256]. In addition, various antibiotics at subinhibitory concentrations, including ceftazidime, ciprofloxacin, amikacin, and colistin, were found to be effective inhibitors of E. coli biofilm formation [257,258]. While the use of subinhibitory concentrations of antibiotics is a promising approach to attenuate bacterial virulence and biofilm formation, it is important to note that the effects can vary depending on the bacterial species, even bacterial strains, and the specific antibiotic used. In some cases, subinhibitory concentrations can stimulate the production of virulence factors, as observed with certain antibiotics in P. aeruginosa [259]. Therefore, careful selection of the type and concentration of antibiotic is crucial when considering this strategy for applications.
5.6. Quorum Quenching Activity of Nanoparticles
Nanoparticles, which are usually between 1 and 100 nm in size, have unique chemical and physical properties due to their size, shape, and large surface area. They can be engineered for targeted drug delivery, prolonged circulation, and co-delivery of multiple drugs, and represent a promising alternative or adjunct to traditional antibiotics [260]. Nanoparticles exhibit a broad-spectrum of antimicrobial activity, including against multidrug-resistant strains, but their QQ and QS-mediated inhibition of virulence and biofilm has also been documented [261]. In contrast to conventional antibiotics, nanoparticles act through multiple mechanisms such as membrane disruption, induction of oxidative stress, and interference with cellular processes, making it more difficult for bacteria to develop resistance. Several types of nanoparticles have anti-QS and antibiofilm properties, including carbon-based, polymer-based, and lipid-based nanoparticles, as well as the most extensively studied metal-based nanoparticles.
Silver nanoparticles have shown promising antibiofilm and antivirulence activity against multidrug-resistant P. aeruginosa in several studies [262,263,264,265,266]. Kamer and colleagues discovered that the combination of silver nanoparticles with antibiotics gentamicin, ceftazidime, and ciprofloxacin resulted in a synergistic effect against this pathogen [264]. A combination of silver and curcumin nanoparticles was also effective in eliminating an established mature biofilm and inhibiting biofilm formation of P. aeruginosa [266]. In another study, it was postulated that phyto-synthesized, biocompatible silver nanoparticles act as antibiofilm agents against P. aeruginosa and E. coli [265]. These nanoparticles have been shown to adhere to the cytoplasmic membrane and cell wall of microorganisms, cause structural disruption, interact with cellular components and biomolecules, and induce the formation of reactive oxygen species and free radicals [262]. Silver nanoparticles also possess antibiofilm and antivirulence activity against A. baumannii and K. pneumoniae [267,268,269]. Another important metal-based nanoparticle is zinc oxide. It effectively inhibits biofilm formation and the production of virulence factors, thereby reducing the patogenicity of P. aeruginosa both in vitro and in vivo [270,271,272]. Zinc oxide nanoparticles also showed promising antibiofilm activity against K. pneumoniae and E coli [273]. Chitosan-polyacrylic acid and chitosan–carvacol nanoparticles inhibited biofilm formation of P. aeruginosa and E. coli [274,275]. Graphene oxide nanoparticles, which are able to physically disrupt biofilms by penetrating the EPS matrix, showed promising antibiofilm activity against Gram-negative bacteria [276,277,278,279]. In addition, biofilms formed by A. baumannii were effectively removed by chitosan nanoparticles loaded with plant essential oils, which act as inactivators of biofilm metabolites [280]. Finally, liposome-based nanoparticles have been reported to be effective antibiofilm agents against P. aeruginosa, A. baumannii, E. coli, and K. pneumoniae [281].
6. In Vivo and Clinical Testing of Quorum Quenching Molecules
Initial testing of the potential of novel compounds is often based on in vitro methods only. However, this approach has some limitations, particularly related to the potential toxicity of the compounds studied, poor pharmacokinetic properties, and lower or no efficacy in vivo. Therefore, in vivo evaluation is crucial for the potential clinical application of these molecules. In vivo models allow researchers to evaluate the bioavailability, stability, and potential immunogenicity of QQ molecules and gain insight into their therapeutic efficacy within complex biological systems that more accurately reflect clinical conditions. In addition, the transition from in vitro and in vivo trials to well-designed clinical studies is essential to determine safety, dosing, and real-world efficacy in patients. Without this process, the therapeutic potential of QQ molecules remains hypothetical, hindering their clinical development and application in combating antimicrobial resistance.
6.1. In Vivo Model Systems of Infection for Testing the Activity of Quorum Quenching Molecules
In this review, we describe commonly used in vivo model systems for evaluating QQ molecules effective against Gram-negative clinical pathogens, C. elegans, G. mellonella, zebrafish (Danio rerio), and mouse models.
Over the last two decades, C. elegans has become a valuable model organism for drug research, especially for studying the mechanisms of action of antimicrobial substances. Its use is in line with the 3R principles (Replacement, Reduction, Refinement) in scientific research as it reduces the dependence on vertebrate models, enables high-throughput screening due to its rapid life cycle, and allows non-invasive observation of biological processes thanks to its optical transparency [282]. C. elegans has been used extensively to study the role of QS in bacterial virulence and to evaluate the efficacy of QSI and QQE. This invertebrate model offers significant advantages for high-throughput screening of bacteria and QQ compounds and provides deep insights into the regulation of virulence and its modulation by anti-infective agents. Studies have shown that the QQ strategy can effectively reduce mortality in C. elegans infected with various bacterial pathogens, providing in vivo evidence for QQ with antivirulence potential in multicellular organisms. The QQ activity of plant extracts and molecules such as tea polyphenols, baicalin, sesamin and sesamolin, wogonin, grapefruit, and mandarin essential oils [122,173,174,175,188] (Table 2) against P. aeruginosa, K. pneumoniae, and B. cenocepacia was confirmed using C. elegans as an in vivo model system. All these molecules increased the survival rate of infected C. elegans. The fungal compounds curvularin and the extracellular protein of Aspergillus oryzae, potent antagonists of the QS system of P. aeruginosa and K. pneumoniae, were also studied in the C. elegans model system [111,131]. In addition, synthetic QQ compounds, butanamide, a synthetic AHL analog, and a vanillin derivative with promising biofilm and antivirulence potential prolonged the lifespan of worms infected with P. aeruginosa [120,121]. This model system was also used to evaluate the activity of various QQ enzymes—YtnP lactonase, MomL lactonase, and HacB acylase [139,141,158]. All mentioned enzymes show no toxicity and prolong the survival of C. elegans infected with clinical and laboratory isolates of P. aeruginosa.
G. mellonella is another valuable in vivo model for studying bacterial virulence and QQ strategies due to its innate immune system, which has functional similarities to that of mammals. The larvae can be incubated at 37 °C, which facilitates the study of human pathogens under physiologically relevant conditions. In addition, G. mellonella offers practical advantages, including low maintenance costs and fewer ethical concerns compared to vertebrate models, consistent with the principles of the 3Rs (Replacement, Reduction, Refinement) in animal research. This model allows for rapid evaluation of antimicrobial and antivirulence compounds and often provides results consistent with those from mammalian studies, thus serving as an effective platform for the evaluation of early-stage therapeutics [283]. Baicalin hydrate, which is effective against P. aeruginosa and B. cenocepacia, has been tested on G. mellonella in addition to other in vivo systems [122]. 1H-pyrrole-2,5-dicarboxylic acid (PT22), isolated from Perenniporia tephropora, reduces the production of virulence factors such as pyocyanin and rhamnolipid and inhibits biofilm formation in P. aeruginosa PAO1. PT22 significantly attenuates the expression of QS-related genes and significantly increases the survival rate of G. mellonella larvae infected with P. aeruginosa [123]. The FDA-approved drug Clofoctol specifically inhibits the expression of pqs-driven virulence traits in P. aeruginosa, including pyocyanin production, swarming motility, biofilm formation, and genes involved in siderophore production. In addition, Clofoctol protected G. melonella larvae from P. aeruginosa infection and effectively inhibits the pqs-QS system in clinical P. aeruginosa isolates from cystic fibrosis patients [113]. Furanone-C30 leads to a significant reduction in the pathogenicity of uropathogenic E. coli in the G. mellonella infection model [136]. G. mellonella is frequently used for the evaluation of the pathogenicity of A. baumannii and K. pneumoniae and for the evaluation of potential therapeutic agents against these pathogens. The indole derivative 7-hydroxyindole, which has been shown to have an antibiofilm effect and reduce the expression of the QS genes abaI and abaR, improves the survival rate of G. mellonella infected with XDR A. baumannii [130]. Tryptanthrin, a natural alkaloid, has been shown to affect various stages of biofilm formation, including inhibition of surface motility and eDNA release in A. baumannii, but also reduces the expression of QS and virulence genes in A. baumannii. In addition, tryptanthrin showed a reduction in virulence of A. baumannii in the G. mellonella infection model without significant toxicity, emphasizing its potential as a safe and effective antivirulence agent [192]. Another natural compound, chlorogenic acid, reduces the production of extracellular protease, capsular polysaccharides, and biofilm formation of carbapenem-resistant K. pneumoniae and improves the survival rate of larvae in the G. mellonella infection model [195]. Finally, G. mellonella was used to evaluate the efficacy of the QQ enzyme PvdQ acylase against B. cenocepacia and A. baumannii [162,163].
The zebrafish (Danio rerio) is an attractive model system for the study of virulence and QQ inhibition due to its unique combination of biological and practical advantages. As a vertebrate, it shares many physiological and genetic similarities with humans, including a conserved innate immune system, making it highly relevant for the study of infections and antimicrobial effects. Its transparency during early development enables non-invasive, real-time visualization of host–pathogen interactions, QS-regulated infection processes, and the effects of QQ treatments. Zebrafish embryos are also suitable for high-throughput screening, are inexpensive to maintain and raise, and pose fewer ethical concerns compared to mammalian models [284]. The plant constituents luteolin and daidzeindimethyl ether downregulate the QS system of P. aeruginosa and thereby reduce the production of virulence factors and biofilm formation. At the same time, these compounds increase the survival rate of zebrafish and stimulate the host’s immune system [170,171]. Octoprohibitin, a synthetic antimicrobial peptide, has been shown to inhibit biofilm formation and eliminate established biofilms of MDR A. baumannii in a dose-dependent manner. Histological analysis of a zebrafish infection model showed that fewer pathological changes occurred in the intestinal, kidney, and gill tissue after treatment with octoprohibitin [129]. The essential oil of Plectranthus amboinicus has a significant inhibitory effect on the formation of K. pneumoniae biofilms. Treatment with the essential oil resulted in significantly improved survival rates, reduced bacterial load, alleviation of visible signs of inflammation, and attenuation of infection-related damage in various organs, which was confirmed by histopathological analyses in a zebrafish infection model [197]. Finally, YtnP lactonase from Bacillus paralicheniformis significantly reduced the production of virulence factors and biofilm formation and effectively rescued zebrafish embryos from lethal systemic infection with P. aeruginosa and partially cleared the bacterial biofilms from the injured tail tissue [142].
Mammalian models such as rats or mice are also commonly used to study the effects of QQ on bacterial infections, as they have both innate and adaptive immune systems and physiological similarities to humans. In addition, they are usually required for preclinical evaluation before clinical trials in humans are initiated. At this stage, QQ strategies have shown considerable efficacy in reducing morbidity and mitigating the deleterious effects associated with various infections [285]. However, unlike simpler organisms such as C. elegans and other previously mentioned model systems, the use of mouse models in high-throughput screening applications is limited due to ethical considerations and logistical constraints. Numerous plant-derived compounds with antibiofilm and antivirulence properties, including the polyphenol vitexin, green tea polyphenols, the isothiocyanates iberin and baicalin, have been studied in various mouse models—catheter-associated mouse biofilm model (vitexin, P. aeruginosa), excision wound infection model (green tea polyphenols, P. aeruginosa), intraperitoneal foreign body infection model (iberin, P. aeruginosa), lung infection and intraperitoneal/intramuscular infection model (baicalin, P. aeruginosa, B. cenocepacia, E. coli) [122,167,172,173,203]. Promising results are also obtained by synthetic compounds, including halogenated anthranilic acid analogs, which inhibit the biosynthesis of HAQ, a part of the pqs-QS system, and interfere with MvfR-dependent gene expression in P. aeruginosa. In addition, valuable results were also obtained by synthetic compounds, including halogenated anthranilic acid analogs that inhibit biosynthesis of HAQ, part of the pqs QS system, and disrupt MvfR-dependent gene expression in P. aeruginosa. These compounds effectively limited the systemic dissemination and mortality associated with P. aeruginosa infections in mice, without affecting bacterial viability [118]. Another synthetic compound, halogenated furanone 5-(dibromomethylene)-2(5H) (named GBr), acts as a LasR antagonist, effectively reducing pyocyanin production, biofilm formation, and swarming motility of P. aeruginosa. In a murine cutaneous abscess model, GBr treatment significantly decreased necrosis formation at the inoculation site and prevented bacterial establishment [119]. Furthermore, (Z)-4-bromo-5-(bromomethylene)-3-methylfuran-2(5H)-one (named BF8) prevents AI-2 synthesis and disperses biofilm of E. coli and increases survival of infected mice [135]. Additionally, erythromycin, levamisole, chloroquine, and propranolol have been identified as QQ agents that effectively reduce the synthesis of virulence factors in A. baumannii, leading to improved survival rates in infected mice [286]. Finally, QQEs such as SsoPox-I and AiiM lactonase, along with PvdQ acylase, have demonstrated significant antivirulence and antibiofilm activities against P. aeruginosa in vitro. In vivo studies further reveal that these enzymes enhance rat and mouse survival rates in rat pneumonia and mouse pulmonary infection models, underscoring their potential as therapeutic agents [145,148,159].
