Tumor Microenvironment: An Emerging Landscape for Lung Cancer Therapy
Abstract
1. Introduction
2. Tumor-Infiltrating Immune Cells
2.1. Tumor-Infiltrating Lymphocytes (TIL)
Cytotoxic CD8+ T Lymphocytes
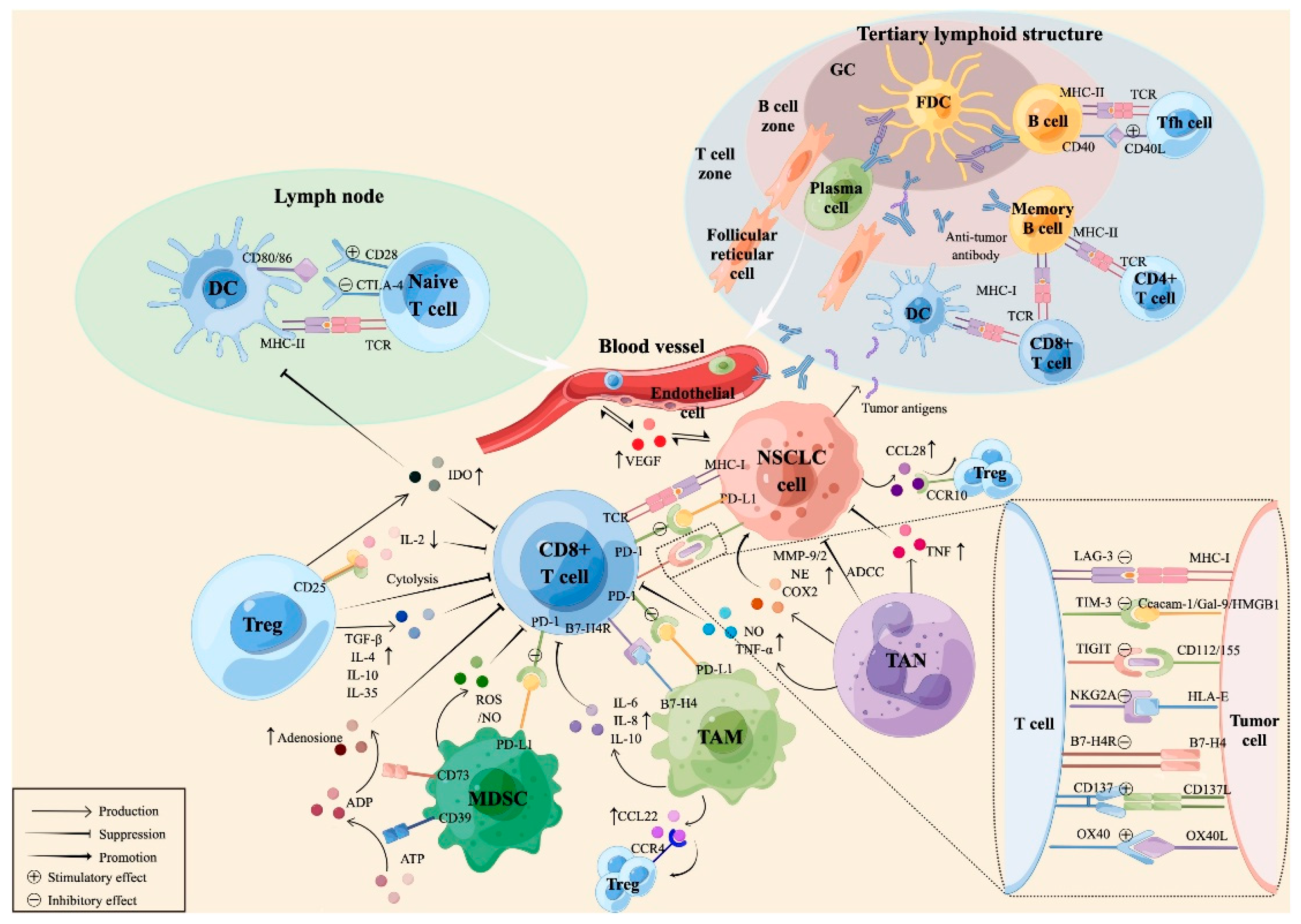
3. Tumor Expressing Cytokines
4. Lung Tumor Microenvironment
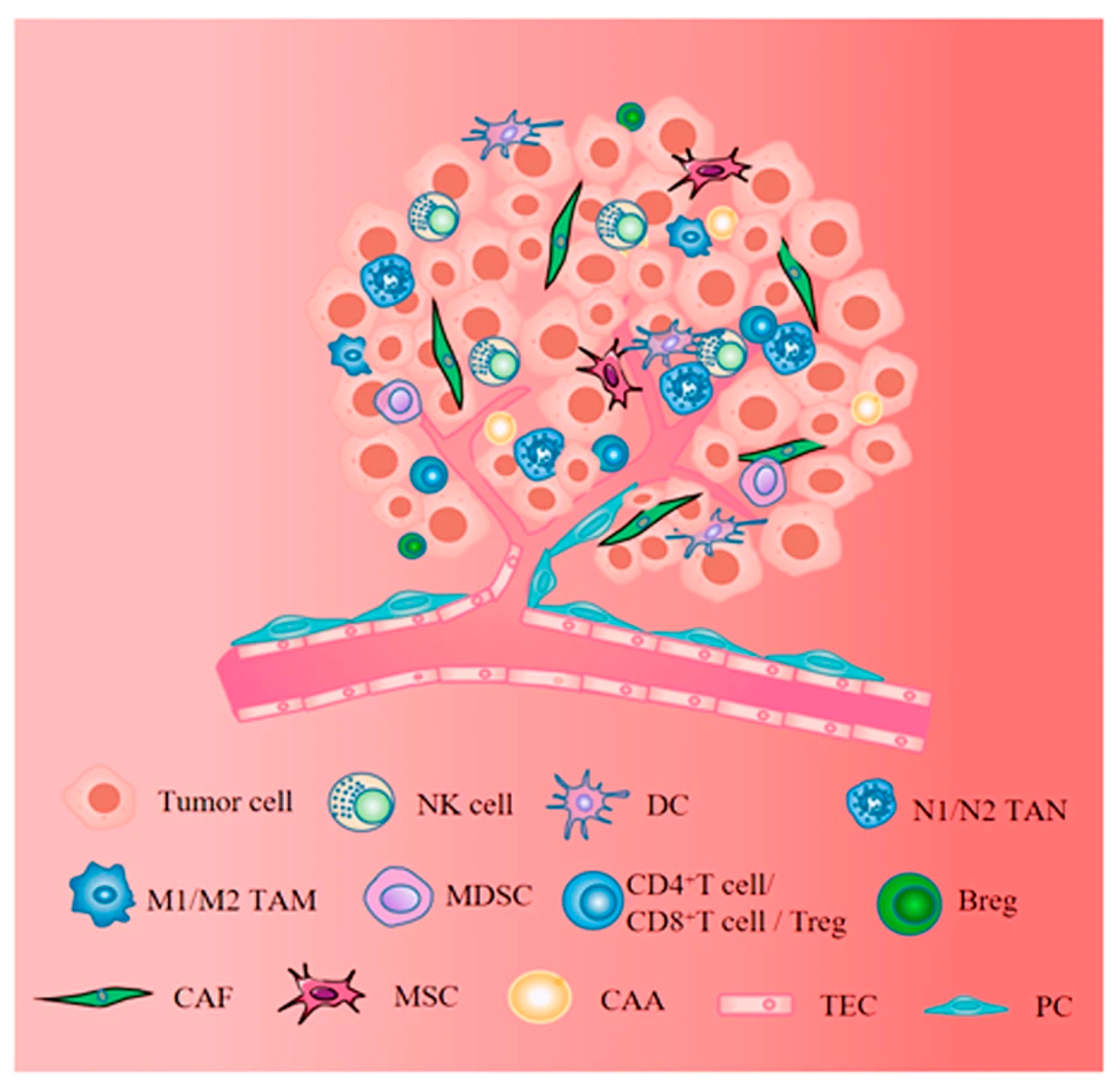
5. Cells of the Stroma
5.1. Fibroblast Cells
5.2. Immune Cells
5.2.1. T Cells
5.2.2. Macrophage

5.2.3. Mast Cells
5.2.4. Dendritic Cells
5.2.5. Vascular Cell
6. Extracellular Molecules
6.1. Cytokines
6.2. Growth Factors
6.3. Matrix Metalloproteinase
7. Immune Regulation by Stroma
8. Hypoxia and Tumor Microenvironment
9. Role of microRNAs in Regulating the Tumor Microenvironment
10. Role of Cells in the Tumor Microenvironment
11. The Antitumor Properties of Natural Extracts and Phytochemicals Against Lung Cancer
12. Therapeutic Strategies and Challenges in Modulating the Tumor Microenvironment in Lung Cancer
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| SCLC | Small cell lung cancer |
| TME | Tumor microenvironment |
| ICB | Immune checkpoint blockade |
| MSCs | Mesenchymal stromal/stem cells |
| TAFs | Tumor-associated fibroblasts |
| Tregs | Regulatory T cells |
| MDSCs | Myeloid-derived suppressor cells |
| NK | Natural killer cells |
| DCs | Dendritic cells |
| ECM | Extracellular matrix |
| HIFs | Hypoxia-inducible factors |
| MMPs | Matrix metalloproteinases |
| MHC | Major histocompatibility complex |
| ICI | Immune checkpoint inhibitors |
| TIL | Tumor-infiltrating lymphocyte |
| CAFs | Cancer-associated fibroblasts |
| MDSCs | Myeloid-derived suppressor cells |
| TAMs | Tumor-associated macrophages |
References
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Mimi, M.A.; Hasan, M.M.; Takanashi, Y.; Waliullah, A.S.; Al Mamun, M.; Chi, Z.; Kahyo, T.; Aramaki, S.; Takatsuka, D.; Koizumi, K.; et al. UBL3 overexpression enhances EV-mediated Achilles protein secretion in conditioned media of MDA-MB-231 cells. Biochem. Biophys. Res. Commun. 2024, 738, 150559. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Remon, J.; Faivre-Finn, C.; Garassino, M.C.; Heymach, J.V.; Kerr, K.M.; Tan, D.S.; Veronesi, G.; Reck, M. Non-small cell lung cancer. Nat. Rev. Dis. Prim. 2024, 10, 71. [Google Scholar] [CrossRef]
- Xi, Z.; Dai, R.; Ze, Y.; Jiang, X.; Liu, M.; Xu, H. Traditional Chinese medicine in lung cancer treatment. Mol. Cancer 2025, 24, 57. [Google Scholar]
- Jung, H.; Paust, S. Chemokines in the tumor microenvironment: Implications for lung cancer and immunotherapy. Front. Immunol. 2024, 15, 1443366. [Google Scholar] [CrossRef] [PubMed]
- Arenberg, D.A.; Keane, M.P.; DiGiovine, B.; Kunkel, S.L.; Morris, S.B.; Xue, Y.Y.; Burdick, M.D.; Glass, M.C.; Iannettoni, M.D.; Strieter, R.M.; et al. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer (NSCLC). J. Clin. Investig. 1998, 102, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, J.; Lee, P.; Sharma, S.; Mao, J.T.; Meissner, H.; Uyemura, K.; Modlin, R.; Wollman, J.; Dubinett, S.M. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995, 55, 3847–3853. [Google Scholar]
- Jover, R.; Nguyen, T.P.; Pérez–Carbonell, L.; Zapater, P.; Payá, A.; Alenda, C.; Rojas, E.; Cubiella, J.; Balaguer, F.; Morillas, J.D.; et al. 5-Fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology 2011, 140, 1174–1181. [Google Scholar] [CrossRef]
- Srinivasarao, D.A.; Shah, S.; Famta, P.; Vambhurkar, G.; Jain, N.; Pindiprolu, S.K.; Sharma, A.; Kumar, R.; Padhy, H.P.; Kumari, M.; et al. Unravelling the role of tumor microenvironment responsive nanobiomaterials in spatiotemporal controlled drug delivery for lung cancer therapy. Drug Deliv. Transl. Res. 2025, 15, 407–435. [Google Scholar] [CrossRef]
- Sohag, S.M.; Toma, S.N.; Morshed, M.N.; Imon, M.A.; Islam, M.M.; Piash, M.I.; Shahria, N.; Mahmud, I. Exploration of analgesic and anthelmintic activities of Artocarpus chaplasha ROXB. leaves supported by in silico molecular docking. Phytomed. Plus 2025, 5, 100761. [Google Scholar] [CrossRef]
- Huang, S.; Chung, J.Y.F.; Li, C.; Wu, Y.; Qiao, G.; To, K.-F.; Tang, P.M.-K. Cellular dynamics of tumor microenvironment driving immunotherapy resistance in non-small-cell lung carcinoma. Cancer Lett. 2024, 604, 217272. [Google Scholar] [CrossRef] [PubMed]
- Riera-Domingo, C.; Audigé, A.; Granja, S.; Cheng, W.-C.; Ho, P.-C.; Baltazar, F.; Stockmann, C.; Mazzone, M. Immunity, hypoxia, and metabolism–the Ménage à Trois of cancer: Implications for immunotherapy. Physiol. Rev. 2020, 100, 1–102. [Google Scholar] [PubMed]
- Saeed Issa, B.; Adhab, A.H.; Salih Mahdi, M.; Kyada, A.; Ganesan, S.; Bhanot, D.; Naidu, K.S.; Kaur, S.; Mansoor, A.S.; Radi, U.K.; et al. Decoding the complex web: Cellular and molecular interactions in the lung tumour microenvironment. J. Drug Target. 2025, 33, 666–690. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M.; Vaillant, F.; Simpson, K.J.; Stingl, J.; Smyth, G.K.; Asselin-Labat, M.L.; Wu, L.; Lindeman, G.J.; Visvader, J.E. Generation of a functional mammary gland from a single stem cell. Nature 2006, 439, 84–88. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Yang, Y.; Zhao, M.; Zhu, G.; Hou, Z. The prognostic landscape of tumor-infiltrating immune cell and immunomodulators in lung cancer. Biomed. Pharmacother. 2017, 95, 55–61. [Google Scholar] [CrossRef]
- van der Bruggen, P.; Van Pel, A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994, 12, 337–365. [Google Scholar]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Cheng, C.; Nguyen, T.T.; Tang, M.; Wang, X.; Jiang, C.; Liu, Y.; Gorlov, I.; Gorlova, O.; Iafrate, J.; Lanuti, M.; et al. Immune Infiltration in Tumor and Adjacent Non-Neoplastic Regions Codetermines Patient Clinical Outcomes in Early-Stage Lung Cancer. J. Thorac. Oncol. 2023, 18, 1184–1198. [Google Scholar] [CrossRef]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The prognostic value of infiltrating immune cells in cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, X.; Liu, H.; Ding, J.; Chu, Y.; Zhou, X. The relationship between tumor infiltrating immune cells and the prognosis of patients with lung adenocarcinoma. J. Thorac. Dis. 2023, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Kayser, C.; Roesner, A.; Rawluk, J.; Schmid, S.; Gkika, E.; Kayser, G. Low densities of immune cells indicate unfavourable overall survival in patients suffering from squamous cell carcinoma of the lung. Sci. Rep. 2024, 14, 14250. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.M.; Kim, Y.; Lee, K.Y.; Kim, S.Y.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; Ahn, M.J. Tumor infiltrated immune cell types support distinct immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. Eur. J. Immunol. 2021, 51, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Shao, Y.; He, W.; Hu, W.; Xu, Y.; Chen, J.; Wu, C.; Jiang, J. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: A meta-analysis. Cell Physiol. Biochem. 2015, 37, 1560–1571. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 35, pp. S185–S198. [Google Scholar]
