Drug Target Validation in Polyamine Metabolism and Drug Discovery Advancements to Combat Tuberculosis
Abstract
1. Tuberculosis as a Global Treat
1.1. Common Features of Pathogenic and Non-Pathogenic Actinobacteria
1.2. Distribution of Tuberculosis and Its Pathogenesis
2. Polyamine Metabolism in M. tuberculosis as a Part of Nitrogen Metabolism for Survival and Pathogenicity
2.1. Nitrogen Assimilation and Control in Mycobacterium tuberculosis
2.2. GS-like Enzymes GlnA2, GlnA3, and GlnA4 in M. tuberculosis
2.3. Polyamine Metabolism in Actinobacteria
3. Current Tuberculosis Drug Targets and Validated Drug Candidates in M. tuberculosis
3.1. Targeting DNA Replication and Protein Synthesis (Transcription and Translation)
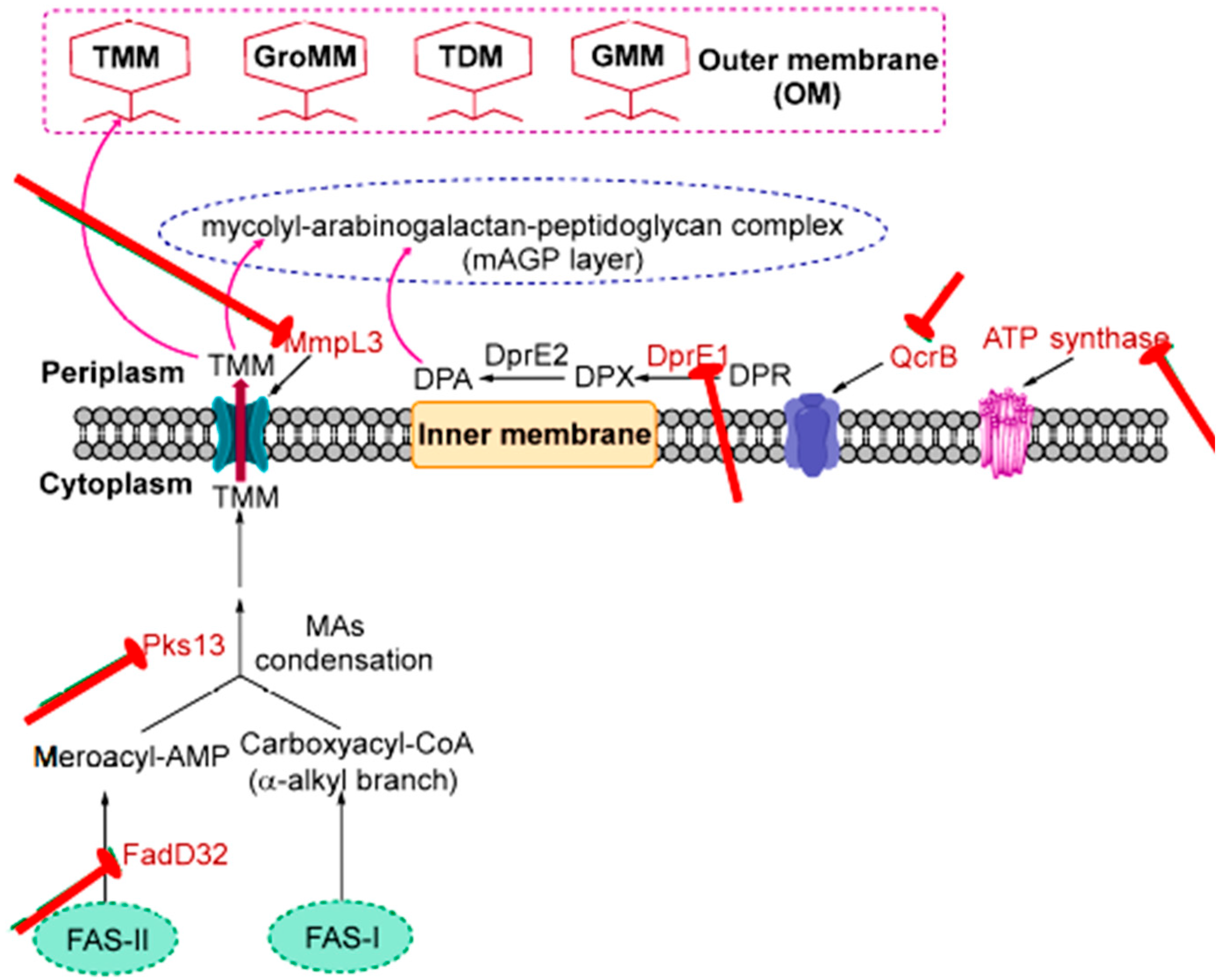
3.2. Targeting Cell Wall/Peptidoglycan Biosynthesis
3.3. Targeting Arabinogalactan
3.4. Targeting Cytochrome B Subunit QcrB
3.5. Targeting Clp Proteases
| Target | Function | References |
|---|---|---|
| Gyrase B, rRNA, Leucyl tRNA synthetase, RNA Polymerase | DNA replication and protein synthesis | [62] |
| MurX, l,d-traspeptidases, l,d-transpeptidases + β lactamase, Lipid II | peptidoglycan biosynthesis | [88] |
| WecA, DprE1 (Covalent inhibitors), DprE1 (Noncovalent inhibitors) | arabinogalactan biosynthesis | [88] |
| InhA, MmpL3, β-ketoacyl-ACP synthase (kasA), Inhibition of methoxy and keto mycolic acid (exact target unknown) | mycolic acid biosynthesis | [9] |
| ATP synthase (AtpE), Cytochrome bc1/aa3 super, NDH-2, MenA, MenG, Isocitrate lyase (ICL) | energy metabolism | [92] |
| ClpC | proteolysis | [91] |
| Glutamine synthetase GlnA1 | Primary metabolism, glutamine synthesis | [94,95,96,97] |