6.2. Quorum Quenching Molecules Application in Medical Devices
Bacterial colonization and biofilm formation on medical implants and devices are a major clinical problem. Preventing bacterial adhesion is critical to reducing infections, improving patient outcomes, and lowering healthcare costs [287]. The QQ strategy offers innovative solutions by locally releasing biomolecules that disrupt bacterial communication, inhibiting biofilm formation and virulence. This localized approach improves the efficacy of systemic antibiotics and reduces their required dosage and duration, but also contributes to the maintenance of beneficial microbiota. Several QQ-based applications have been investigated, including various biomedical implants and devices.
Urinary catheters coated with acylase from Aspergillus melleus showed a significant reduction in P. aeruginosa biofilm after 24 h and maintained their antibacterial properties for up to 7 days. By combining this acylase with α-amylase in multilayer coatings, the efficacy was further increased, and a reduction in biofilm was achieved over one week in a rabbit model [288]. These nanocoatings significantly reduced biofilm formation of single species (P. aeruginosa) and mixed species (P. aeruginosa and E. coli) biofilms on silicone catheters under static and dynamic conditions. The same enzyme was applied to sandblasted polydimethylsiloxane (PDMS) surfaces. This material is commonly used for permanent medical devices such as urinary catheters, which are often susceptible to microbial contamination and biofilm formation. Acylase-functionalized PMDS surfaces significantly reduced bacterial adherence and biofilm formation of P. aeruginosa [289]. In addition, the lactonase SsoPox immobilized in polyurethane coatings by glutaraldehyde crosslinking led to a sevenfold reduction in biofilm formation of P. aeruginosa compared to uncoated controls [147].
Dihydropyrrolones (DHPs), a class of QS inhibitors, were immobilized on glass surfaces to reduce P. aeruginosa adhesion. In one study, DHPs were covalently bound to glass via a copper-catalyzed azide-alkyne-1,3-dipolar cycloaddition, resulting in a significant reduction in bacterial adhesion. These surface-bound DHPs reduced the expression of a lasB-gfp reporter fusion in P. aeruginosa without affecting cell viability, suggesting QS inhibition via a membrane-based pathway [290]. Subsequently, DHPs and furanones were immobilized on glass surfaces using azide/nitrene chemistry and showed considerable efficacy in reducing surface adhesion of P. aeruginosa. This technique represents a promising approach for the development of biomaterial surfaces with increased resistance to bacterial colonization [291]. In addition, the multifunctional coating with polyethylene glycol (PEG) enables the covalent incorporation of DHP on the surface, resulting in reduced cell attachment and bacterial adhesion, as confirmed by colonization tests. This coating significantly reduces biofilm formation of P. aeruginosa, demonstrating that DHP retains its QSI activity after covalent integration into the coating [292].
Antibiofilm and QSI properties are also exhibited by thiazolinyl-picolinamide-based palladium(II) complexes. This complex downregulated the expression of QS-mediated virulence genes in biofilms of A. baumannii colonized on titanium alloy implants [293]. The QS antagonists 3-methyl-2(5H)-furanone and 2′-hydroxycinnamic acid effectively reduced the adherence and biofilm formation of K. pneumoniae on the PVC urethral catheter. In particular, 3-methyl-2(5H)-furanone inhibited the biofilm-promoting effect of the autoinducer C6-HSL, suggesting its potential as a QS inhibitor to prevent catheter-associated infections [132].
QQ-based medical devices have shown promise in combating hospital-acquired infections by attenuating bacterial virulence and inhibiting biofilm formation. Despite encouraging in vitro results, comprehensive in vivo and clinical evaluations are needed to validate their efficacy in different clinical isolates. Although medical devices are generally subject to less stringent regulatory requirements compared to pharmaceuticals, rigorous testing is essential to ensure their safety and efficacy. The far-reaching potential of QQEs and QSIs provides a good basis for innovation in the development of next-generation anti-infective medical devices.
6.3. Quroum Quenching Molecules and Clinical Application
Although an enormous number of QQ molecules have been described in the literature, so far, only approved or commercialized compounds with QQ properties have been used in clinical trials. Remarkably, for most of them, the primary approved use is unrelated to antibiofilm or QQ activity; instead, they were originally recognized for their antimicrobial or antitumor properties.
For example, FDA-approved antibiotic clofoctol, traditionally used to treat respiratory infections caused by Gram-positive bacteria, is a promising candidate for repurposing as a therapeutic agent targeting QS-mediated pathogenicity in Gram-negative bacterial lung infections [113]. Another example is azithromycin, a macrolide antibiotic that has been used for the treatment of cystic fibrosis and ventilator-associated pneumonia. Although it showed antivirulent effects by interrupting QS in P. aeruginosa in a high-risk group of patients, the results were not significant enough [294]. Similarly, garlic extract, which is known for its QQ properties, was tested in cystic fibrosis patients. However, the study did not provide conclusive evidence of its therapeutic efficacy against QS-mediated infections [295]. Another example is palmitoleic acid with proven QQ activity in the mouse lung infection model. In a clinical study of bronchiectasis patients infected with P. aeruginosa for the first time, palmitoleic acid levels in bronchoalveolar lavage fluid correlated negatively with concentrations of C4-HSL and 3OC12-HSL and positively with forced vital capacity and forced expiratory volume in the first second of patients, indicating the protective role of palmitoleic acid against lung damage caused by bacterial virulence [296]. Then, 5-fluorouracil, an anticancer agent, was shown to have a QS inhibitory effect on P. aeruginosa in vitro. It was applied as a coating to urinary catheters, resulting in reduced biofilm formation in clinical evaluations [297]. Finally, a short-term clinical evaluation examined the safety of fimbrolide-coated contact lenses in human volunteers and guinea pigs against P. aeruginosa. Its efficacy in minimizing bacterial adhesion was demonstrated in a 30-day trial in animals and a 24 h trial in humans. The adhesion of all tested strains was significantly reduced, supporting its potential as an antipathogenic agent. In addition, the authors reported no significant ocular reactions during the trial period, indicating the safety of fimbrolide-coated lenses [298].
These studies highlight the potential of reusing existing drugs with QQ properties for clinical applications. However, in order to increase the number of QQ molecules enrolled in clinical trials, additional efforts are needed to validate this approach in clinical phases and confirm its therapeutic relevance.
7. Bioinformatics Approach in Quorum Quenching Experiments
The development of novel high-throughput omics methods has generated a large amount of data that can be used to decipher key molecules and mechanisms for biofilm formation, as well as molecules and mechanisms that disrupt the process of biofilm formation. While some databases exist, they are collected and curated separately, making it difficult to adequately understand the above processes and, in particular, to decipher complex regulatory relationships, including quorum sensing. Therefore, systematic workflows based on machine learning have been developed and deployed to collect, augment, and analyse QS-related data [299]. Machine learning algorithms have been used to analyse large datasets in the areas of microbial genomics, transcriptomics and proteomics, clinical data, and drug data and interactions, providing outcome data used by scientists and healthcare professionals.
The search for inhibitors of biofilm formation is a challenging task, but improvements have been made in the rational improvement of inhibitors and drug prediction through machine learning [300,301,302].
8. Conclusions
Antimicrobial resistance is one of the greatest challenges to global public health today and is becoming a major threat to the future socio-economic development of modern societies. The rapid increase in the infection rate associated with antibiotic resistance is attributed to misuse and over-prescription in agriculture, animal husbandry, and veterinary medicine. In addition, globalisation, horizontal gene transfer, and biofilm formation significantly contribute to the rapid spread of resistant strains. The rate of biofilm-associated infections with Gram-negative bacteria is very high, especially in intensive care units, and is associated with high morbidity and mortality. Alternative therapies include targeting the quorum sensing system, which leads to a reduction in virulence and biofilm formation, usually by inactivating signalling molecules. Therapies based on QS interference could be used in patients as monotherapies or as part of a combinatorial therapy, together with antibiotics. To develop new QS interference systems, knowledge gaps in the regulation of the biofilm cycle in various Gram-negative, clinically relevant pathogens should be closed. As QS interference systems have a lower probability of developing a resistance phenotype, they could even be used for coating medical devices or prophylactically to prevent the development of biofilms.
9. Future Directions
The development of efficient therapeutics based on QS interference needs to be linked with bioinformatics, machine learning, and general computational biology to perpetuate the discovery and resolution of regulatory networks and molecules relevant to biofilm development, as well as the modelling and prediction of novel QS inhibitors. Further progress needs to be made in the application of structural chemistry and pharmacology methods to define stable and efficient formulations of novel quorum-quenching therapeutics. Animal models to test the efficacy and safety of novel QS inhibitors should be developed, or existing models should be adapted and standardized for this purpose. Finally, considerable efforts should be made to introduce, standardize, and improve clinical trials for inhibitors of quorum sensing.
Author Contributions
Acquisition, analysis, and interpretation of literature data, drafting the work, final approval: B.J. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia [Grant No. 451-03-136/2025-03/200042 and Grant No. 451-03-137/2025-03/200178].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Macesic, N.; Uhlemann, A.-C.; Peleg, A.Y. Multidrug-resistant Gram-negative bacterial infections. Lancet 2025, 405, 257–272. [Google Scholar] [CrossRef]
- Flannery, D.D.; Chiotos, K.; Gerber, J.S.; Puopolo, K.M. Neonatal multidrug-resistant gram-negative infection: Epidemiology, mechanisms of resistance, and management. Pediatr. Res. 2022, 91, 380–391. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2023 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. 2024. Available online: https://www.who.int/publications/i/item/9789240094000 (accessed on 20 April 2025).