- Ye, B.; Stary, C.M.; Gao, Q.; Wang, Q.; Zeng, Z.; Jian, Z.; Gu, L.; Xiong, X. Genetically Modified T-Cell-Based Adoptive Immunotherapy in Hematological Malignancies. J. Immunol. Res. 2017, 2017, 5210459. [Google Scholar] [CrossRef]
- Keenan, T.E.; Burke, K.P.; Van Allen, E.M. Genomic correlates of response to immune checkpoint blockade. Nat. Med. 2019, 25, 389–402. [Google Scholar] [CrossRef]
- Walunas, T.L.; Lenschow, D.J.; Bakker, C.Y.; Linsley, P.S.; Freeman, G.J.; Green, J.M.; Thompson, C.B.; Bluestone, J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994, 1, 405–413. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Wang, H.; Xu, Z.; Wang, Y.; Li, S.; Liu, J.; Chen, Y.; Luo, H.; Wu, L.; et al. Massive PD-L1 and CD8 double positive TILs characterize an immunosuppressive microenvironment with high mutational burden in lung cancer. J. Immunother. Cancer 2021, 9, e002356. [Google Scholar] [CrossRef]
- Wang, F.; Yang, M.; Luo, W.; Zhou, Q. Characteristics of tumor microenvironment and novel immunotherapeutic strategies for non-small cell lung cancer. J. Natl. Cancer Cent. 2022, 2, 243–262. [Google Scholar] [CrossRef]
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef]
- Rochman, Y.; Spolski, R.; Leonard, W.J. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 2009, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Essogmo, F.E.; Zhilenkova, A.V.; Tchawe, Y.S.; Owoicho, A.M.; Rusanov, A.S.; Boroda, A.; Pirogova, Y.N.; Sangadzhieva, Z.D.; Sanikovich, V.D.; Bagmet, N.N.; et al. Cytokine profile in lung cancer patients: Antitumor and oncogenic cytokines. Cancers 2023, 15, 5383. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V.; Pestka, S. Jak-Stat signal transduction pathway through the eyes of cytokine class II receptor complexes. Oncogene 2000, 19, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Vilcek, J. Novel interferons. Nat. Immunol. 2003, 4, 8–9. [Google Scholar] [CrossRef]
- Steen, H.C.; Gamero, A.M. Interferon-lambda as a potential therapeutic agent in cancer treatment. J. Interferon Cytokine Res. 2010, 30, 597–602. [Google Scholar] [CrossRef]
- Langer, C.J.; Besse, B.; Gualberto, A.; Brambilla, E.; Soria, J.C. The evolving role of histology in the management of advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 5311–5320. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Karin, M. Inflammation and oncogenesis: A vicious connection. Curr. Opin. Genet. Dev. 2010, 20, 65–71. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Azzali, G. The modality of transendothelial passage of lymphocytes and tumor cells in the absorbing lymphatic vessel. Eur. J. Histochem. 2007, 51, 73. [Google Scholar] [PubMed]
- Chunhacha, P.; Chanvorachote, P. Roles of caveolin-1 on anoikis resistance in non-small cell lung cancer. Int. J. Physiol. Pathophysiol. Pharmacol. 2012, 4, 149. [Google Scholar] [PubMed]
- Al-Mehdi, A.B.; Tozawa, K.; Fisher, A.B.; Shientag, L.; Lee, A.; Muschel, R. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat. Med. 2000, 6, 100–102. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Nguyen, D.X.; Chiang, A.C.; Bos, P.D.; Kim, J.Y.; Nadal, C.; Gomis, R.R.; Manova-Todorova, K.; Massagué, J. Mediators of vascular remodeling co-opted for sequential steps in lung metastasis. Nature 2007, 446, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Psaila, B.; Lyden, D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef]
- Erler, J.T.; Bennewith, K.L.; Cox, T.R.; Lang, G.; Bird, D.; Koong, A.; Le, Q.T.; Giaccia, A.J. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the pre-metastatic niche. Cancer Cell 2009, 15, 35–44. [Google Scholar] [CrossRef]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Bos, P.D.; Massagué, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef]
- Bozkurt, A.S. Publication status of mouse embryonic fibroblast cells in scientific journals. Eur. J. Ther. 2021, 27, 135–141. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Parsonage, G.; Filer, A.D.; Haworth, O.; Nash, G.B.; Rainger, G.E.; Salmon, M.; Buckley, C.D. A stromal address code defined by fibroblasts. Trends Immunol. 2005, 26, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Rodemann, H.P.; Müller, G.A. Characterization of human renal fibroblasts in health and disease: II, In vitro growth, differentiation, and collagen synthesis of fibroblasts from kidneys with interstitial fibrosis. Am. J. Kidney Dis. 1991, 17, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Chi, J.T.; Dudoit, S.; Bondre, C.; Van De Rijn, M.; Botstein, D.; Brown, P.O. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA 2002, 99, 12877–12882. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Guyot, C.; Gabbiani, G. The stroma reaction myofibroblast: A key player in the control of tumor cell behavior. Int. J. Dev. Biol. 2004, 48, 509–517. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Schliekelman, M.J.; Creighton, C.J.; Baird, B.N.; Chen, Y.; Banerjee, P.; Bota-Rabassedas, N.; Ahn, Y.H.; Roybal, J.D.; Chen, F.; Zhang, Y.; et al. Thy-1+ cancer-associated fibroblasts adversely impact lung cancer prognosis. Sci. Rep. 2017, 7, 6478. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Zhu, L.; Li, J.; Ma, S. Cancer-associated fibroblasts in non-small cell lung cancer: Recent advances and future perspectives. Cancer Lett. 2021, 514, 38–47. [Google Scholar] [CrossRef]