| Gamma-glutamylpolyamine synthetase GlnA3 | Polyamine metabolism | [23] |
3.6. Targeting Primary Metabolism
3.6.1. Targeting Carbon Metabolism
3.6.2. Targeting Sulfur Metabolism
3.6.3. Targeting Phosphate/ATP Metabolism
3.6.4. Targeting Nitrogen and the Metabolism of Polyamines
4. Drug Repurposing Targeting Polyamines in M. tuberculosis
4.1. Reactive Oxygen Species (ROS) as Drug Targets for TB
4.2. The Synergy of Abscisic Acid and Nitric Oxide as a Therapeutic Target
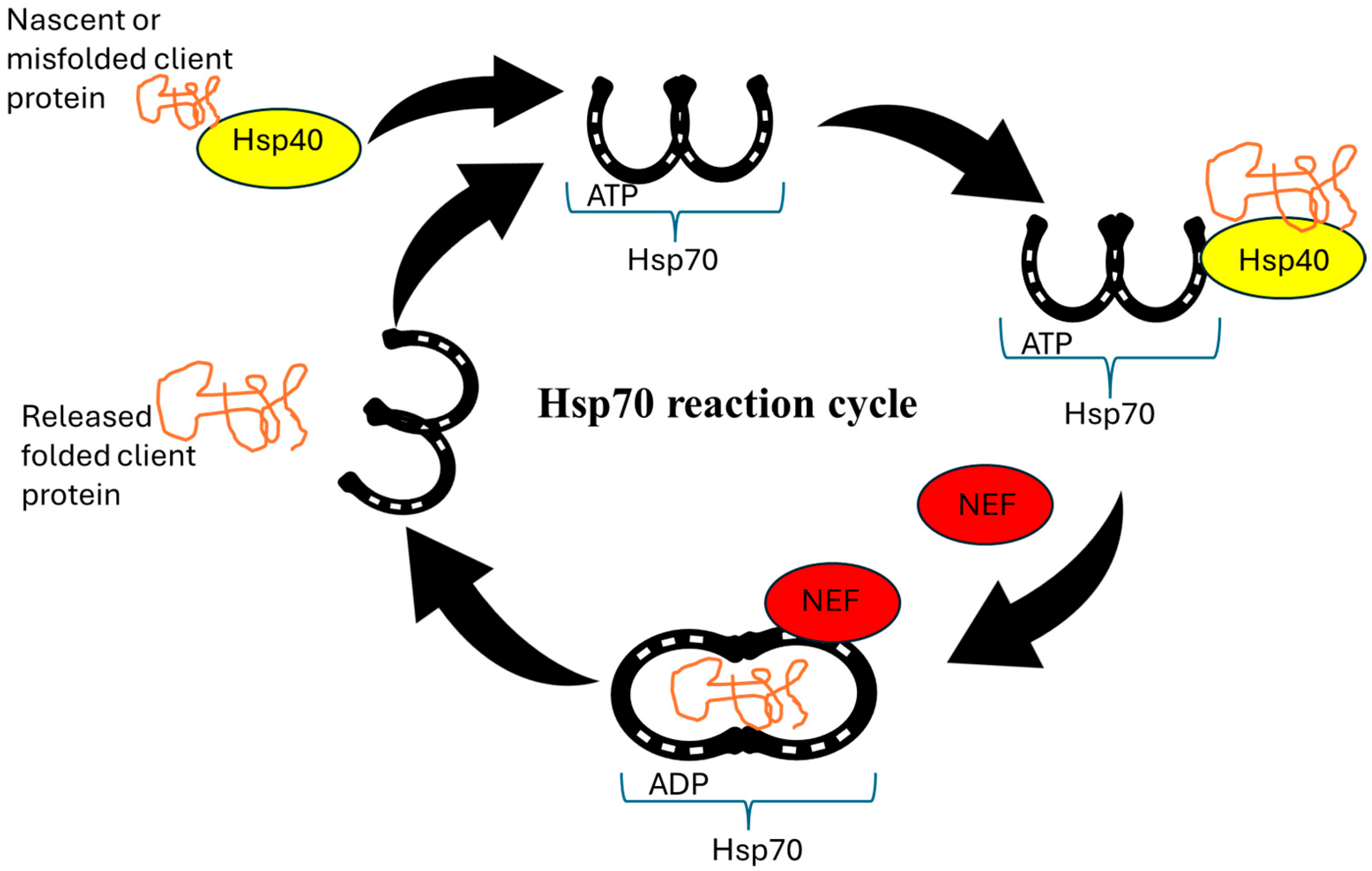
4.3. Molecular Chaperones as Drug Targets
4.4. Targeting Chaperone Networks
5. Combination Therapies for the Treatment of Tuberculosis
6. Conclusions: Perspectives on Drug Target Validation in Polyamine Metabolism and Tuberculosis Drug Discovery
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siavashifar, M.; Rezaei, F.; Motallebirad, T.; Azadi, D.; Absalan, A.; Naserramezani, Z.; Golshani, M.; Jafarinia, M.; Ghaffari, K. Species diversity and molecular analysis of opportunistic Mycobacterium, Nocardia and Rhodococcus isolated from the hospital environment in a developing country, a potential resources for nosocomial infection. Genes Environ. 2021, 43, 2. [Google Scholar] [CrossRef] [PubMed]
- Kubica, G.P.; Kim, T.H.; Dunbar, F.P. Designation of strain H37Rv as the neotype of Mycobacterium tuberculosis. Int. J. Syst. Evol. Microbiol. 1972, 22, 99–106. [Google Scholar] [CrossRef]