- Dutescu, I.A.; Hillier, S.A. Encouraging the Development of New Antibiotics: Are Financial Incentives the Right Way Forward? A Systematic Review and Case Study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef]
- Lau, W.Y.V.; Taylor, P.K.; Brinkman, F.S.L.; Lee, A.H.Y. Pathogen-associated gene discovery workflows for novel antivirulence therapeutic development. eBioMedicine 2023, 88, 104429. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Mandal, M. Attenuation of biofilm and quorum sensing regulated virulence factors of an opportunistic pathogen Pseudomonas aeruginosa by phytofabricated silver nanoparticles. Microb. Pathog. 2023, 185, 106433. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Singh, A.K.; Singh, S.; Chakravortty, D.; Das, D. Enzymatic dispersion of biofilms: An emerging biocatalytic avenue to combat biofilm-mediated microbial infections. J. Biol. Chem. 2022, 298, 102352. [Google Scholar] [CrossRef]
- Silpe, J.E.; Duddy, O.P.; Bassler, B.L. Natural and synthetic inhibitors of a phage-encoded quorum-sensing receptor affect phage–host dynamics in mixed bacterial communities. Proc. Natl. Acad. Sci. USA 2022, 119, e2217813119. [Google Scholar] [CrossRef]
- Ivanova, K.; Ivanova, A.; Hoyo, J.; Pérez-Rafael, S.; Tzanov, T. Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 7632. [Google Scholar] [CrossRef]
- Hawas, S.; Verderosa, A.D.; Totsika, M. Combination Therapies for Biofilm Inhibition and Eradication: A Comparative Review of Laboratory and Preclinical Studies. Front. Cell. Infect. Microbiol. 2022, 12, 850030. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Carthey, A.J.R.; Blumstein, D.T.; Gallagher, R.V.; Tetu, S.G.; Gillings, M.R. Conserving the holobiont. Funct. Ecol. 2020, 34, 764–776. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, A.; Head, I.M.; Tsesmetzis, N. Damage to offshore production facilities by corrosive microbial biofilms. Appl. Microbiol. Biotechnol. 2018, 102, 2525–2533. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Goswami, P.; Pant, D.; Sevda, S. The role of biofilm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: An overview of surveillance, outbreaks, and prevention. World J. Microbiol. Biotechnol. 2021, 37, 36. [Google Scholar] [CrossRef]
- Jiang, Z.; Nero, T.; Mukherjee, S.; Olson, R.; Yan, J. Searching for the Secret of Stickiness: How Biofilms Adhere to Surfaces. Front. Microbiol. 2021, 12, 686793. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Sepehrnia, N.; Bachmann, J.; Hajabbasi, M.A.; Afyuni, M.; Horn, M.A. Modeling Escherichia coli and Rhodococcus erythropolis transport through wettable and water repellent porous media. Colloids Surf. B Biointerfaces 2018, 172, 280–287. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid. Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Yu, C.; Li, J.; Zhou, X. Biofilm formation: Mechanistic insights and therapeutic targets. Mol. Biomed. 2023, 4, 49. [Google Scholar] [CrossRef]
- Almatroudi, A. Investigating Biofilms: Advanced Methods for Comprehending Microbial Behavior and Antibiotic Resistance. Front. Biosci. (Landmark Ed.) 2024, 29, 133. [Google Scholar] [CrossRef]
- Hazrin-Chong, N.H.; Das, T.; Manefield, M. Surface physico-chemistry governing microbial cell attachment and biofilm formation on coal. Int. J. Coal Geol. 2021, 236, 103671. [Google Scholar] [CrossRef]
- Floyd, K.A.; Eberly, A.R.; Hadjifrangiskou, M. 3—Adhesion of bacteria to surfaces and biofilm formation on medical devices. In Biofilms and Implantable Medical Devices; Deng, Y., Lv, W., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 47–95. [Google Scholar]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Bruzaud, J.; Tarrade, J.; Coudreuse, A.; Canette, A.; Herry, J.-M.; Taffin de Givenchy, E.; Darmanin, T.; Guittard, F.; Guilbaud, M.; Bellon-Fontaine, M.-N. Flagella but not type IV pili are involved in the initial adhesion of Pseudomonas aeruginosa PAO1 to hydrophobic or superhydrophobic surfaces. Colloids Surf. B Biointerfaces 2015, 131, 59–66. [Google Scholar] [CrossRef]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef] [PubMed]
- Persat, A. Bacterial mechanotransduction. Curr. Opin. Microbiol. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Utada, A.S.; Bennett, R.R.; Fong, J.C.N.; Gibiansky, M.L.; Yildiz, F.H.; Golestanian, R.; Wong, G.C.L. Vibrio cholerae use pili and flagella synergistically to effect motility switching and conditional surface attachment. Nat. Commun. 2014, 5, 4913. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Agarwal, H.; Gurnani, B.; Pippal, B.; Jain, N. Capturing the micro-communities: Insights into biogenesis and architecture of bacterial biofilms. BBA Adv. 2025, 7, 100133. [Google Scholar] [CrossRef]
- Van Gerven, N.; Klein, R.D.; Hultgren, S.J.; Remaut, H. Bacterial Amyloid Formation: Structural Insights into Curli Biogensis. Trends Microbiol. 2015, 23, 693–706. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Seviour, T.; Winnerdy, F.R.; Wong, L.L.; Shi, X.; Mugunthan, S.; Foo, Y.H.; Castaing, R.; Adav, S.S.; Subramoni, S.; Kohli, G.S.; et al. The biofilm matrix scaffold of Pseudomonas aeruginosa contains G-quadruplex extracellular DNA structures. npj Biofilms Microbiomes 2021, 7, 27. [Google Scholar] [CrossRef]
- Mugunthan, S.; Wong, L.L.; Winnerdy, F.R.; Summers, S.; Bin Ismail, M.H.; Foo, Y.H.; Jaggi, T.K.; Meldrum, O.W.; Tiew, P.Y.; Chotirmall, S.H.; et al. RNA is a key component of extracellular DNA networks in Pseudomonas aeruginosa biofilms. Nat. Commun. 2023, 14, 7772. [Google Scholar] [CrossRef]
- Serra, D.O.; Hengge, R. Stress responses go three dimensional—The spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 2014, 16, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.N.H.; Davies, D.G.; Sauer, K. Control of Biofilms with the Fatty Acid Signaling Molecule cis-2-Decenoic Acid. Pharmaceuticals 2015, 8, 816–835. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Rao, H.; Choo, S.; Rajeswari Mahalingam, S.R.; Adisuri, D.S.; Madhavan, P.; Md. Akim, A.; Chong, P.P. Approaches for Mitigating Microbial Biofilm-Related Drug Resistance: A Focus on Micro- and Nanotechnologies. Molecules 2021, 26, 1870. [Google Scholar] [CrossRef]
- Shenkutie, A.M.; Yao, M.Z.; Siu, G.K.; Wong, B.K.; Leung, P.H. Biofilm-Induced Antibiotic Resistance in Clinical Acinetobacter baumannii Isolates. Antibiotics 2020, 9, 817. [Google Scholar] [CrossRef]
- Nahum, Y.; Gross, N.; Cerrone, A.; Matouš, K.; Nerenberg, R. Effect of biofilm physical characteristics on their susceptibility to antibiotics: Impacts of low-frequency ultrasound. npj Biofilms Microbiomes 2024, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Xiu, W.; Wan, L.; Yang, K.; Li, X.; Yuwen, L.; Dong, H.; Mou, Y.; Yang, D.; Wang, L. Potentiating hypoxic microenvironment for antibiotic activation by photodynamic therapy to combat bacterial biofilm infections. Nat. Commun. 2022, 13, 3875. [Google Scholar] [CrossRef]
- Whelan, M.V.X.; Ardill, L.; Koide, K.; Nakajima, C.; Suzuki, Y.; Simpson, J.C.; Ó Cróinín, T. Acquisition of fluoroquinolone resistance leads to increased biofilm formation and pathogenicity in Campylobacter jejuni. Sci. Rep. 2019, 9, 18216. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Reygaert, W.C. Gram-Negative Bacteria. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorge, A.G.; et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 2022, 77, 1015. [Google Scholar] [CrossRef]
- PHS Consulting Ltd. Quantification of Market Sectors Engaging With Biofilm Technologies; PHS Consulting Ltd.: Tarvin, UK, 2021. [Google Scholar]
- WHO. Global Spending on Health: A World in Transition. 2019. Available online: https://www.who.int/publications/i/item/WHO-HIS-HGF-HFWorkingPaper-19.4 (accessed on 20 April 2025).
- Perry, E.K.; Tan, M.-W. Bacterial biofilms in the human body: Prevalence and impacts on health and disease. Front. Cell. Infect. Microbiol. 2023, 13, 1237164. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2014, 4, 373–381. [Google Scholar] [CrossRef]
- Romanova, Y.M.; Mulabaev, N.S.; Tolordava, E.R.; Seregin, A.V.; Seregin, I.V.; Alexeeva, N.V.; Stepanova, T.V.; Levina, G.A.; Barkhatova, O.I.; Gamova, N.A.; et al. Microbial communities on kidney stones. Mol. Genet. Microbiol. Virol. 2015, 30, 78–84. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Rajab, A.A.H.; Abdallah, M.H.; Rizvi, S.M.; Moin, A.; Khafagy, E.-S.; Tabrez, S.; Hegazy, W.A.H. Biofilm Lifestyle in Recurrent Urinary Tract Infections. Life 2023, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Haller, S.; Quinten, C.; Kärki, T.; Zacher, B.; Eckmanns, T.; Abu Sin, M.; Plachouras, D.; Kinross, P.; Suetens, C.; et al. Healthcare-associated pneumonia in acute care hospitals in European Union/European Economic Area countries: An analysis of data from a point prevalence survey, 2011 to 2012. Eurosurveillance 2018, 23, 1700843. [Google Scholar] [CrossRef]
- Mishra, A.; Aggarwal, A.; Khan, F. Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies. Antibiotics 2024, 13, 623. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Potter, R.F.; McCoy, W.H.t.; Wildenthal, J.A.; Katumba, G.L.; Mucha, P.J.; Dantas, G.; Henderson, J.P. E. coli catheter-associated urinary tract infections are associated with distinctive virulence and biofilm gene determinants. JCI Insight 2023, 8, e161461. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. npj Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; Fullwood, C.; Zaman, M.; Qamruddin, A.; Cunnington, C.; Mamas, M.A.; Sandoe, J.; Motwani, M.; Zaidi, A. Cardiac implantable electronic device (CIED) infections are expensive and associated with prolonged hospitalisation: UK Retrospective Observational Study. PLoS ONE 2019, 14, e0206611. [Google Scholar] [CrossRef]
- Kaier, K.; Heister, T.; Wolff, J.; Wolkewitz, M. Mechanical ventilation and the daily cost of ICU care. BMC Health Serv. Res. 2020, 20, 267. [Google Scholar] [CrossRef]
- Sandiford, N.A.; Hutt, J.R.; Kendoff, D.O.; Mitchell, P.A.; Citak, M.; Granger, L. Prolonged suppressive antibiotic therapy is successful in the management of prosthetic joint infection. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 313–321. [Google Scholar] [CrossRef]
- Lamagni, T. Epidemiology and burden of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. S1), i5–i10. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef] [PubMed]
- Markowska, K.; Szymanek-Majchrzak, K.; Pituch, H.; Majewska, A. Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities. Int. J. Mol. Sci. 2024, 25, 12808. [Google Scholar] [CrossRef]
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef]
- Mukherjee, S.; Moustafa, D.; Smith, C.D.; Goldberg, J.B.; Bassler, B.L. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLOS Pathog. 2017, 13, e1006504. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, A.; Marcos-Torres, F.J.; Llamas, M.A. Mechanisms of iron homeostasis in Pseudomonas aeruginosa and emerging therapeutics directed to disrupt this vital process. Microb. Biotechnol. 2023, 16, 1475–1491. [Google Scholar] [CrossRef]