- Shintani, Y.; Kimura, T.; Funaki, S.; Ose, N.; Kanou, T.; Fukui, E. Therapeutic targeting of cancer-associated fibroblasts in the non-small cell lung cancer tumor microenvironment. Cancers 2023, 15, 335. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y.; et al. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, S.; Liu, Q.; Qian, C.; Liu, Z.; Luo, D. Exosomal microRNA remodels the tumor microenvironment. PeerJ 2017, 5, e4196. [Google Scholar] [CrossRef]
- Seager, R.J.; Hajal, C.; Spill, F.; Kamm, R.D.; Zaman, M.H. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg. Sci. Phys. Oncol. 2017, 3, 34002. [Google Scholar] [CrossRef]
- Green, J.A.; Arpaia, N.; Schizas, M.; Dobrin, A.; Rudensky, A.Y. A non-immune function of T cells in promoting lung tumor progression. J. Exp. Med. 2017, 214, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Huang, K.; Lin, H.; Xia, Z.; Zhang, J.; Li, D.; Jin, J. Mogroside IIE inhibits digestive enzymes via suppression of interleukin 9/interleukin 9 receptor signaling in acute pancreatitis. Front. Pharmacol. 2020, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Lin, Y.; Navin, N. Advancing cancer research and medicine with single-cell genomics. Cancer Cell 2020, 37, 456–470. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, R.; Kratochvill, F.; Murray, P.J.; Natoli, G. Macrophages and cancer: From mechanisms to therapeutic implications. Trends Immunol. 2015, 36, 229–239. [Google Scholar] [CrossRef]
- Mazur, A.; Holthoff, E.; Vadali, S.; Kelly, T.; Post, S.R. Cleavage of type I collagen by fibroblast activation protein-α enhances class A scavenger receptor mediated macrophage adhesion. PLoS ONE 2016, 11, e0150287. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Bingle, L.; Brown, N.J.; Lewis, C.E. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2002, 196, 254–265. [Google Scholar] [CrossRef]
- Conway, E.M.; Pikor, L.A.; Kung, S.H.; Hamilton, M.J.; Lam, S.; Lam, W.L.; Bennewith, K.L. Macrophages, inflammation, and lung cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 116–130. [Google Scholar] [CrossRef]
- Edin, S.; Wikberg, M.L.; Oldenborg, P.A.; Palmqvist, R. Macrophages: Good guys in colorectal cancer. Oncoimmunology 2013, 2, e23038. [Google Scholar] [CrossRef] [PubMed]
- Trivanović, D.; Krstić, J.; Djordjević, I.O.; Mojsilović, S.; Santibanez, J.F.; Bugarski, D.; Jauković, A. The roles of mesenchymal stromal/stem cells in tumor microenvironment associated with inflammation. Mediat. Inflamm. 2016, 2016, 7314016. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010, 40, 1843–1851. [Google Scholar] [CrossRef]
- Boldrini, L.; Gisfredi, S.; Ursino, S.; Lucchi, M.; Melfi, F.; Mussi, A.; Basolo, F.; Fontanini, G. Tumour necrosis factor-α: Prognostic role and relationship with interleukin-8 and endothelin-1 in non-small cell lung cancer. Int. J. Mol. Med. 2006, 17, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Redegeld, F.A. Role of mast cells in shaping the tumor microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, A.M.; Tamma, R.; Ruggieri, S.; Nico, B.; Toma, A.; Ribatti, D. Mast cells in breast cancer angiogenesis. Crit. Rev. Oncol. Hematol. 2017, 115, 23–26. [Google Scholar] [CrossRef]
- Jang, G.Y.; Lee, J.W.; Kim, Y.S.; Lee, S.E.; Han, H.D.; Hong, K.J.; Kang, T.H.; Park, Y.M. Interactions between tumor-derived proteins and Toll-like receptors. Exp. Mol. Med. 2020, 52, 1926–1935. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018, 172, 1022–1037. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; e Sousa, C.R.; et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 2019, 179, 846–863. [Google Scholar]
- Canton, J.; Blees, H.; Henry, C.M.; Buck, M.D.; Schulz, O.; Rogers, N.C.; Childs, E.; Zelenay, S.; Rhys, H.; Domart, M.C.; et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat. Immunol. 2021, 22, 140–153. [Google Scholar] [CrossRef]
- Giampazolias, E.; Schulz, O.; Lim, K.H.; Rogers, N.C.; Chakravarty, P.; Srinivasan, N.; Gordon, O.; Cardoso, A.; Buck, M.D.; Poirier, E.Z.; et al. Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity. Cell 2021, 184, 4016–4031. [Google Scholar] [CrossRef]
- Ahluwalia, P.; Ahluwalia, M.; Mondal, A.K.; Sahajpal, N.S.; Kota, V.; Rojiani, M.V.; Kolhe, R. Natural killer cells and dendritic cells: Expanding clinical relevance in the non-small cell lung cancer (NSCLC) tumor microenvironment. Cancers 2021, 13, 4037. [Google Scholar] [CrossRef]
- Saintigny, P.; Kambouchner, M.; Ly, M.; Gomes, N.; Sainte-Catherine, O.; Vassy, R.; Czernichow, S.; Letoumelin, P.; Breau, J.L.; Bernaudin, J.F.; et al. Vascular endothelial growth factor-C and its receptor VEGFR-3 in non-small-cell lung cancer: Concurrent expression in cancer cells from primary tumour and metastatic lymph node. Lung Cancer 2007, 58, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef]
- Dvorak, H.F. Angiogenesis: Update 2005. J. Thromb. Haemost. 2005, 3, 1835–1842. [Google Scholar] [CrossRef]
- Ribatti, D.; Ennas, M.G.; Vacca, A.; Ferreli, F.; Nico, B.; Orru, S.; Sirigu, P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur. J. Clin. Investig. 2003, 33, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Hogan, K.A.; Cho, D.S.; Arneson, P.C.; Samani, A.; Palines, P.; Yang, Y.; Doles, J.D. Tumor-derived cytokines impair myogenesis and alter the skeletal muscle immune microenvironment. Cytokine 2018, 107, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and chemokine receptors: New targets for cancer immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Sarode, P.; Schaefer, M.B.; Grimminger, F.; Seeger, W.; Savai, R. Macrophage and tumor cell cross-talk is fundamental for lung tumor progression: We need to talk. Front. Oncol. 2020, 10, 324. [Google Scholar] [CrossRef]
- Boersma, B.; Jiskoot, W.; Lowe, P.; Bourquin, C. The interleukin-1 cytokine family members: Role in cancer pathogenesis and potential therapeutic applications in cancer immunotherapy. Cytokine Growth Factor Rev. 2021, 62, 1–14. [Google Scholar] [CrossRef]
- Garon, E.B.; Chih-Hsin Yang, J.; Dubinett, S.M. The Role of Interleukin 1β in the Pathogenesis of Lung Cancer. JTO Clin. Res. Rep. 2020, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, K.; Wang, X.; Zhao, Y.; Shi, J.; Liu, Z. Roles of IL-4, IL-13, and their receptors in lung cancer. J. Interf. Cytokine Res. 2024, 44, 399–407. [Google Scholar] [CrossRef]