- Alam, K.; Mazumder, A.; Sikdar, S.; Zhao, Y.M.; Hao, J.; Song, C.; Wang, Y.; Sarkar, R.; Islam, S.; Zhang, Y.; et al. Streptomyces: The biofactory of secondary metabolites. Front. Microbiol. 2022, 13, 968053. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S.; Wohlleben, W. Polyamine and ethanolamine metabolism in bacteria as an important component of nitrogen assimilation for survival and pathogenicity. Med. Sci. 2022, 10, 40. [Google Scholar] [CrossRef]
- Krysenko, S.; Wohlleben, W. Role of Carbon, Nitrogen, Phosphate and Sulfur Metabolism in Secondary Metabolism Precursor Supply in Streptomyces spp. Microorganisms 2024, 12, 1571. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Foundation Norwich: Berlin, Germany, 2000; Volume 291. [Google Scholar]
- World Health Organization. World Health Statistics 2024: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Natarajan, A.; Beena, P.M.; Devnikar, A.V.; Mali, S. A systemic review on tuberculosis. Indian. J. Tuberc. 2020, 67, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, S.S.R.; Gunosewoyo, H. Tuberculosis: Pathogenesis, Current Treatment Regimens and New Drug Targets. Int. J. Mol. Sci. 2023, 24, 5202. [Google Scholar] [CrossRef]
- Vasiliu, A.; Martinez, L.; Gupta, R.K.; Hamada, Y.; Ness, T.; Kay, A.; Bonnet, M.; Sester, M.; Kaufmann, S.H.E.; Lange, C.; et al. Tuberculosis prevention: Current strategies and future directions. Clin. Microbiol. Infect. 2024, 30, 1123–1130. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef]
- Latour, Y.L.; Gobert, A.P.; Wilson, K.T. The role of polyamines in the regulation of macrophage polarization and function. Amino Acids 2020, 52, 151–160. [Google Scholar] [CrossRef]