- Cornelis, P. Putting an end to the Pseudomonas aeruginosa IQS controversy. MicrobiologyOpen 2020, 9, e962. [Google Scholar] [CrossRef] [PubMed]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Saipriya, K.; Swathi, C.H.; Ratnakar, K.S.; Sritharan, V. Quorum-sensing system in Acinetobacter baumannii: A potential target for new drug development. J. Appl. Microbiol. 2020, 128, 15–27. [Google Scholar] [CrossRef] [PubMed]
- López-Martín, M.; Dubern, J.-F.; Alexander Morgan, R.; Williams, P. AbaM Regulates Quorum Sensing, Biofilm Formation, and Virulence in Acinetobacter baumannii. J. Bacteriol. 2021, 203, 10–1128. [Google Scholar] [CrossRef]
- Dou, Y.; Song, F.; Guo, F.; Zhou, Z.; Zhu, C.; Xiang, J.; Huan, J. Acinetobacter baumannii quorum-sensing signalling molecule induces the expression of drug-resistance genes. Mol. Med. Rep. 2017, 15, 4061–4068. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Han, K. AbaR is a LuxR type regulator essential for motility and the formation of biofilm and pellicle in Acinetobacter baumannii. Genes. Genom. 2020, 42, 1339–1346. [Google Scholar] [CrossRef]
- Cui, B.; Guo, Q.; Li, X.; Song, S.; Wang, M.; Wang, G.; Yan, A.; Zhou, J.; Deng, Y. A response regulator controls Acinetobacter baumannii virulence by acting as an indole receptor. PNAS Nexus 2023, 2, pgad274. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- Kalferstova, L.; Kolar, M.; Fila, L.; Vavrova, J.; Drevinek, P. Gene Expression Profiling of Burkholderia cenocepacia at the Time of Cepacia Syndrome: Loss of Motility as a Marker of Poor Prognosis? J. Clin. Microbiol. 2015, 53, 1515–1522. [Google Scholar] [CrossRef]
- Sousa, S.A.; Feliciano, J.R.; Pita, T.; Guerreiro, S.I.; Leitão, J.H. Burkholderia cepacia Complex Regulation of Virulence Gene Expression: A Review. Genes 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, S.; Kong, X.; Chen, X.; Zhao, Z.; Lin, Z.; Jia, Y.; Zhang, Y.; Luo, H.-B.; Wang, Q.-P.; et al. Regulation of Burkholderia cenocepacia virulence by the fatty acyl-CoA ligase DsfR as a response regulator of quorum sensing signal. Cell Rep. 2024, 43, 114223. [Google Scholar] [CrossRef]
- Suppiger, A.; Nadine, S.; Claudio, A.; Gabriella, P.; Eberl, L. Two quorum sensing systems control biofilm formation and virulence in members of the Burkholderia cepacia complex. Virulence 2013, 4, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Shah, A.A.; Alwashmi, A.S.S.; Abalkhail, A.; Alkahtani, A.M. Emerging challenges in Klebsiella pneumoniae: Antimicrobial resistance and novel approach. Microb. Pathog. 2025, 202, 107399. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, D.; Haagensen Janus, A.J.; Rich, C.; Forestier, C. Characterization of Type 2 Quorum Sensing in Klebsiella pneumoniae and Relationship with Biofilm Formation. J. Bacteriol. 2005, 187, 2870–2880. [Google Scholar] [CrossRef]
- Kendall Melissa, M.; Sperandio, V. Cell-to-Cell Signaling in Escherichia coli and Salmonella. EcoSal Plus 2014, 6, 10–1128. [Google Scholar] [CrossRef]
- Guilhen, C.; Charbonnel, N.; Parisot, N.; Gueguen, N.; Iltis, A.; Forestier, C.; Balestrino, D. Transcriptional profiling of Klebsiella pneumoniae defines signatures for planktonic, sessile and biofilm-dispersed cells. BMC Genom. 2016, 17, 237. [Google Scholar] [CrossRef]
- De Araujo, C.; Balestrino, D.; Roth, L.; Charbonnel, N.; Forestier, C. Quorum sensing affects biofilm formation through lipopolysaccharide synthesis in Klebsiella pneumoniae. Res. Microbiol. 2010, 161, 595–603. [Google Scholar] [CrossRef]
- Pacheco, T.; Gomes, A.É.I.; Siqueira, N.M.G.; Assoni, L.; Darrieux, M.; Venter, H.; Ferraz, L.F.C. SdiA, a Quorum-Sensing Regulator, Suppresses Fimbriae Expression, Biofilm Formation, and Quorum-Sensing Signaling Molecules Production in Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 597735. [Google Scholar] [CrossRef]
- Lee, K.W.K.; Periasamy, S.; Mukherjee, M.; Xie, C.; Kjelleberg, S.; Rice, S.A. Biofilm development and enhanced stress resistance of a model, mixed-species community biofilm. ISME J. 2014, 8, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Subramoni, S.; Muzaki, M.Z.B.M.; Booth, S.C.M.; Kjelleberg, S.; Rice, S.A. N-Acyl Homoserine Lactone-Mediated Quorum Sensing Regulates Species Interactions in Multispecies Biofilm Communities. Front. Cell. Infect. Microbiol. 2021, 11, 646991. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Silva, V.; Dapkevicius, M.D.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Borges, A.; Flament-Simon, S.-C.; Simões, M. Quorum sensing architecture network in Escherichia coli virulence and pathogenesis. FEMS Microbiol. Rev. 2023, 47, fuad031. [Google Scholar] [CrossRef]
- Bez, C.; Geller Alexander, M.; Levy, A.; Venturi, V. Cell-Cell Signaling Proteobacterial LuxR Solos: A Treasure Trove of Subgroups Having Different Origins, Ligands, and Ecological Roles. mSystems 2023, 8, e01039-22. [Google Scholar] [CrossRef]
- Jani, S.; Seely, A.L.; Peabody, V.G.L.; Jayaraman, A.; Manson, M.D. Chemotaxis to self-generated AI-2 promotes biofilm formation in Escherichia coli. Microbiology 2017, 163, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wood, T.K. The Primary Physiological Roles of Autoinducer 2 in Escherichia coli Are Chemotaxis and Biofilm Formation. Microorganisms 2021, 9, 386. [Google Scholar] [CrossRef]
- Lustri Bruna, C.; Sperandio, V.; Moreira Cristiano, G. Bacterial Chat: Intestinal Metabolites and Signals in Host-Microbiota-Pathogen Interactions. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef]
- Cui, B.; Chen, X.; Guo, Q.; Song, S.; Wang, M.; Liu, J.; Deng, Y. The Cell–Cell Communication Signal Indole Controls the Physiology and Interspecies Communication of Acinetobacter baumannii. Microbiol. Spectr. 2022, 10, e01027-22. [Google Scholar] [CrossRef]
- Baldelli, V.; Francesca, D.A.; Viola, P.; Vita, F.E.; Paolo, V.; Giordano, R.; Leoni, L. Identification of FDA-approved antivirulence drugs targeting the Pseudomonas aeruginosa quorum sensing effector protein PqsE. Virulence 2020, 11, 652–668. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Xu, J.-L.; Li, X.-Z.; Zhang, L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef] [PubMed]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in Bacterial Quorum Sensing: A Biopharmaceutical Perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Malešević, M.; Di Lorenzo, F.; Filipić, B.; Stanisavljević, N.; Novović, K.; Senerovic, L.; Polović, N.; Molinaro, A.; Kojić, M.; Jovčić, B. Pseudomonas aeruginosa quorum sensing inhibition by clinical isolate Delftia tsuruhatensis 11304: Involvement of N-octadecanoylhomoserine lactones. Sci. Rep. 2019, 9, 16465. [Google Scholar] [CrossRef]
- Cugini, C.; Calfee, M.W.; Farrow Iii, J.M.; Morales, D.K.; Pesci, E.C.; Hogan, D.A. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 896–906. [Google Scholar] [CrossRef]
- Choi, H.-Y.; Le, D.D.; Kim, W.-G. Curvularin Isolated From Phoma macrostoma Is an Antagonist of RhlR Quorum Sensing in Pseudomonas aeruginosa. Front. Microbiol. 2022, 13, 913882. [Google Scholar] [CrossRef]
- Bulman, Z.; Le, P.; Hudson, A.O.; Savka, M.A. A novel property of propolis (bee glue): Anti-pathogenic activity by inhibition of N-acyl-homoserine lactone mediated signaling in bacteria. J. Ethnopharmacol. 2011, 138, 788–797. [Google Scholar] [CrossRef]
- D’Angelo, F.; Baldelli, V.; Halliday, N.; Pantalone, P.; Polticelli, F.; Fiscarelli, E.; Williams, P.; Visca, P.; Leoni, L.; Rampioni, G. Identification of FDA-Approved Drugs as Antivirulence Agents Targeting the pqs Quorum-Sensing System of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Leitão, M.M.; Gonçalves, A.S.C.; Sousa, S.F.; Borges, F.; Simões, M.; Borges, A. Two cinnamic acid derivatives as inhibitors of Pseudomonas aeruginosa las and pqs quorum-sensing systems: Impact on biofilm formation and virulence factors. Biomed. Pharmacother. 2025, 187, 118090. [Google Scholar] [CrossRef]
- McInnis, C.E.; Blackwell, H.E. Thiolactone modulators of quorum sensing revealed through library design and screening. Bioorganic Med. Chem. 2011, 19, 4820–4828. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.; Park, H.-Y.; Park, H.-J.; Lee, J.H.; Kim, C.K.; Yoon, J. Furanone derivatives as quorum-sensing antagonists of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 80, 37–47. [Google Scholar] [CrossRef]
- Kato, N.; Tanaka, T.; Nakagawa, S.; Morohoshi, T.; Hiratani, K.; Ikeda, T. Control of virulence factor expression in opportunistic pathogens using cyclodextrin immobilized gel. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 419–423. [Google Scholar] [CrossRef]
- Lesic, B.; Lépine, F.; Déziel, E.; Zhang, J.; Zhang, Q.; Padfield, K.; Castonguay, M.-H.; Milot, S.; Stachel, S.; Tzika, A.A.; et al. Inhibitors of Pathogen Intercellular Signals as Selective Anti-Infective Compounds. PLOS Pathog. 2007, 3, e126. [Google Scholar] [CrossRef]
- Muñoz-Cázares, N.; Castillo-Juárez, I.; García-Contreras, R.; Castro-Torres, V.A.; Díaz-Guerrero, M.; Rodríguez-Zavala, J.S.; Quezada, H.; González-Pedrajo, B.; Martínez-Vázquez, M. A Brominated Furanone Inhibits Pseudomonas aeruginosa Quorum Sensing and Type III Secretion, Attenuating Its Virulence in a Murine Cutaneous Abscess Model. Biomedicines 2022, 10, 1847. [Google Scholar] [CrossRef]
- Wei, Z.; Li, T.; Gu, Y.; Zhang, Q.; Wang, E.; Li, W.; Wang, X.; Li, Y.; Li, H. Design, Synthesis, and Biological Evaluation of N-Acyl-Homoserine Lactone Analogs of Quorum Sensing in Pseudomonas aeruginosa. Front. Chem. 2022, 10, 948687. [Google Scholar] [CrossRef] [PubMed]
- Shastry, R.P.; Ghate, S.D.; Sukesh Kumar, B.; Srinath, B.S.; Kumar, V. Vanillin derivative inhibits quorum sensing and biofilm formation in Pseudomonas aeruginosa: A study in a Caenorhabditis elegans infection model. Nat. Prod. Res. 2022, 36, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Cos, P.; Maes, L.; Nelis Hans, J.; Coenye, T. Quorum Sensing Inhibitors Increase the Susceptibility of Bacterial Biofilms to Antibiotics In Vitro and In Vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Zeng, Y.; Wang, W.; Tang, S.; Jia, A. 1H-Pyrrole-2,5-dicarboxylic acid, a quorum sensing inhibitor from one endophytic fungus in Areca catechu L. acts as antibiotic accelerant against Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2024, 14, 1413728. [Google Scholar] [CrossRef]
- Khadke, S.K.; Lee, J.-H.; Kim, Y.-G.; Raj, V.; Lee, J. Assessment of Antibiofilm Potencies of Nervonic and Oleic Acid against Acinetobacter baumannii Using In Vitro and Computational Approaches. Biomedicines 2021, 9, 1133. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Shaldam, M.A.; Shaaban, M.I. Ketoprofen, piroxicam and indomethacin-suppressed quorum sensing and virulence factors in Acinetobacter baumannii. J. Appl. Microbiol. 2022, 133, 2182–2197. [Google Scholar] [CrossRef]
- Nicol, M.; Alexandre, S.; Luizet, J.-B.; Skogman, M.; Jouenne, T.; Salcedo, S.P.; Dé, E. Unsaturated Fatty Acids Affect Quorum Sensing Communication System and Inhibit Motility and Biofilm Formation of Acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 214. [Google Scholar] [CrossRef]
- Stacy, D.M.; Welsh, M.A.; Rather, P.N.; Blackwell, H.E. Attenuation of Quorum Sensing in the Pathogen Acinetobacter baumannii Using Non-native N-Acyl Homoserine Lactones. ACS Chem. Biol. 2012, 7, 1719–1728. [Google Scholar] [CrossRef]