- Huang, M.; Stolina, M.; Sharma, S.; Mao, J.T.; Zhu, L.; Miller, P.W.; Wollman, J.; Herschman, H.; Dubinett, S.M. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: Up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998, 58, 1208–1216. [Google Scholar] [PubMed]
- Brenner, D.R.; Fanidi, A.; Grankvist, K.; Muller, D.C.; Brennan, P.; Manjer, J.; Byrnes, G.; Hodge, A.; Severi, G.; Giles, G.G.; et al. Inflammatory cytokines and lung cancer risk in 3 prospective studies. Am. J. Epidemiol. 2017, 185, 86–95. [Google Scholar] [CrossRef]
- Matanić, D.; Beg-Zec, Z.; Stojanović, D.; Matakorić, N.; Flego, V.; Milevoj-Ribić, F. Cytokines in patients with lung cancer. Scand. J. Immunol. 2003, 57, 173–178. [Google Scholar] [CrossRef]
- Ramachandran, S.; Verma, A.K.; Dev, K.; Goyal, Y.; Bhatt, D.; Alsahli, M.A.; Rahmani, A.H.; Almatroudi, A.; Almatroodi, S.A.; Alrumaihi, F.; et al. Role of cytokines and chemokines in NSCLC immune navigation and proliferation. Oxid. Med. Cell. Longev. 2021, 2021, 5563746. [Google Scholar] [CrossRef]
- Hendler, F.J.; Ozanne, B.W. Human squamous cell lung cancers express increased epidermal growth factor receptors. J. Clin. Investig. 1984, 74, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S.; et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Hodkinson, P.S.; MacKinnon, A.; Sethi, T. Targeting growth factors in lung cancer. Chest 2008, 133, 1209–1216. [Google Scholar] [CrossRef]
- Zandi, R.; Larsen, A.B.; Andersen, P.; Stockhausen, M.T.; Poulsen, H.S. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell. Signal. 2007, 19, 2013–2023. [Google Scholar] [CrossRef]
- Liu, T.C.; Jin, X.; Wang, Y.; Wang, K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am. J. Cancer Res. 2017, 7, 187. [Google Scholar] [PubMed]
- Sakuma, Y.; Matsukuma, S.; Yoshihara, M.; Nakamura, Y.; Noda, K.; Nakayama, H.; Kameda, Y.; Tsuchiya, E.; Miyagi, Y. Distinctive evaluation of non-mucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: Confirmation of the correlations with histologic subtypes and gene mutations. Am. J. Clin. Pathol. 2007, 128, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Natale, R.B.; Herbst, R.S.; Lynch, T.J., Jr.; Prager, D.; Belani, C.P.; Schiller, J.H.; Kelly, K.; Spiridonidis, H.; Sandler, A.; et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. Jama 2003, 290, 2149–2158. [Google Scholar] [CrossRef]
- Fukuoka, M.; Yano, S.; Giaccone, G.; Tamura, T.; Nakagawa, K.; Douillard, J.Y.; Nishiwaki, Y.; Vansteenkiste, J.; Kudoh, S.; Rischin, D.; et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2003, 21, 2237–2246. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005, 366, 1527–1537. [Google Scholar]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodeling. Nat. Rev. Mol Cell. Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Rintoul, R.C.; Sethi, T. The role of extracellular matrix in small-cell lung cancer. Lancet Oncol. 2001, 2, 437–442. [Google Scholar] [CrossRef]
- Merchant, N.; Nagaraju, G.P.; Rajitha, B.; Lammata, S.; Jella, K.K.; Buchwald, Z.S.; Lakka, S.S.; Ali, A.N. Matrix metalloproteinases: Their functional role in lung cancer. Carcinogenesis 2017, 38, 766–780. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The Role of Tumor Stroma in Cancer Progression and Prognosis: Emphasis on Carcinoma-Associated Fibroblasts and Non-small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef]
- Hughes, C.C.W. Endothelial–stromal interactions in angiogenesis. Curr. Opin. Hematol. 2008, 15, 204–209. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.; Huang, L.; He, J.; Liu, G.; Ma, S.; Weng, Y.; Huang, S. Systemic immune microenvironment and regulatory network analysis in patients with lung adenocarcinoma. Transl. Cancer Res. 2021, 10, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The Myofibroblast: One Function, Multiple Origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- McAnulty, R.J. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int. J. Biochem. Cell Biol. 2007, 39, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Östman, A.; Augsten, M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr. Opin. Genet. Dev. 2009, 19, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Stigliano, E.; Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004, 14, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, X.F. Hypoxia and the tumor microenvironment. Technol. Cancer Res. Treat. 2021, 20, 15330338211036304. [Google Scholar] [CrossRef]
- Bedogni, B.; Powell, M.B. Hypoxia, melanocytes and melanoma—Survival and tumor development in the permissive microenvironment of the skin. Pigment Cell Melanoma Res. 2009, 22, 166–174. [Google Scholar] [CrossRef]
- Chan, D.A.; Sutphin, P.D.; Denko, N.C.; Giaccia, A.J. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. J. Biol. Chem. 2002, 277, 40112–40117. [Google Scholar] [CrossRef]
- Masson, N.; Willam, C.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001, 20, 5197–5206. [Google Scholar] [CrossRef]
- Goyal, A.; Afzal, M.; Goyal, K.; Ballal, S.; Sharma, G.C.; Kavitha, V.; Maharana, L.; Devi, A.; Rana, M.; Kumar, K.B.; et al. miR-210: A non-invasive biomarker for hypoxia-driven lung cancer diagnosis and therapy. Clin. Chim. Acta 2025, 571, 120215. [Google Scholar] [CrossRef] [PubMed]
- Tse, S.W.; Tan, C.F.; Park, J.E.; Gnanasekaran, J.; Gupta, N.; Low, J.K.; Yeoh, K.W.; Chng, W.J.; Tay, C.Y.; McCarthy, N.E.; et al. Microenvironmental hypoxia induces dynamic changes in lung cancer synthesis and secretion of extracellular vesicles. Cancers 2020, 12, 2917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, J.; Zhu, W.; Yang, Z.; Wang, P.; Zeng, Y. Hypoxia studies in non-small cell lung cancer: Pathogenesis and clinical implications. Oncol. Rep. 2024, 53, 29. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Chemical radiosensitizers for use in radiotherapy. Clin. Oncol. 2007, 19, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Wu, J.; Zhang, H.; Cai, H.; Liu, X.; Li, Q. Hypoxia-guided treatment planning for lung cancer with dose painting by numbers. J. Appl. Clin. Med. Phys. 2025, 26, e14609. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Jain, R.K. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 18632–18637. [Google Scholar] [CrossRef]