- Saroj Sunil, D.; Rather Philip, N. Streptomycin Inhibits Quorum Sensing in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, E.H.T.T.; Rajapaksha, D.C.; Nikapitiya, C.; Lee, J.; De Zoysa, M.; Whang, I. Novel Antimicrobial Peptide “Octoprohibitin” against Multidrug Resistant Acinetobacter baumannii. Pharmaceuticals 2022, 15, 928. [Google Scholar] [CrossRef]
- Li, J.; Xie, L.; Lin, F.; Ling, B. Indole derivatives display antimicrobial and antibiofilm effects against extensively drug-resistant Acinetobacter baumannii. Microbiol. Spectr. 2025, 13, e03388-24. [Google Scholar] [CrossRef] [PubMed]
- Rachma, L.N.; Fitri, L.E.; Prawiro, S.R.; Mardining Raras, T.Y. Aspergillus oryzae attenuates quorum sensing -associated virulence factors and biofilm formation in Klebsiella pneumoniae extended-spectrum beta-lactamases. F1000Res 2022, 11, 1148. [Google Scholar] [CrossRef]
- Cadavid, E.; Echeverri, F. The Search for Natural Inhibitors of Biofilm Formation and the Activity of the Autoinductor C6-AHL in Klebsiella pneumoniae ATCC 13884. Biomolecules 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Muteeb, G.; Khan, S.; Alqahtani, A.S.; Somvanshi, P.; Alqahtani, M.S.; Ameta, K.L.; Haque, S. Identifying novel inhibitor of quorum sensing transcriptional regulator (SdiA) of Klebsiella pneumoniae through modelling, docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2021, 39, 3594–3604. [Google Scholar] [CrossRef]
- Ren, D.; Bedzyk, L.A.; Ye, R.W.; Thomas, S.M.; Wood, T.K. Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli. Biotechnol. Bioeng. 2004, 88, 630–642. [Google Scholar] [CrossRef]
- Pan, J.; Xie, X.; Tian, W.; Bahar, A.A.; Lin, N.; Song, F.; An, J.; Ren, D. (Z)-4-Bromo-5-(bromomethylene)-3-methylfuran-2(5H)-one sensitizes Escherichia coli persister cells to antibiotics. Appl. Microbiol. Biotechnol. 2013, 97, 9145–9154. [Google Scholar] [CrossRef]
- Henly, E.L.; Norris, K.; Rawson, K.; Zoulias, N.; Jaques, L.; Chirila, P.G.; Parkin, K.L.; Kadirvel, M.; Whiteoak, C.; Lacey, M.M.; et al. Impact of long-term quorum sensing inhibition on uropathogenic Escherichia coli. J. Antimicrob. Chemother. 2021, 76, 909–919. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Xuan, C.-G.; Lu, C.-H.; Guo, S.; Yu, J.-F.; Asif, M.; Jiang, W.-J.; Zhou, Z.-G.; Luo, Z.-Q.; Zhang, L.-Q. AidB, a Novel Thermostable N-Acylhomoserine Lactonase from the Bacterium bosea sp. Appl. Environ. Microbiol. 2019, 85, e02065-19. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yu, M.; Shan, H.; Tian, X.; Zheng, Y.; Xue, C.; Zhang, X.-H. Characterization of a Novel N-Acylhomoserine Lactonase RmmL from Ruegeria mobilis YJ3. Mar. Drugs 2018, 16, 370. [Google Scholar] [CrossRef]
- Tang, K.; Su, Y.; Brackman, G.; Cui, F.; Zhang, Y.; Shi, X.; Coenye, T.; Zhang, X.-H. MomL, a Novel Marine-Derived N-Acyl Homoserine Lactonase from Muricauda olearia. Appl. Environ. Microbiol. 2015, 81, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Malešević, M.; Stanisavljević, N.; Novović, K.; Polović, N.; Vasiljević, Z.; Kojić, M.; Jovčić, B. Burkholderia cepacia YtnP and Y2-aiiA lactonases inhibit virulence of Pseudomonas aeruginosa via quorum quenching activity. Microb. Pathog. 2020, 149, 104561. [Google Scholar] [CrossRef]
- Curcic, J.; Dinic, M.; Novovic, K.; Vasiljevic, Z.; Kojic, M.; Jovcic, B.; Malesevic, M. A novel thermostable YtnP lactonase from Stenotrophomonas maltophilia inhibits Pseudomonas aeruginosa virulence in vitro and in vivo. Int. J. Biol. Macromol. 2024, 264, 130421. [Google Scholar] [CrossRef] [PubMed]
- Djokic, L.; Stankovic, N.; Galic, I.; Moric, I.; Radakovic, N.; Šegan, S.; Pavic, A.; Senerovic, L. Novel Quorum Quenching YtnP Lactonase from Bacillus paralicheniformis Reduces Pseudomonas aeruginosa Virulence and Increases Antibiotic Efficacy in vivo. Front. Microbiol. 2022, 13, 906312. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, J.; Guo, X.; Kong, D.; Zhang, Q.; Zhou, Y.; Liu, X.; Zhao, S.; Ruan, Z. Characterization of AiiK, an AHL lactonase, from Kurthia huakui LAM0618T and its application in quorum quenching on Pseudomonas aeruginosa PAO1. Sci. Rep. 2018, 8, 6013. [Google Scholar] [CrossRef]
- Anandan, K.; Vittal, R.R. Quorum quenching activity of AiiA lactonase KMMI17 from endophytic Bacillus thuringiensis KMCL07 on AHL- mediated pathogenic phenotype in Pseudomonas aeruginosa. Microb. Pathog. 2019, 132, 230–242. [Google Scholar] [CrossRef]
- Migiyama, Y.; Kaneko, Y.; Yanagihara, K.; Morohoshi, T.; Morinaga, Y.; Nakamura, S.; Miyazaki, T.; Hasegawa, H.; Izumikawa, K.; Kakeya, H.; et al. Efficacy of AiiM, an N-Acylhomoserine Lactonase, against Pseudomonas aeruginosa in a Mouse Model of Acute Pneumonia. Antimicrob. Agents Chemother. 2013, 57, 3653–3658. [Google Scholar] [CrossRef]
- Fan, X.; Liang, M.; Wang, L.; Chen, R.; Li, H.; Liu, X. Aii810, a Novel Cold-Adapted N-Acylhomoserine Lactonase Discovered in a Metagenome, Can Strongly Attenuate Pseudomonas aeruginosa Virulence Factors and Biofilm Formation. Front. Microbiol. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Guendouze, A.; Plener, L.; Bzdrenga, J.; Jacquet, P.; Rémy, B.; Elias, M.; Lavigne, J.-P.; Daudé, D.; Chabrière, E. Effect of Quorum Quenching Lactonase in Clinical Isolates of Pseudomonas aeruginosa and Comparison with Quorum Sensing Inhibitors. Front. Microbiol. 2017, 8, 227. [Google Scholar] [CrossRef]
- Hraiech, S.; Hiblot, J.; Lafleur, J.; Lepidi, H.; Papazian, L.; Rolain, J.-M.; Raoult, D.; Elias, M.; Silby, M.W.; Bzdrenga, J.; et al. Inhaled Lactonase Reduces Pseudomonas aeruginosa Quorum Sensing and Mortality in Rat Pneumonia. PLoS ONE 2014, 9, e107125. [Google Scholar] [CrossRef]
- Mayer, C.; Muras, A.; Romero, M.; López, M.; Tomás, M.; Otero, A. Multiple Quorum Quenching Enzymes Are Active in the Nosocomial Pathogen Acinetobacter baumannii ATCC17978. Front. Cell. Infect. Microbiol. 2018, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Chow Jeng, Y.; Yang, Y.; Tay Song, B.; Chua Kim, L.; Yew Wen, S. Disruption of Biofilm Formation by the Human Pathogen Acinetobacter baumannii Using Engineered Quorum-Quenching Lactonases. Antimicrob. Agents Chemother. 2014, 58, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Mayer, C.; Fernández-García, L.; Blasco, L.; Muras, A.; Ruiz, F.M.; Bou, G.; Otero, A.; Tomás, M.; GEIH-GEMARA (SEIMC). Quorum sensing network in clinical strains of A. baumannii: AidA is a new quorum quenching enzyme. PLoS ONE 2017, 12, e0174454. [Google Scholar] [CrossRef]
- Bergonzi, C.; Schwab, M.; Naik, T.; Daudé, D.; Chabrière, E.; Elias, M. Structural and Biochemical Characterization of AaL, a Quorum Quenching Lactonase with Unusual Kinetic Properties. Sci. Rep. 2018, 8, 11262. [Google Scholar] [CrossRef]
- Bergonzi, C.; Schwab, M.; Naik, T.; Elias, M. The Structural Determinants Accounting for the Broad Substrate Specificity of the Quorum Quenching Lactonase GcL. ChemBioChem 2019, 20, 1848–1855. [Google Scholar] [CrossRef]
- Gupta, K.; Chhibber, S. Biofunctionalization of Silver Nanoparticles With Lactonase Leads to Altered Antimicrobial and Cytotoxic Properties. Front. Mol. Biosci. 2019, 6, 63. [Google Scholar] [CrossRef]
- Mayer, C.; Romero, M.; Muras, A.; Otero, A. Aii20J, a wide-spectrum thermostable N-acylhomoserine lactonase from the marine bacterium Tenacibaculum sp. 20J, can quench AHL-mediated acid resistance in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 9523–9539. [Google Scholar] [CrossRef]
- Nithya, C.; Aravindraja, C.; Pandian, S.K. Bacillus pumilus of Palk Bay origin inhibits quorum-sensing-mediated virulence factors in Gram-negative bacteria. Res. Microbiol. 2010, 161, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Xu, J.-L.; Hu, J.; Wang, L.-H.; Ong, S.L.; Leadbetter, J.R.; Zhang, L.-H. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 2003, 47, 849–860. [Google Scholar] [CrossRef]
- Wahjudi, M.; Papaioannou, E.; Hendrawati, O.; van Assen, A.H.G.; van Merkerk, R.; Cool, R.H.; Poelarends, G.J.; Quax, W.J. PA0305 of Pseudomonas aeruginosa is a quorum quenching acylhomoserine lactone acylase belonging to the Ntn hydrolase superfamily. Microbiology 2011, 157, 2042–2055. [Google Scholar] [CrossRef]
- Utari, P.D.; Setroikromo, R.; Melgert, B.N.; Quax, W.J. PvdQ Quorum Quenching Acylase Attenuates Pseudomonas aeruginosa Virulence in a Mouse Model of Pulmonary Infection. Front. Cell. Infect. Microbiol. 2018, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kang, H.-O.; Jang, H.-S.; Lee, J.-K.; Koo, B.-T.; Yum, D.-Y. Identification of Extracellular N-Acylhomoserine Lactone Acylase from a Streptomyces sp. and Its Application to Quorum Quenching. Appl. Environ. Microbiol. 2005, 71, 2632–2641. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.C.; Romero, M.; Salto, R.; Cámara, M.; Llamas, I.; AhaP, A. Quorum Quenching Acylase from Psychrobacter sp. M9-54-1 That Attenuates Pseudomonas aeruginosa and Vibrio coralliilyticus Virulence. Mar. Drugs 2021, 19, 16. [Google Scholar] [CrossRef]
- Vogel, J.; Jansen, L.; Setroikromo, R.; Cavallo, F.M.; van Dijl, J.M.; Quax, W.J. Fighting Acinetobacter baumannii infections with the acylase PvdQ. Microbes Infect. 2022, 24, 104951. [Google Scholar] [CrossRef]
- Koch, G.; Nadal-Jimenez, P.; Reis, C.R.; Muntendam, R.; Bokhove, M.; Melillo, E.; Dijkstra, B.W.; Cool, R.H.; Quax, W.J. Reducing virulence of the human pathogen Burkholderia by altering the substrate specificity of the quorum-quenching acylase PvdQ. Proc. Natl. Acad. Sci. USA 2014, 111, 1568–1573. [Google Scholar] [CrossRef]
- Bijtenhoorn, P.; Mayerhofer, H.; Müller-Dieckmann, J.; Utpatel, C.; Schipper, C.; Hornung, C.; Szesny, M.; Grond, S.; Thürmer, A.; Brzuszkiewicz, E.; et al. A Novel Metagenomic Short-Chain Dehydrogenase/Reductase Attenuates Pseudomonas aeruginosa Biofilm Formation and Virulence on Caenorhabditis elegans. PLoS ONE 2011, 6, e26278. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N.; Kisch, M.J.; Pinnow, N.; Liese, A.; Schmitz, R.A. Highly Effective Inhibition of Biofilm Formation by the First Metagenome-Derived AI-2 Quenching Enzyme. Front. Microbiol. 2016, 7, 1098. [Google Scholar] [CrossRef]
- Bodede, O.; Shaik, S.; Chenia, H.; Singh, P.; Moodley, R. Quorum sensing inhibitory potential and in silico molecular docking of flavonoids and novel terpenoids from Senegalia nigrescens. J. Ethnopharmacol. 2018, 216, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen Tim, H.; Bragason Steinn, K.; Phipps Richard, K.; Christensen Louise, D.; van Gennip, M.; Alhede, M.; Skindersoe, M.; Larsen Thomas, O.; Høiby, N.; Bjarnsholt, T.; et al. Food as a Source for Quorum Sensing Inhibitors: Iberin from Horseradish Revealed as a Quorum Sensing Inhibitor of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2012, 78, 2410–2421. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Krishnan, T.; Wang, H.; Chen, Y.; Yin, W.-F.; Chong, Y.-M.; Tan, L.Y.; Chong, T.M.; Chan, K.-G. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep. 2014, 4, 7245. [Google Scholar] [CrossRef]
- Kim, J.; Shin, M.; Song, W.; Park, S.; Ryu, J.; Jung, J.; Choi, S.; Yu, Y.; Kweon, J.; Lee, J.-H. Application of quorum sensing inhibitors for improving anti-biofouling of polyamide reverse osmosis membranes: Direct injection versus surface modification. Sep. Purif. Technol. 2021, 255, 117736. [Google Scholar] [CrossRef]
- Nayak, S.P.R.R.; Boopathi, S.; Priya, P.S.; Pasupuleti, M.; Pachaiappan, R.; Almutairi, B.O.; Arokiyaraj, S.; Arockiaraj, J. Luteolin, a promising quorum quencher mitigates virulence factors production in Pseudomonas aeruginosa—In vitro and In vivoapproach. Microb. Pathog. 2023, 180, 106123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Seenivasan, B.; Li, R.; Li, C.; Zhang, Y.; Ravichandran, V.; Zhong, L.; Li, A. Exploring daidzein dimethyl ether from Albizzia lebbeck as a novel quorum sensing inhibitor against Pseudomonas aeruginosa: Insights from in vitro and in vivo studies. Bioorganic Chem. 2025, 156, 108168. [Google Scholar] [CrossRef]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Akhter, Y.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef]
- Yin, H.; Deng, Y.; Wang, H.; Liu, W.; Zhuang, X.; Chu, W. Tea polyphenols as an antivirulence compound Disrupt Quorum-Sensing Regulated Pathogenicity of Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 16158. [Google Scholar] [CrossRef]
- Anju, V.T.; Busi, S.; Ranganathan, S.; Ampasala, D.R.; Kumar, S.; Suchiang, K.; Kumavath, R.; Dyavaiah, M. Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors. Microb. Pathog. 2021, 155, 104912. [Google Scholar] [CrossRef]
- D’Almeida, R.E.; Sued, N.; Arena, M.E. Citrus paradisi and Citrus reticulata essential oils interfere with Pseudomonas aeruginosa quorum sensing in vivo on Caenorhabditis elegans. Phytomedicine Plus 2022, 2, 100160. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria—Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Nain, Z.; Jasin, M.F.; Bin, S.S.; Ariful, I.M.; Hiroyuki, A.; Rezuanul, I.M.; Karim, M.M. Inhibition of biofilm formation, quorum sensing and other virulence factors in Pseudomonas aeruginosa by polyphenols of Gynura procumbens leaves. J. Biomol. Struct. Dyn. 2022, 40, 5357–5371. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, H.M.; Bashir, G.I.; Sachin, K.; Anwar, S.; Ismail, C.; Shahid, M. Anti-quorum sensing, antibiofilm, and antibacterial activities of extracts of Centella asiatica L. leaves, and in vitro derived leaves-calli through tissue culture: A potential for biofouling-prevention. Biofouling 2022, 38, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Merghni, A.; Alreshidi, M.M.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef] [PubMed]
- Mozirandi, W.; Tagwireyi, D.; Mukanganyama, S. Evaluation of antimicrobial activity of chondrillasterol isolated from Vernonia adoensis (Asteraceae). BMC Complement. Altern. Med. 2019, 19, 249. [Google Scholar] [CrossRef]
- Ghosh, C.; Bhowmik, J.; Ghosh, R.; Das, M.C.; Sandhu, P.; Kumari, M.; Acharjee, S.; Daware, A.V.; Akhter, Y.; Banerjee, B.; et al. The anti-biofilm potential of triterpenoids isolated from Sarcochlamys pulcherrima (Roxb.) Gaud. Microb. Pathog. 2020, 139, 103901. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Zhou, L.; Ji, H.; Zhao, L.; Yu, W.; Gong, Q. Falcarindiol Isolated from Notopterygium incisum Inhibits the Quorum Sensing of Pseudomonas aeruginosa. Mol. 2021, 26, 5896. [Google Scholar] [CrossRef] [PubMed]
- Sagar, P.K.; Sharma, P.; Singh, R. Inhibition of Quorum Sensing Regulated Virulence Factors and Biofilm Formation by Eucalyptus globulus against Multidrug-Resistant Pseudomonas aeruginosa. J. Pharmacopunct. 2022, 25, 37–45. [Google Scholar] [CrossRef]
- Chatterjee, B.; Vittal, R.R. Quorum sensing modulatory and biofilm inhibitory activity of Plectranthus barbatus essential oil: A novel intervention strategy. Arch. Microbiol. 2021, 203, 1767–1778. [Google Scholar] [CrossRef]
- Naga, N.G.; Zaki, A.A.; El-Badan, D.E.; Rateb, H.S.; Ghanem, K.M.; Shaaban, M.I. Methoxyisoflavan derivative from Trigonella stellata inhibited quorum sensing and virulence factors of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2022, 38, 156. [Google Scholar] [CrossRef]
- Cosa, S.; Rakoma, J.R.; Yusuf, A.A.; Tshikalange, T.E. Calpurnia aurea (Aiton) Benth Extracts Reduce Quorum Sensing Controlled Virulence Factors in Pseudomonas aeruginosa. Mol. 2020, 25, 2283. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, V.; Thirunanasambandham, R.; Thangaraj, G. Anti-quorum sensing and antibiofilm potential of 1,8-cineole derived from Musa paradisiaca against Pseudomonas aeruginosa strain PAO1. World J. Microbiol. Biotechnol. 2021, 37, 66. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Y.; Han, X.; Cai, X.; Yang, L.; Liu, C.; Shen, L. Inhibition of Virulence Factors and Biofilm Formation by Wogonin Attenuates Pathogenicity of Pseudomonas aeruginosa PAO1 via Targeting pqs Quorum-Sensing System. Int. J. Mol. Sci. 2021, 22, 12699. [Google Scholar] [CrossRef]
- Elamary, R.B.; Albarakaty, F.M.; Salem, W.M. Efficacy of Acacia nilotica aqueous extract in treating biofilm-forming and multidrug resistant uropathogens isolated from patients with UTI syndrome. Sci. Rep. 2020, 10, 11125. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Singh, S.P.; Anupam, S.; Prince, S.; Capalash, N. Attenuation of Quorum Sensing-Mediated Virulence of Acinetobacter baumannii by Glycyrrhiza Glabra Flavonoids. Future Microbiol. 2015, 10, 1953–1968. [Google Scholar] [CrossRef]
- Güran, M.; Çakıral, K.; Teralı, K.; Kandemir, T.; Şanlıtürk, G.; Öcal, M.M.; Nagiyev, T.; Köksal, F. Meropenem in combination with baicalein exhibits synergism against extensively drug resistant and pan-drug-resistant Acinetobacter baumannii clinical isolates in vitro. Pathog. Dis. 2023, 81, ftad007. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhou, N.; Yang, L.; Wang, Z.; Huan, C.; Lin, T.; Bao, G.; Hu, J.; Li, G. Acinetobacter baumannii biofilm was inhibited by tryptanthrin through disrupting its different stages and genes expression. iScience 2024, 27, 109942. [Google Scholar] [CrossRef]
- Mohammadi, M.; Masoumipour, F.; Hassanshahian, M.; Jafarinasab, T. Study the antibacterial and antibiofilm activity of Carum copticum against antibiotic-resistant bacteria in planktonic and biofilm forms. Microb. Pathog. 2019, 129, 99–105. [Google Scholar] [CrossRef]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Hamat, R.A.; Pichika, M.R.; Lung, L.T.T.; Mohd Fauzi, F.; Chigurupati, S.; van Belkum, A.; Neela, V. Antibacterial and Antibiofilm Activities of Nonpolar Extracts of Allium stipitatum Regel. against Multidrug Resistant Bacteria. BioMed Res. Int. 2018, 2018, 9845075. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Liu, Y.; Xu, M.; Yao, Z.; Zhang, X.; Sun, Y.; Zhou, T.; Shen, M. Effects of chlorogenic acid on antimicrobial, antivirulence, and anti-quorum sensing of carbapenem-resistant Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 997310. [Google Scholar] [CrossRef]
- Liu, W.; Lu, H.; Chu, X.; Lou, T.; Zhang, N.; Zhang, B.; Chu, W. Tea polyphenols inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances resistance to Klebsiella pneumoniae infection in Caenorhabditis elegans model. Microb. Pathog. 2020, 147, 104266. [Google Scholar] [CrossRef] [PubMed]
- Augustus, A.R.; Jana, S.; Samsudeen, M.B.; Nagaiah, H.P.; Shunmugiah, K.P. In vitro and in vivo evaluation of the anti-infective potential of the essential oil extracted from the leaves of Plectranthus amboinicus (lour.) spreng against Klebsiella pneumoniae and elucidation of its mechanism of action through proteomics approach. J. Ethnopharmacol. 2024, 330, 118202. [Google Scholar] [CrossRef]
- Gopu, V.; Kothandapani, S.; Shetty, P.H. Quorum quenching activity of Syzygium cumini (L.) Skeels and its anthocyanin malvidin against Klebsiella pneumoniae. Microb. Pathog. 2015, 79, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Thinina, A.C.; Karim, H.; Alia, M.M.; Karim, A. Evaluation and quantification of the inhibition of biofilm and planktonic forms of Klebsiella pneumoniae by the polyphenolic extract of Pulicaria crispa. J. Adv. Pharm. Technol. Res. 2020, 11, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.S.; Branco Santos, J.C.; Castro Junior, J.A.; Wakui, V.G.; Rodrigues, J.F.S.; Arruda, M.O.; Monteiro, A.D.; Monteiro-Neto, V.; Bomfim, M.R.; Kato, L.; et al. Himatanthus drasticus Leaves: Chemical Characterization and Evaluation of Their Antimicrobial, Antibiofilm, Antiproliferative Activities. Molecules 2017, 22, 910. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Shi, M.; Wei, X.; Zhou, X.; Li, B.; Zhang, J. Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli. Int. J. Mol. Sci. 2022, 23, 8809. [Google Scholar] [CrossRef]
- Mashamba, T.G.; Adeosun, I.J.; Baloyi, I.T.; Tshikalange, E.T.; Cosa, S. Quorum sensing modulation and inhibition in biofilm forming foot ulcer pathogens by selected medicinal plants. Heliyon 2022, 8, e09303. [Google Scholar] [CrossRef]
- Zong, B.; Xiao, Y.; Wang, P.; Liu, W.; Ren, M.; Li, C.; Fu, S.; Zhang, Y.; Qiu, Y. Baicalin Weakens the Virulence of Porcine Extraintestinal Pathogenic Escherichia coli by Inhibiting the LuxS/AI-2 Quorum-Sensing System. Biomolecules 2024, 14, 452. [Google Scholar] [CrossRef]
- Sharifi, A.; Nayeri Fasaei, B. Selected plant essential oils inhibit biofilm formation and luxS- and pfs-mediated quorum sensing by Escherichia coli O157:H7. Lett. Appl. Microbiol. 2022, 74, 916–923. [Google Scholar] [CrossRef]
- Sood, H.; Kumar, Y.; Gupta, V.K.; Arora, D.S. Bioprospecting the antimicrobial, antibiofilm and antiproliferative activity of Symplocos racemosa Roxb. Bark phytoconstituents along with their biosafety evaluation and detection of antimicrobial components by GC-MS. BMC Pharmacol. Toxicol. 2020, 21, 78. [Google Scholar] [CrossRef]
- Vazquez, N.M.; Mariani, F.; Torres, P.S.; Moreno, S.; Galván, E.M. Cell death and biomass reduction in biofilms of multidrug resistant extended spectrum β-lactamase-producing uropathogenic Escherichia coli isolates by 1,8-cineole. PLoS ONE 2020, 15, e0241978. [Google Scholar] [CrossRef] [PubMed]
- Gleńsk, M.; Tichaczek-Goska, D.; Środa-Pomianek, K.; Włodarczyk, M.; Wesolowski, C.A.; Wojnicz, D. Differing antibacterial and antibiofilm properties of Polypodium vulgare L. Rhizome aqueous extract and one of its purified active ingredients–osladin. J. Herb. Med. 2019, 17–18, 100261. [Google Scholar] [CrossRef]
- Ghazal, T.S.; Schelz, Z.; Vidács, L.; Szemerédi, N.; Veres, K.; Spengler, G.; Hohmann, J. Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes. Plants 2022, 11, 14322. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-G.; Liu, Y.-C.; Chang, C.-Y. Inhibiting N-acyl-homoserine lactone synthesis and quenching Pseudomonas quinolone quorum sensing to attenuate virulence. Front. Microbiol. 2015, 6, 1173. [Google Scholar] [CrossRef]
- Storz, M.P.; Maurer, C.K.; Zimmer, C.; Wagner, N.; Brengel, C.; de Jong, J.C.; Lucas, S.; Müsken, M.; Häussler, S.; Steinbach, A.; et al. Validation of PqsD as an Anti-biofilm Target in Pseudomonas aeruginosa by Development of Small-Molecule Inhibitors. J. Am. Chem. Soc. 2012, 134, 16143–16146. [Google Scholar] [CrossRef]