- Halpin-Veszeleiova, K.; Mallouh, M.P.; Williamson, L.M.; Apro, A.C.; Botticello-Romero, N.R.; Bahr, C.; Shin, M.; Ward, K.M.; Rosenberg, L.; Ritov, V.B.; et al. Oxygen-carrying nanoemulsions and respiratory hyperoxia eliminate tumor hypoxia–induced immunosuppression. JCI Insight 2025, 10, e174675. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M.M.; Mamun, M.A.; Islam, A.; Hasan, M.M.; Waliullah, A.S.; Tamannaa, Z.; Sato, T.; Kahyo, T.; Setou, M. Mass spectrometry in the lipid study of cancer. Expert Rev. Proteom. 2021, 18, 201–219. [Google Scholar] [CrossRef]
- Sahu, A.; Kwon, I.; Tae, G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials 2020, 228, 119578. [Google Scholar] [CrossRef] [PubMed]
- Nishii, K.; Ohashi, K.; Watanabe, H.; Makimoto, G.; Nakasuka, T.; Higo, H.; Ninomiya, K.; Kato, Y.; Kubo, T.; Rai, K.; et al. Triple therapy with osimertinib, bevacizumab and cetuximab in EGFR-mutant lung cancer with HIF-1α/TGF-α expression. Oncol. Lett. 2021, 22, 639. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hasan, M.M.; Zhang, H.; Zhai, Q.; Waliullah, A.S.; Ping, Y.; Zhang, C.; Oyama, S.; Mimi, M.A.; Tomochika, Y.; et al. UBL3 Interacts with Alpha-synuclein in Cells and the Interaction is Downregulated by the EGFR Pathway Inhibitor Osimertinib. Biomedicines 2023, 11, 1685. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V.; The, C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.J.; Plasterk, R.H.A.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Guz, M.; Rivero-Müller, A.; Okoń, E.; Stenzel-Bembenek, A.; Polberg, K.; Słomka, M.; Stepulak, A. MicroRNAs-role in lung cancer. Dis. Markers 2014, 2014, 218169. [Google Scholar] [CrossRef]
- Palmero, E.I.; de Campos, S.G.; Campos, M.; Souza, N.C.; Guerreiro, I.D.; Carvalho, A.L.; Marques, M.M. Mechanisms and role of microRNA deregulation in cancer onset and progression. Genet. Mol. Biol. 2011, 34, 363–370. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Garzon, R.; Calin, G.A.; Croce, C.M. MicroRNAs in cancer. Annu. Rev. Med. 2009, 60, 167–179. [Google Scholar] [CrossRef]
- Balca-Silva, J.; Neves, S.S.; Goncalves, A.C.; Abrantes, A.M.; Casalta-Lopes, J.; Botelho, M.F.; Sarmento-Ribeiro, A.B.; Silva, H.C. Effect of miR-34b overexpression on the radiosensitivity of non-small cell lung cancer cell lines. Anticancer Res. 2012, 32, 1603–1609. [Google Scholar] [PubMed]
- Feng, S.; Cong, S.; Zhang, X.; Bao, X.; Wang, W.; Li, H.; Wang, Z.; Wang, G.; Xu, J.; Du, B.; et al. MicroRNA-192 targeting retinoblastoma 1 inhibits cell proliferation and induces cell apoptosis in lung cancer cells. Nucleic Acids Res. 2011, 9, 6669–6678. [Google Scholar] [CrossRef]
- Lee, J.H.; Voortman, J.; Dingemans, A.M.; Voeller, D.M.; Pham, T.; Wang, Y.; Giaccone, G.; Cho, W.C.S. MicroRNA expression and clinical outcome of small cell lung cancer. PLoS ONE 2011, 6, e21300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wei, X.; Xu, L. miR-150 promotes the proliferation of lung cancer cells by targeting P53. FEBS Lett. 2013, 587, 2346–2351. [Google Scholar] [CrossRef]
- Green, D.R.; Evan, G.I. A matter of life and death. Cancer Cell 2002, 1, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.; Yang, S.; Zhang, W.; He, S.; Hu, C.; Zhu, H.; Quan, L.; Bai, J.; Xu, N.; et al. TNF-α is a novel target of miR-19a. Int. J. Oncol. 2011, 38, 1013–1022. [Google Scholar][Green Version]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Garofalo, M.; Jeon, Y.J.; Nuovo, G.J.; Middleton, J.; Secchiero, P.; Joshi, P.; Alder, H.; Nazaryan, N.; Di Leva, G.; Romano, G.; et al. MiR-34a/c-dependent PDGFR-α/β downregulation inhibits tumorigenesis and enhances TRAIL-induced apoptosis in lung cancer. PLoS ONE 2013, 8, e67581. [Google Scholar] [CrossRef]
- Incoronato, M.; Garofalo, M.; Urso, L.; Romano, G.; Quintavalle, C.; Zanca, C.; Iaboni, M.; Nuovo, G.; Croce, C.M.; Condorelli, G.; et al. MiR-212 increases tumor necrosis factor–related apoptosis-inducing ligand sensitivity in non-small cell lung cancer by targeting the antiapoptotic protein PED. Cancer Res. 2010, 70, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Quintavalle, C.; Di Leva, G.; Zanca, C.; Romano, G.; Taccioli, C.; Liu, C.G.; Croce, C.M.; Condorelli, G. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene 2008, 27, 3845–3855. [Google Scholar] [CrossRef]
- Romano, G.; Acunzo, M.; Garofalo, M.; Di Leva, G.; Cascione, L.; Zanca, C.; Bolon, B.; Condorelli, G.; Croce, C.M. MiR-494 is regulated by ERK1/2 and modulates TRAIL-induced apoptosis in non-small-cell lung cancer through BIM down-regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 16570–16575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Lu, J.; Mercer, K.L.; Golub, T.R.; Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 2007, 39, 673–677. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Chen, L.; Zhao, J.; Guo, M.; Zhao, X.; Wen, Z.; He, Z.; Chen, C.; Xu, L. MiRNA-based therapies for lung cancer: Opportunities and challenges? Biomolecules 2023, 13, 877. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, W.C.; Shen, W.H.; Xu, K.; Hu, Y.Y.; Han, G.H.; Liu, Y.B. Thalidomide suppresses angiogenesis and immune evasion via lncRNA FGD5-AS1/miR-454–3p/ZEB1 axis-mediated VEGFA expression and PD-1/PD-L1 checkpoint in NSCLC. Chem. Biol. Interact. 2021, 349, 109652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ning, F.; Cai, Y.; Sheng, H.; Zheng, R.; Yin, X.; Lu, Z.; Su, L.; Chen, X.; Zeng, C.; et al. The EGFR-P38 MAPK axis up-regulates PD-L1 through miR-675-5p and down-regulates HLA-ABC via hexokinase-2 in hepatocellular carcinoma cells. Cancer Commun. 2021, 41, 62–78. [Google Scholar] [CrossRef]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Hung, J.Y.; Chang, W.A.; Jian, S.F.; Lin, Y.S.; Pan, Y.C.; Wu, C.Y.; Kuo, P.L. Hypoxic lung-cancer-derived extracellular vesicle microRNA-103a increases the oncogenic effects of macrophages by targeting PTEN. Mol. Ther. 2018, 26, 568–581. [Google Scholar] [CrossRef]