- Chung, J.; Goo, E.; Yu, S.; Choi, O.; Lee, J.; Kim, J.; Kim, H.; Igarashi, J.; Suga, H.; Moon, J.S.; et al. Small-molecule inhibitor binding to an N-acyl-homoserine lactone synthase. Proc. Natl. Acad. Sci. USA 2011, 108, 12089–12094. [Google Scholar] [CrossRef]
- Christensen, Q.H.; Grove, T.L.; Booker, S.J.; Greenberg, E.P. A high-throughput screen for quorum-sensing inhibitors that target acyl-homoserine lactone synthases. Proc. Natl. Acad. Sci. USA 2013, 110, 13815–13820. [Google Scholar] [CrossRef]
- Kaufmann, G.F.; Sartorio, R.; Lee, S.-H.; Mee, J.M.; Altobell, L.J.; Kujawa, D.P.; Jeffries, E.; Clapham, B.; Meijler, M.M.; Janda, K.D. Antibody Interference with N-Acyl Homoserine Lactone-Mediated Bacterial Quorum Sensing. J. Am. Chem. Soc. 2006, 128, 2802–2803. [Google Scholar] [CrossRef]
- Roy, V.; Adams, B.L.; Bentley, W.E. Developing next generation antimicrobials by intercepting AI-2 mediated quorum sensing. Enzym. Microb. Technol. 2011, 49, 113–123. [Google Scholar] [CrossRef]
- Chen, F.; Gao, Y.; Chen, X.; Yu, Z.; Li, X. Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection. Int. J. Mol. Sci. 2013, 14, 17477–17500. [Google Scholar] [CrossRef]
- Murugayah, S.A.; Gerth, M.L. Engineering quorum quenching enzymes: Progress and perspectives. Biochem. Soc. Trans. 2019, 47, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Wang, C.; Mulchandani, A.; Ge, X. Engineering Soluble Human Paraoxonase 2 for Quorum Quenching. ACS Chem. Biol. 2016, 11, 3122–3131. [Google Scholar] [CrossRef] [PubMed]
- LaSarre, B.; Federle Michael, J. Exploiting Quorum Sensing To Confuse Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef]
- Fetzner, S. Quorum quenching enzymes. J. Biotechnol. 2015, 201, 2–14. [Google Scholar] [CrossRef]
- Utari, P.D.; Vogel, J.; Quax, W.J. Deciphering Physiological Functions of AHL Quorum Quenching Acylases. Front. Microbiol. 2017, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. BioMed Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef]
- Zhang, X.; Ou-Yang, S.; Wang, J.; Liao, L.; Wu, R.; Wei, J. Construction of Antibacterial Surface Via Layer-by-Layer Method. Curr. Pharm. Des. 2018, 24, 926–935. [Google Scholar] [CrossRef]
- Kaufmann, G.F.; Park, J.; Mee, J.M.; Ulevitch, R.J.; Janda, K.D. The quorum quenching antibody RS2-1G9 protects macrophages from the cytotoxic effects of the Pseudomonas aeruginosa quorum sensing signalling molecule N-3-oxo-dodecanoyl-homoserine lactone. Mol. Immunol. 2008, 45, 2710–2714. [Google Scholar] [CrossRef]
- Palliyil, S.; Downham, C.; Broadbent, I.; Charlton, K.; Porter Andrew, J. High-Sensitivity Monoclonal Antibodies Specific for Homoserine Lactones Protect Mice from Lethal Pseudomonas aeruginosa Infections. Appl. Environ. Microbiol. 2014, 80, 462–469. [Google Scholar] [CrossRef]
- Pathak, A. New Vaccines for Infectious Diseases: Immunological Targeting of the Quorum Sensing System of Pseudomonas aeruginosa. Doctoral Dissertation, University of Nottingham, Nottingham, UK, 2012. [Google Scholar]
- Chen, X.; Kremmer, E.; Gouzy, M.-F.; Clausen, E.; Starke, M.; Wöllner, K.; Pfister, G.; Hartmann, A.; Krämer, P.M. Development and characterization of rat monoclonal antibodies for N-acylated homoserine lactones. Anal. Bioanal. Chem. 2010, 398, 2655–2667. [Google Scholar] [CrossRef]
- Ray, V.A.; Hill, P.J.; Stover, C.K.; Roy, S.; Sen, C.K.; Yu, L.; Wozniak, D.J.; DiGiandomenico, A. Anti-Psl Targeting of Pseudomonas aeruginosa Biofilms for Neutrophil-Mediated Disruption. Sci. Rep. 2017, 7, 16065. [Google Scholar] [CrossRef] [PubMed]
- Kurbatfinski, N.; Goodman Steven, D.; Bakaletz Lauren, O. A Humanized Monoclonal Antibody Potentiates Killing of Diverse Biofilm-Forming Respiratory Tract Pathogens by Antibiotics. Antimicrob. Agents Chemother. 2022, 66, e01877-21. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Q.; Estellés, A.; Li, L.; Abdelhady, W.; Gonzales, R.; Bayer, A.S.; Tenorio, E.; Leighton, A.; Ryser, S.; Kauvar, L.M. A Human Biofilm-Disrupting Monoclonal Antibody Potentiates Antibiotic Efficacy in Rodent Models of both Staphylococcus aureus and Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2017, 61, e00904-17. [Google Scholar] [CrossRef]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef]
- Fattahian, Y.; Rasooli, I.; Mousavi Gargari, S.L.; Rahbar, M.R.; Darvish Alipour Astaneh, S.; Amani, J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap). Microb. Pathog. 2011, 51, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef]
- Diago-Navarro, E.; Motley Michael, P.; Ruiz-Peréz, G.; Yu, W.; Austin, J.; Seco Bruna, M.S.; Xiao, G.; Chikhalya, A.; Seeberger Peter, H.; Fries Bettina, C. Novel, Broadly Reactive Anticapsular Antibodies against Carbapenem-Resistant Klebsiella pneumoniae Protect from Infection. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chang, C.-s.; Pennini, M.; Pelletier, M.; Rajan, S.; Zha, J.; Chen, Y.; Cvitkovic, R.; Sadowska, A.; Heidbrink Thompson, J.; et al. Target-Agnostic Identification of Functional Monoclonal Antibodies Against Klebsiella pneumoniae Multimeric MrkA Fimbrial Subunit. J. Infect. Dis. 2016, 213, 1800–1808. [Google Scholar] [CrossRef]
- Monteiro, R.; Chafsey, I.; Caccia, N.; Ageorges, V.; Leroy, S.; Viala, D.; Hébraud, M.; Livrelli, V.; Pizza, M.; Pezzicoli, A.; et al. Specific Proteomic Identification of Collagen-Binding Proteins in Escherichia coli O157:H7: Characterisation of OmpA as a Potent Vaccine Antigen. Cells 2023, 12, 1634. [Google Scholar] [CrossRef]
- Shirmohammadpour, M.; Mehrasbi, M.R.; Noshiranzadeh, N.; Afshar, D.; Mansori, K.; Mirzaei, B. Investigation of the effect of anti-PIA/PNAG antibodies on biofilm formation in Escherichia coli. Front. Microbiol. 2025, 16, 1552670. [Google Scholar] [CrossRef]
- Kisiela, D.I.; Avagyan, H.; Friend, D.; Jalan, A.; Gupta, S.; Interlandi, G.; Liu, Y.; Tchesnokova, V.; Rodriguez, V.B.; Sumida, J.P.; et al. Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic E. coli. PLOS Pathog. 2015, 11, e1004857. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Zhang, C.; Yang, R.; Boo, Z.Z.; Tan, S.K.; Nielsen, T.E.; Givskov, M.; Liu, X.-W.; Bin, W.; Su, H.; et al. Combination Therapy Strategy of Quorum Quenching Enzyme and Quorum Sensing Inhibitor in Suppressing Multiple Quorum Sensing Pathways of P. aeruginosa. Sci. Rep. 2018, 8, 1155. [Google Scholar] [CrossRef]
- Rezzoagli, C.; Archetti, M.; Mignot, I.; Baumgartner, M.; Kümmerli, R. Combining antibiotics with antivirulence compounds can have synergistic effects and reverse selection for antibiotic resistance in Pseudomonas aeruginosa. PLOS Biol. 2020, 18, e3000805. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yin, B.; Qian, L.; Zeng, Z.; Yang, Z.; Li, H.; Lu, Y.; Zhou, S. Screening for novel quorum-sensing inhibitors to interfere with the formation of Pseudomonas aeruginosa biofilm. J. Med. Microbiol. 2011, 60, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Xu, Z.-H.; Zhang, Y.-Q.; Tian, J.; Weng, L.-X.; Wang, L.-H. A new quorum-sensing inhibitor attenuates virulence and decreases antibiotic resistance in Pseudomonas aeruginosa. J. Microbiol. 2012, 50, 987–993. [Google Scholar] [CrossRef]
- Mion, S.; Rémy, B.; Plener, L.; Brégeon, F.; Chabrière, E.; Daudé, D. Quorum Quenching Lactonase Strengthens Bacteriophage and Antibiotic Arsenal Against Pseudomonas aeruginosa Clinical Isolates. Front. Microbiol. 2019, 10, 2049. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Feng, L.; Xu, M.; Wen, H.; Yao, Z.; Shi, S.; Wu, Q.; Zhou, C.; Cao, J.; et al. In vitro and in vivo synergistic effect of chrysin in combination with colistin against Acinetobacter baumannii. Front. Microbiol. 2022, 13, 961498. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef]
- Beasley, J.-M.; Dorjsuren, D.; Jain, S.; Rath, M.; Scheufen Tieghi, R.; Tropsha, A.; Simeonov, A.; Zakharov, A.V.; Muratov, E. Breaking the Phalanx: Overcoming Bacterial Drug Resistance with Quorum Sensing Inhibitors that Enhance Therapeutic Activity of Antibiotics. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef]
- Al Marjani, M.F.; Aziz, S.N.; Al-Kadmy, I.M.S. Synergistic effects of combination indole and ciprofloxacin aantibiotic against persistence Klebsiella pneumoniae isolates. AIP Conf. Proc. 2022, 2386, 020005. [Google Scholar] [CrossRef]
- Dera, A.A.; Ahmad, I.; Rajagopalan, P.; Shahrani, M.A.; Saif, A.; Alshahrani, M.Y.; Alraey, Y.; Alamri, A.M.; Alasmari, S.; Makkawi, M.; et al. Synergistic efficacies of thymoquinone and standard antibiotics against multi-drug resistant isolates. Saudi Med. J. 2021, 42, 196. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, D.; Tichaczek-Goska, D.; Kicia, M. Pentacyclic triterpenes combined with ciprofloxacin help to eradicate the biofilm formed in vitro by Escherichia coli. Indian. J. Med. Res. 2015, 141, 343–353. [Google Scholar] [CrossRef]
- de la Fuente-Núñez, C.; Reffuveille, F.; Fairfull-Smith Kathryn, E.; Hancock Robert, E.W. Effect of Nitroxides on Swarming Motility and Biofilm Formation, Multicellular Behaviors in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4877–4881. [Google Scholar] [CrossRef] [PubMed]
- Reffuveille, F.; de la Fuente-Núñez, C.; Fairfull-Smith, K.E.; Hancock, R.E.W. Potentiation of ciprofloxacin action against Gram-negative bacterial biofilms by a nitroxide. Pathog. Dis. 2015, 73, ftv016. [Google Scholar] [CrossRef] [PubMed]
- Morgaan, H.A.; Omar, H.M.G.; Zakaria, A.S.; Mohamed, N.M. Repurposing carvacrol, cinnamaldehyde, and eugenol as potential anti-quorum sensing agents against uropathogenic Escherichia coli isolates in Alexandria, Egypt. BMC Microbiol. 2023, 23, 300. [Google Scholar] [CrossRef]
- Kumar, L.; Brenner, N.; Brice, J.; Klein-Seetharaman, J.; Sarkar, S.K. Cephalosporins Interfere With Quorum Sensing and Improve the Ability of Caenorhabditis elegans to Survive Pseudomonas aeruginosa Infection. Front. Microbiol. 2021, 12, 598498. [Google Scholar] [CrossRef]
- Tateda, K.; Comte, R.; Pechere, J.-C.; Köhler, T.; Yamaguchi, K.; Van Delden, C. Azithromycin Inhibits Quorum Sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2001, 45, 1930–1933. [Google Scholar] [CrossRef]
- Babić, F.; Venturi, V.; Maravić-Vlahoviček, G. Tobramycin at subinhibitory concentration inhibits the RhlI/R quorum sensing system in a Pseudomonas aeruginosa environmental isolate. BMC Infect. Dis. 2010, 10, 148. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Neubauer, D.; Kazor, K.; Bartoszewska, S.; Kamysz, W. Antimicrobial Activity of Selected Antimicrobial Peptides Against Planktonic Culture and Biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Proteins 2019, 11, 317–324. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Yue, C.; Liu, Y.; Li, J. The Anti-Virulence Effect of Sub-Minimal Inhibitory Concentrations of Levofloxacin on Hypervirulent Klebsiella pneumoniae. Infect. Drug Resist. 2022, 15, 3513–3522. [Google Scholar] [CrossRef]