- Martino, M.T.D.; Tagliaferri, P.; Tassone, P. MicroRNA in cancer therapy: Breakthroughs and challenges in early clinical applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Guo, P.; Wen, W.-C.; Wong, H.L. Lipid-based nanocarriers for RNA delivery. Curr. Pharm. Des. 2015, 21, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in drug delivery systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Xi, S.; Xu, H.; Shan, J.; Tao, Y.; Hong, J.A.; Inchauste, S.; Zhang, M.; Kunst, T.F.; Mercedes, L.; Schrump, D.S.; et al. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J. Clin. Investig. 2013, 123, 1241–1261. [Google Scholar] [CrossRef] [PubMed]
- Rusek, A.M.; Abba, M.; Eljaszewicz, A.; Moniuszko, M.; Niklinski, J.; Allgayer, H. MicroRNA modulators of epigenetic regulation, the tumor microenvironment and the immune system in lung cancer. Mol. Cancer 2015, 14, 34. [Google Scholar] [CrossRef]
- Peng, Y.; Dai, Y.; Hitchcock, C.; Yang, X.; Kassis, E.S.; Liu, L.; Luo, Z.; Sun, H.L.; Cui, R.; Wei, H.; et al. Insulin growth factor signaling is regulated by microRNA-486, an underexpressed microRNA in lung cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 15043–15048. [Google Scholar] [CrossRef]
- Lujambio, A.; Ropero, S.; Ballestar, E.; Fraga, M.F.; Cerrato, C.; Setién, F.; Casado, S.; Suarez-Gauthier, A.; Sanchez-Cespedes, M.; Gitt, A.; et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007, 67, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Ho, J.Y.; Hung, S.H.; Yu, D.S. miR-429 expression in bladder cancer and its correlation with tumor behavior and clinical outcome. Kaohsiung J. Med. Sci. 2018, 34, 335–340. [Google Scholar] [CrossRef]
- Hubaux, R.; Becker-Santos, D.D.; Enfield, K.S.S.; Lam, S.; Lam, W.L.; Martinez, V.D. MicroRNAs As Biomarkers For Clinical Features Of Lung Cancer. Metabolomics Open Access 2012, 2, 1000108. [Google Scholar] [CrossRef]
- San Ho, C.; Noor, S.M.; Nagoor, N.H. MiR-378 and MiR-1827 regulate tumor invasion, migration and angiogenesis in human lung adenocarcinoma by targeting RBX1 and CRKL, respectively. J. Cancer 2018, 9, 331. [Google Scholar]
- Chen, M.J.; Wu, D.W.; Wang, G.C.; Wang, Y.C.; Chen, C.Y.; Lee, H. MicroRNA-630 may confer favorable cisplatin-based chemotherapy and clinical outcomes in non-small cell lung cancer by targeting Bcl-2. Oncotarget 2018, 9, 13758. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sempere, L.F.; Ouyang, H.; Memoli, V.A.; Andrew, A.S.; Luo, Y.; Demidenko, E.; Korc, M.; Shi, W.; Preis, M.; et al. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J. Clin. Investig. 2010, 120, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Zhang, A.; Wang, Y.; Han, L.; You, Y.; Pu, P.; Kang, C. PUMA is a novel target of miR-221/222 in human epithelial cancers. Int. J. Oncol. 2010, 37, 1621–1626. [Google Scholar] [CrossRef]
- Fujita, Y.; Yagishita, S.; Hagiwara, K.; Yoshioka, Y.; Kosaka, N.; Takeshita, F.; Fujiwara, T.; Tsuta, K.; Nokihara, H.; Tamura, T.; et al. The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant Non-small cell lung cancer. Mol. Ther. 2015, 23, 717–727. [Google Scholar] [CrossRef]
- Chen, G.; Umelo, I.A.; Lv, S.; Teugels, E.; Fostier, K.; Kronenberger, P.; Dewaele, A.; Sadones, J.; Geers, C.; De Grève, J.; et al. miR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS ONE 2013, 8, e60317. [Google Scholar] [CrossRef] [PubMed]
- Masciale, V.; Grisendi, G.; Banchelli, F.; D’Amico, R.; Maiorana, A.; Sighinolfi, P.; Pinelli, M.; Lovati, E.; Stefani, A.; Morandi, U.; et al. Correlating tumor-infiltrating lymphocytes and lung cancer stem cells: A cross-sectional study. Ann. Transl. Med. 2019, 7, 619. [Google Scholar] [CrossRef]
- Odarenko, K.V.; Zenkova, M.A.; Markov, A.V. The nexus of inflammation-induced epithelial-mesenchymal transition and lung cancer progression: A roadmap to pentacyclic triterpenoid-based therapies. Int. J. Mol. Sci. 2023, 24, 17325. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, J.; Rao, S.; Guo, S.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; et al. Tumor infiltrating lymphocyte (TIL) therapy for solid tumor treatment: Progressions and challenges. Cancers 2022, 14, 4160. [Google Scholar] [CrossRef]
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-associated fibroblasts: Overview, progress, challenges, and directions. Cancer Gene Ther. 2021, 28, 984–999. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, T.; Sun, L.; Yuan, Y.; Zhu, Y. Potential mechanisms of cancer-associated fibroblasts in therapeutic resistance. Biomed. Pharmacother. 2023, 166, 115425. [Google Scholar] [CrossRef]
- Yan, C.; Chang, J.; Song, X.; Qi, Y.; Ji, Z.; Liu, T.; Yu, W.; Wei, F.; Yang, L.; Ren, X.; et al. Lung cancer-associated mesenchymal stem cells promote tumor metastasis and tumorigenesis by induction of epithelial–mesenchymal transition and stem-like reprogram. Aging 2021, 13, 9780. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Tian, C.; Zhao, M.; Sun, Y.; Huang, C. Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int. 2021, 21, 595. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Annan, D.A.; Hida, Y. Contribution of tumor endothelial cells in cancer progression. Int. J. Mol. Sci. 2018, 19, 1272. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.P. Aneuploid circulating tumor-derived endothelial cell (CTEC): A novel versatile player in tumor neovascularization and cancer metastasis. Cells 2020, 9, 1539. [Google Scholar] [CrossRef]
- Yao, X.; Zeng, Y. Tumour associated endothelial cells: Origin, characteristics and role in metastasis and anti-angiogenic resistance. Front. Physiol. 2023, 14, 1199225. [Google Scholar] [CrossRef]
- Marzagalli, M.; Fontana, F.; Raimondi, M.; Limonta, P. Cancer stem cells—Key players in tumor relapse. Cancers 2021, 13, 376. [Google Scholar] [CrossRef]
- Lei, Z.N.; Teng, Q.X.; Koya, J.; Liu, Y.; Chen, Z.; Zeng, L.; Chen, Z.S.; Fang, S.; Wang, J.; Liu, Y.; et al. The correlation between cancer stem cells and epithelial-mesenchymal transition: Molecular mechanisms and significance in cancer theragnosis. Front. Immunol. 2024, 15, 1417201. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Grubba, M.; Balihodzic, A.; Szot, O.; Sobocki, B.K.; Perdyan, A. The role of regulatory T cells in cancer treatment resistance. Int. J. Mol. Sci. 2023, 24, 14114. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Z.; Li, G.; Zhang, Y.; Liu, X.; Li, B.; Wang, J.; Li, X. Cancer stem cells and their niche in cancer progression and therapy. Cancer Cell Int. 2023, 23, 305. [Google Scholar] [CrossRef]
- Basak, U.; Sarkar, T.; Mukherjee, S.; Chakraborty, S.; Dutta, A.; Dutta, S.; Nayak, D.; Kaushik, S.; Das, T.; Sa, G.; et al. Tumor-associated macrophages: An effective player of the tumor microenvironment. Front. Immunol. 2023, 14, 1295257. [Google Scholar]
- McWhorter, R.; Bonavida, B. The role of TAMs in the regulation of tumor cell resistance to chemotherapy. Crit. Rev. Oncog. 2024, 29, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Imon, M.A.; Toma, S.N.; Sohag, S.M.; Islam, M.J.; Islam, M.M.; Sohag, M.S.; Mahmud, I.; Shahria, N.; Dutta, S. Evaluation of anthelmintic and antioxidant efficacy of green-synthesized copper nanoparticles derived from Erioglossum rubiginosum leaf and seed aqueous extracts. Eur. J. Med. Chem. Rep. 2024, 12, 100181. [Google Scholar] [CrossRef]
- Monteiro, L.D.S.; Bastos, K.X.; Barbosa-Filho, J.M.; de Athayde-Filho, P.F.; de Fátima Formiga Melo Diniz, M.; Sobral, M.V. Medicinal Plants and Other Living Organisms with Antitumor Potential against Lung Cancer. Evid.-Based Complement. Altern. Med. 2014, 2014, 604152. [Google Scholar] [CrossRef]
- Wattanathamsan, O.; Hayakawa, Y.; Pongrakhananon, V. Molecular mechanisms of natural compounds in cell death induction and sensitization to chemotherapeutic drugs in lung cancer. Phytother. Res. 2019, 33, 2531–2547. [Google Scholar] [CrossRef]
- Saleem, N.; Habib, A.; Shafi, A.; Al-Qaneh, A.M.; Jabbar, A.A.J. Phytocompounds as Promising Weapons against Lung Cancer: A Review. Phytopharm. Commun. 2024, 4, 57–68. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Akassh, S.; Rithiga, S.B.; Prithika, S.; Gunasekaran, R.; Shin, H.; Kumar, R.; Baskar, V.; Kim, J. Integrated omics profiling and network pharmacology uncovers the prognostic genes and multi-targeted therapeutic bioactives to combat lung cancer. Eur. J. Pharmacol. 2023, 940, 175479. [Google Scholar] [CrossRef]
- Khosravifarsani, M.; Tolouian, R.; Yadollahifarsani, S.; Soleimani, P.; Sarazen, M.; Mostafizi, P.; Tolouian, A.; Philip, A.K.; Hotkani, Z.G. Medical plants for lung cancer: An overview of current knowledge. Immunopathol. Persa 2022, 9, e38455. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.X.; Ma, L.R.; Xu, D.H.; Wang, P.; Li, L.Q.; Yu, L.L.; Li, Y.; Li, R.Z.; Zhang, H.; et al. Traditional herbal medicine: A potential therapeutic approach for adjuvant treatment of non-small cell lung cancer in the future. Integr. Cancer Ther. 2022, 21, 15347354221144312. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Lin, C.H.; Wu, L.Y.; Wang, J.S.; Chung, M.C.; Chang, J.F.; Chao, M.W.; Wang, Y.-J. Potential combinational anticancer therapy in non-small cell lung cancer with traditional Chinese medicine Sun-Bai-Pi extract and cisplatin. PLoS ONE 2016, 11, e0155469. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, C.; Zhang, Y.; Liu, F.; Wang, X.; Zhao, M.; Hoffman, R.M.; Sun, B. Comparison of efficacy and toxicity of traditional Chinese medicine (TCM) herbal mixture LQ and conventional chemotherapy on lung cancer metastasis and survival in mouse models. PLoS ONE 2014, 9, e109814. [Google Scholar] [CrossRef]
- Sounni, N.E.; Noel, A. Targeting the tumor microenvironment for cancer therapy. Clin. Chem. 2013, 59, 85–93. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, W.K.K.; Loong, H.H.F.; Cho, W.C.S.; To, K.K.W. Drug combination approach to overcome resistance to EGFR tyrosine kinase inhibitors in lung cancer. Cancer Lett. 2017, 405, 100–110. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hao, J.; Li, B.; Li, M.; Xiuwen, W. Lung cancer combination therapy: Co-delivery of paclitaxel and doxorubicin by nanostructured lipid carriers for synergistic effect. Drug Deliv. 2016, 23, 1398–1403. [Google Scholar] [CrossRef]
- Xue, W.; Dahlman, J.E.; Tammela, T.; Khan, O.F.; Sood, S.; Dave, A.; Cai, W.; Chirino, L.M.; Yang, G.R.; Bronson, R.; et al. Small RNA combination therapy for lung cancer. Proc. Natl. Acad. Sci. USA 2014, 111, E3553–E3561. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
| Immune Cell Type | Main Function in TME | Clinical/Prognostic Association |
|---|---|---|
| CD8+ T cells | Cytotoxic killing of tumor cells | Improved survival, better ICI response [19,20] |
| CD4+ T cells | Helper/regulatory roles; coordinate immune responses | Variable; subset-dependent [20,21] |
| Regulatory T-cells (Tregs) | inhibit anti-tumor immunity | Poorer prognosis [20,22] |
| B cells | Antibody production, antigen presentation | Mixed; high density may predict HPD [19,23] |
| Macrophages (M1/M2) | M1: pro-inflammatory/anti-tumor; M2: immunosuppressive | M1: favorable; M2: poor prognosis [20,21,22] |
| Myeloid-derived suppressor cells (MDSCs) | Inhibit T-cell function, promote tumor growth | Poorer prognosis [20] |
| Natural Killer cells (NKs) | Direct killing of tumor cells (innate immunity) | Generally favorable [21] |
| Dendritic cells (DCs) | Antigen presentation, T-cell activation | It can be immunosuppressive in TME [22] |
| Mast cells | Modulate inflammation, angiogenesis | Prognostic value in LUAD [21] |
| Cytokine | Primary Source | Susceptible Cells | Primary Function |
|---|---|---|---|
| IL-1 | Macrophages, monocytes, fibroblasts | Endothelium, hypothalamus, T/B cells | Co-stimulation, inflammation, fever |
| IL-2 | T cells, NK cells | T/B/NK cells, monocytes | Growth and activation of immune cells |
| IL-4 | T cells | T/B cells | Th2 differentiation, IgE switching |
| IL-6 | Macrophages, T cells | T/B cells, liver | Acute phase response, inflammation |
| IL-10 | Th2 cells | Macrophages, T cells | Anti-inflammatory, suppresses APCs |
| IL-12 | NK cells, macrophages | T cells | Promotes Th1 differentiation |
| IL-17 | NKT cells, ILCs | Epithelial, endothelial cells | Inflammation, infection control |
| IL-21 | CD4+ T cells, NKT cells | T/B/NK cells | Enhances immune responses |
| IL-23 | APCs | T cells, NK cells | Promotes chronic inflammation via Th17 |