- Sun, F.; Qian, Y.; Yu, W.; Lin, C.; Xiaoyu, L.; Wei, F.; Xia, P. Sub-minimum inhibitory concentration ceftazidime inhibits Escherichia coli biofilm formation by influencing the levels of the ibpA gene and extracellular indole. J. Chemother. 2020, 32, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, D.; Tichaczek-Goska, D. Effect of sub-minimum inhibitory concentrations of ciprofloxacin, amikacin and colistin on biofilm formation and virulence factors of Escherichia coli planktonic and biofilm forms isolated from human urine. Braz. J. Microbiol. 2013, 44, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Mojsoska, B.; Ghoul, M.; Perron, G.G.; Jenssen, H.; Alatraktchi, F.A. Changes in toxin production of environmental Pseudomonas aeruginosa isolates exposed to sub-inhibitory concentrations of three common antibiotics. PLoS ONE 2021, 16, e0248014. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical applications and therapeutic potentials of advanced nanoparticles: A comprehensive review on completed human clinical trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Qais, F.A.; Khan, M.S.; Ahmad, I. Nanoparticles as Quorum Sensing Inhibitor: Prospects and Limitations. In Biotechnological Applications of Quorum Sensing Inhibitors; Kalia, V.C., Ed.; Springer Singapore: Singapore, 2018; pp. 227–244. [Google Scholar]
- de Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; Medeiros, S.M.d.F.R.d.S.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef]
- Chegini, Z.; Shariati, A.; Alikhani, M.Y.; Safaiee, M.; Rajaeih, S.; Arabestani, M.; Azizi, M. Antibacterial and antibiofilm activity of silver nanoparticles stabilized with C-phycocyanin against drug-resistant Pseudomonas aeruginosa and Staphylococcus aureus. Front. Bioeng. Biotechnol. 2024, 12, 1455385. [Google Scholar] [CrossRef]
- Kamer, A.M.A.; El Maghraby, G.M.; Shafik, M.M.; Al-Madboly, L.A. Silver nanoparticle with potential antimicrobial and antibiofilm efficiency against multiple drug resistant, extensive drug resistant Pseudomonas aeruginosa clinical isolates. BMC Microbiol. 2024, 24, 277. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Loo, C.-Y.; Rohanizadeh, R.; Young, P.M.; Traini, D.; Cavaliere, R.; Whitchurch, C.B.; Lee, W.-H. Combination of Silver Nanoparticles and Curcumin Nanoparticles for Enhanced Anti-biofilm Activities. J. Agric. Food Chem. 2016, 64, 2513–2522. [Google Scholar] [CrossRef]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Ellah, N.H.A.; Ahmed, E.A.; Abd-ellatief, R.B.; El-Masry, E.A.; et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef]
- Scandorieiro, S.; Teixeira, F.M.M.B.; Nogueira, M.C.L.; Panagio, L.A.; de Oliveira, A.G.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella pneumoniae. Antibiotics 2023, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Elashkar, E.; Alfaraj, R.; El-Borady, O.M.; Amer, M.M.; Algammal, A.M.; El-Demerdash, A.S. Novel silver nanoparticle-based biomaterials for combating Klebsiella pneumoniae biofilms. Front. Microbiol. 2025, 15, 1507274. [Google Scholar] [CrossRef]
- Abdelraheem, W.M.; Mohamed, E.S. The effect of Zinc Oxide nanoparticles on Pseudomonas aeruginosa biofilm formation and virulence genes expression. J. Infect. Dev. Ctries. 2021, 15, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Valadbeigi, H.; Sadeghifard, N.; Kaviar, V.H.; Haddadi, M.H.; Ghafourian, S.; Maleki, A. Effect of ZnO nanoparticles on biofilm formation and gene expression of the toxin-antitoxin system in clinical isolates of Pseudomonas aeruginosa. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 89. [Google Scholar] [CrossRef]
- Saleh, M.M.; Sadeq, R.A.; Latif, H.K.A.; Abbas, H.A.; Askoura, M. Zinc oxide nanoparticles inhibits quorum sensing and virulence in Pseudomonas aeruginosa. Afr. Health Sci. 2019, 19, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Udayagiri, H.; Sana, S.S.; Dogiparthi, L.K.; Vadde, R.; Varma, R.S.; Koduru, J.R.; Ghodake, G.S.; Somala, A.R.; Boya, V.K.N.; Kim, S.-C.; et al. Phytochemical fabrication of ZnO nanoparticles and their antibacterial and anti-biofilm activity. Sci. Rep. 2024, 14, 19714. [Google Scholar] [CrossRef]
- Al-Fawares, O.; Alshweiat, A.; Al-Khresieh, R.O.; Alzarieni, K.Z.; Rashaid, A.H.B. A significant antibiofilm and antimicrobial activity of chitosan-polyacrylic acid nanoparticles against pathogenic bacteria. Saudi Pharm. J. 2024, 32, 101918. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Juarez, J.; Valdez, M.A.; Ayala-Zavala, J.F.; Del-Toro-Sánchez, C.L.; Encinas-Basurto, D. Hydrophobic Chitosan Nanoparticles Loaded with Carvacrol against Pseudomonas aeruginosa Biofilms. Molecules 2022, 27, 699. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide against Chronic Wound Microorganisms. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Negi, P.; Chadha, J.; Harjai, K.; Gondil, V.S.; Kumari, S.; Raj, K. Antimicrobial and Antibiofilm Potential of Green-Synthesized Graphene–Silver Nanocomposite against Multidrug-Resistant Nosocomial Pathogens. Biomedicines 2024, 12, 1104. [Google Scholar] [CrossRef]
- Muthuchamy, M.; Govindan, R.; Shine, K.; Thangasamy, V.; Alharbi, N.S.; Thillaichidambaram, M.; Khaled, J.M.; Wen, J.-L.; Alanzi, K.F. Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa and K. pneumoniae. Carbohydr. Polym. 2020, 230, 115646. [Google Scholar] [CrossRef]
- Dezaki, F.S.; Narimani, T.; Ghanadian, M.; Bidram, E.; Poursina, F. Antimicrobial and antibiofilm effects of cyclic dipeptide-rich fraction from Lactobacillus plantarum loaded on graphene oxide nanosheets. Front. Microbiol. 2024, 15, 1391039. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Chackaravarthi, G.; Ramachandran, G.; Chelliah, C.K.; Muthuchamy, M.; Quero, F.; Mothana, R.A.; Noman, O.M.; Siddiqui, N.A.; Li, W.-J. Effective removal of biofilm formation in Acinetobacter baumannii using chitosan nanoparticles loaded plant essential oils. J. King Saud. Univ. Sci. 2022, 34, 101845. [Google Scholar] [CrossRef]
- Makhlouf, Z.; Ali, A.A.; Al-Sayah, M.H. Liposomes-Based Drug Delivery Systems of Anti-Biofilm Agents to Combat Bacterial Biofilm Formation. Antibiotics 2023, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Wellenberg, A.; Weides, L.; Kurzke, J.; Hennecke, T.; Bornhorst, J.; Crone, B.; Karst, U.; Brinkmann, V.; Fritz, G.; Honnen, S. Use of C. elegans as a 3R-compliant in vivo model for the chemoprevention of cisplatin-induced neurotoxicity. Exp. Neurol. 2021, 341, 113705. [Google Scholar] [CrossRef]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef]
- Clatworthy Anne, E.; Lee Jenny, S.-W.; Leibman, M.; Kostun, Z.; Davidson Alan, J.; Hung Deborah, T. Pseudomonas aeruginosa Infection of Zebrafish Involves both Host and Pathogen Determinants. Infect. Immun. 2009, 77, 1293–1303. [Google Scholar] [CrossRef]
- Li, Y.; Di Santo James, P. Modeling Infectious Diseases in Mice with a “Humanized” Immune System. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- Seleem, N.M.; Abd El Latif, H.K.; Shaldam, M.A.; El-Ganiny, A. Drugs with new lease of life as quorum sensing inhibitors: For combating MDR Acinetobacter baumannii infections. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1687–1702. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Ivanova, K.; Fernandes, M.M.; Francesko, A.; Mendoza, E.; Guezguez, J.; Burnet, M.; Tzanov, T. Quorum-Quenching and Matrix-Degrading Enzymes in Multilayer Coatings Synergistically Prevent Bacterial Biofilm Formation on Urinary Catheters. ACS Appl. Mater. Interfaces 2015, 7, 27066–27077. [Google Scholar] [CrossRef]
- Silva, C.A.; Moreira, J.; Fernandes, M.; Zille, A.; Cardoso, V.F.; Nine, M.J.; Silva, F.S.; Fernandes, M.M. Acylase-Based Coatings on Sandblasted Polydimethylsiloxane-Based Materials for Antimicrobial Applications. Polymers 2025, 17, 182. [Google Scholar] [CrossRef]
- Ho, K.K.K.; Chen, R.; Willcox, M.D.P.; Rice, S.A.; Cole, N.; Iskander, G.; Kumar, N. Quorum sensing inhibitory activities of surface immobilized antibacterial dihydropyrrolones via click chemistry. Biomaterials 2014, 35, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Taunk, A.H.K.; Iskander, G.; Willcox, M.D.P.; Kumar, N. Surface Immobilization of Antibacterial Quorum Sensing Inhibitors by Photochemical Activation. J. Biotechnol. Biomater. 2016, 6, 38. [Google Scholar] [CrossRef]
- Ozcelik, B.; Ho, K.K.K.; Glattauer, V.; Willcox, M.; Kumar, N.; Thissen, H. Poly(ethylene glycol)-Based Coatings Combining Low-Biofouling and Quorum-Sensing Inhibiting Properties to Reduce Bacterial Colonization. ACS Biomater. Sci. Eng. 2017, 3, 78–87. [Google Scholar] [CrossRef]
- Jothipandiyan, S.; Devarajan, S.; Venkatachalam, S.S.; Subbiah, T.; Kumaravel, S.; Preethi, V.; Saravanan, S.; Shanmugaraj, G.; Karutha, P.S.; Paramasivam, N. Heteroleptic pincer palladium(II) complex coated orthopedic implants impede the AbaI/AbaR quorum sensing system and biofilm development by Acinetobacter baumannii. Biofouling 2022, 38, 55–70. [Google Scholar] [CrossRef]
- van Delden, C.; Köhler, T.; Brunner-Ferber, F.; François, B.; Carlet, J.; Pechère, J.-C. Azithromycin to prevent Pseudomonas aeruginosa ventilator-associated pneumonia by inhibition of quorum sensing: A randomized controlled trial. Intensive Care Med. 2012, 38, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.R.; Cifelli, P.M.; Ortori, C.A.; Righetti, K.; Lewis, S.; Erskine, P.; Holland, E.D.; Givskov, M.; Williams, P.; Cámara, M.; et al. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis—A pilot randomized controlled trial. Pediatr. Pulmonol. 2010, 45, 356–362. [Google Scholar] [CrossRef]
- Han, L.; Ren, J.; Xue, Y.; Xie, G.; Gao, J.; Fu, Q.; Shao, P.; Zhu, H.; Zhang, M.; Ding, F. Palmitoleic acid inhibits Pseudomonas aeruginosa quorum sensing activation and protects lungs from infectious injury. Respir. Res. 2024, 25, 423. [Google Scholar] [CrossRef]
- Walz, J.M.; Avelar, R.L.; Longtine, K.J.; Carter, K.L.; Mermel, L.A.; Heard, S.O.; 5-FU Catheter Study Group. Anti-infective external coating of central venous catheters: A randomized, noninferiority trial comparing 5-fluorouracil with chlorhexidine/silver sulfadiazine in preventing catheter colonization*. Crit. Care Med. 2010, 38, 2095–2102. [Google Scholar] [CrossRef]
- Zhu, H.U.A.; Kumar, A.; Ozkan, J.; Bandara, R.; Ding, A.; Perera, I.; Steinberg, P.; Kumar, N.; Lao, W.; Griesser, S.S.; et al. Fimbrolide-Coated Antimicrobial Lenses: Their In Vitro and In Vivo Effects. Optom. Vis. Sci. 2008, 85, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Feng, J.; Liu, C.; Wu, H.; Qiu, Z.; Ge, J.; Sun, S.; Hong, X.; Li, Y.; Wang, X.; et al. Machine learning aided construction of the quorum sensing communication network for human gut microbiota. Nat. Commun. 2022, 13, 3079. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Magalhães, R.P.; de la Oliva Roque, V.M.; Simões, M.; Pratas, D.; Sousa, S.F. TargIDe: A machine-learning workflow for target identification of molecules with antibiofilm activity against Pseudomonas aeruginosa. J. Comput. Aided Mol. Des. 2023, 37, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.M.M.; Yung, P.L.S.; Heng, X.C.H.; Bee, T.L.; Siaw, S.H.; Palombo, E.A.; Wezen, X.C. A data-driven machine learning approach for discovering potent LasR inhibitors. Bioengineered 2023, 14, 2243416. [Google Scholar] [CrossRef]
- Raya, D.; Shreya, A.; Kumar, A.; Giri, S.K.; Salem, D.R.; Gnimpieba, E.Z.; Gadhamshetty, V.; Dhiman, S.S. Molecular regulation of conditioning film formation and quorum quenching in sulfate reducing bacteria. Front. Microbiol. 2022, 13, 1008536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).