| IFN-γ | T, NK, NKT cells | Monocytes, endothelial cells | MHC upregulation, macrophage induction |
| TNF-α | T cells, macrophages | Immune, endothelial, liver cells | Inflammation, fever, acute-phase response |
| TGF-β | T cells, macrophages | T cells | Suppresses immune activation |
| IL-35 | Tregs | T cells | Immunosuppressive, induces iTr35 |
| IL-37 | DCs, Monocyte | Macrophages, B cells | Dampens excessive inflammation |
| Cytokine | Source | Functions |
|---|---|---|
| IL-6 | T cells, adipocytes, macrophages | Proinflammatory action, promotes differentiation and cytokine production |
| IL-8 | Macrophages, epithelial cells, endothelial cells. | Proinflammatory action, promotes angiogenesis and chemotaxis |
| IL-10 | Monocytes, B cells, T cells | Anti-inflammatory action, inhibits proinflammatory cytokines |
| IL-17 | Th17 cells | Proinflammatory action, enhances cytokine and chemokine production, contributes to antitumor immunity |
| IL-27 | Antigen-presenting cells (APCs) | Anti-inflammatory action, induces IL-10 production |
| IL-35 | Regulatory T cells (Tregs) | Anti-inflammatory action, promotes Treg proliferation, suppresses Th17 cells |
| IL-37 | NK cells, monocytes, epithelial cells, B cells | Anti-inflammatory, antimicrobial, and contributes to antitumor immunity |
| TNF-α | Macrophages, CD4+ lymphocytes, adipocytes, NK cells | Proinflammatory action, induces cell proliferation, cytokine production, and apoptosis |
| IFN-γ | NK cells, T cells | Antiviral and proinflammatory action |
| TGF-β | T cells, macrophages | Anti-inflammatory action, suppresses proinflammatory cytokine production |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF) | T cells, macrophages, fibroblasts | Proinflammatory action, enhances neutrophil and monocyte function, activates macrophages |
| Vascular endothelial growth factor (VEGF) | Macrophages, endothelial cells, platelets | Promotes vasculogenesis, angiogenesis, endothelial chemotaxis, and migration |
| microRNA | Targets | Expression Status (Underexpressed/Overexpressed/ Unchanged) | Comments (If Any) | Reference |
|---|---|---|---|---|
| miR-487b | SUZ12,BM11, MYC | Overexpressed | Tumor suppressor | Xi et al., 2013 [165] |
| miR-449 | HDAC1 | Overexpressed | AM Rusek et al., 2015 [166] | |
| miR-101 | EZH2 | Overexpressed | AM Rusek et al., 2015 [166] | |
| miR-486 | IGF1R | Underexpressed | NSCLC tumor suppressor | C M. Croce et al., 2013 [167] |
| miR-9 | MHC 1 gene | Overexpressed | AM Rusek et al., 2015 [166] | |
| miR-124a | CDK6 | Overexpressed | Tumor suppressor | A Lujambio et al., 2007 [168] |
| miR-221 | TIMP3 | Overexpressed | AM Rusek et al., 2015 [166] | |
| miR-222 | TIMP3 | Overexpressed | AM Rusek et al., 2015 [166] | |
| miR-429 | ZEB1/2 | Overexpressed | NSCLC oncogenic | Wu Cl et al., 2018 [169] |
| miR-128b | EGFR in NSCLC | Underexpressed | Tumor suppressor | Becker-Santos DD et al., 2012 [170] |
| miR-1827 | SK-LU-1, RBX1 in NSCLC | Underexpressed | NSCLC tumor suppressor | SM Noor et al., 2018 [171] |
| miR-378 | RBX1, CRKL in NSCLC | Overexpressed | NSCLC tumor suppressor | SM Noor et al., 2018 [171] |
| miR-630 | Mut-Bcl-2–3′-UTR | Unchanged | NSCLC tumor suppressor | Huei Lee et al., 2018 [172] |
| miR-31 | LATS2/PPP2R2A | Overexpressed | NSCLC oncogenic | Liu et al., 2010 [173] |
| miR-221/222 | PUMA | Overexpressed | NSCLC oncogenic | Zhang et al., 2010 [174] |
| miR-197 | PD-L1 | Overexpressed | NSCLC oncogenic | Fujita et al., 2015 [175] |
| microRNA-146a | EGFR | Overexpressed | NSCLC tumor suppressor | Chen et al., 2013 [176] |
| Cell Types | In the Progression of Lung Cancer | In the Induction of the EMT Process | In the Development of Anticancer Drugs Resistance |
|---|---|---|---|
| Tumor-infiltrating lymphocytes (TILs) | Positive correlation and high levels are associated with better overall survival [24,177] | Induce epithelial–mesenchymal transition (EMT) [178] | Resistance to anti-tumor immunity [179] |
| Cancer-associated fibroblasts (CAFs) | Enhancing the plasticity and self-renewal capabilities of CSCs [58] | Induce epithelial–mesenchymal transition (EMT) [180] | Support cancer cell survival in the presence of anticancer drugs [181] |
| Mesenchymal stem cells (MSCs) | Promote tumor metastasis and tumorigenesis [182] | Induce epithelial–mesenchymal transition (EMT) [182] | Play a significant role in cancer progression and drug resistance [183] |
| Tumor endothelial cells (TECs) | Cancer progression by supporting angiogenesis and metastasis [184] | Crucially engage the hypoxia-triggered epithelial-to-mesenchymal transition (EMT) [185] | Contribute to drug resistance in cancer therapy [186] |
| Regulatory T cells (Tregs) | Interact with cancer stem cells (CSCs) and contribute to tumor relapse [187] | Promote epithelial-mesenchymal transition (EMT) [188] | Play a crucial role in cancer treatment resistance [189] |
| Tumor-associated macrophages (TAMs) | Support cancer stem cells (CSCs) and promotes lung cancer progression [190] | Induce epithelial–mesenchymal transition (EMT) [191] | Promote drug resistance in cancer treatment [192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohag, S.M.; Toma, S.N.; Imon, M.A.-I.; Maihemuti, M.; Ahmed, F.; Mimi, M.A.; Mahmud, I.; Hasan, M.M. Tumor Microenvironment: An Emerging Landscape for Lung Cancer Therapy. Future Pharmacol. 2025, 5, 34. https://doi.org/10.3390/futurepharmacol5030034
Sohag SM, Toma SN, Imon MA-I, Maihemuti M, Ahmed F, Mimi MA, Mahmud I, Hasan MM. Tumor Microenvironment: An Emerging Landscape for Lung Cancer Therapy. Future Pharmacology. 2025; 5(3):34. https://doi.org/10.3390/futurepharmacol5030034
Chicago/Turabian StyleSohag, S. M., Sharmin Nur Toma, Md. Al-Imran Imon, Maiweilan Maihemuti, Famim Ahmed, Mst. Afsana Mimi, Imran Mahmud, and Md. Mahmudul Hasan. 2025. "Tumor Microenvironment: An Emerging Landscape for Lung Cancer Therapy" Future Pharmacology 5, no. 3: 34. https://doi.org/10.3390/futurepharmacol5030034
APA StyleSohag, S. M., Toma, S. N., Imon, M. A.-I., Maihemuti, M., Ahmed, F., Mimi, M. A., Mahmud, I., & Hasan, M. M. (2025). Tumor Microenvironment: An Emerging Landscape for Lung Cancer Therapy. Future Pharmacology, 5(3), 34. https://doi.org/10.3390/futurepharmacol5030034