- Muraille, E.; Leo, O.; Moser, M. TH1/TH2 paradigm extended: Macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front. Immunol. 2014, 5, 603. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Li, J.; Cao, L.; Yan, C. Mycobacterium tuberculosis virulence protein ESAT-6 influences M1/M2 polarization and macrophage apoptosis to regulate tuberculosis progression. Genes Genom. 2024, 46, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Harth, G.; Clemens, D.L.; Horwitz, M.A. Glutamine synthetase of Mycobacterium tuberculosis: Extracellular release and characterization of its enzymatic activity. Proc. Natl. Acad. Sci. USA 1994, 91, 9342–9346. [Google Scholar] [CrossRef] [PubMed]
- Tullius, M.V.; Harth, G.; Horwitz, M.A. Glutamine synthetase GlnA1 is essential for growth of Mycobacterium tuberculosis in human THP-1 macrophages and guinea pigs. Infect. Immun. 2003, 71, 3927–3936. [Google Scholar] [CrossRef]
- Harth, G.; Maslesa-Galic, S.; Tullius, M.V.; Horwitz, M.A. All four Mycobacterium tuberculosis glnA genes encode glutamine synthetase activities but only GlnA1 is abundantly expressed and essential for bacterial homeostasis. Mol. Microbiol. 2005, 58, 1157–1172. [Google Scholar] [CrossRef]
- Tiffert, Y.; Supra, P.; Wurm, R.; Wohlleben, W.; Wagner, R.; Reuther, J. The Streptomyces coelicolor GlnR regulon: Identification of new GlnR targets and evidence for a central role of GlnR in nitrogen metabolism in actinomycetes. Mol. Microbiol. 2008, 67, 861–880. [Google Scholar] [CrossRef]
- Malm, S.; Tiffert, Y.; Micklinghoff, J.; Schultze, S.; Joost, I.; Weber, I.; Horst, S.; Ackermann, B.; Schmidt, M.; Wohlleben, W.; et al. The roles of the nitrate reductase NarGHJI, the nitrite reductase NirBD and the response regulator GlnR in nitrate assimilation of Mycobacterium tuberculosis. Microbiology 2009, 155, 1332–1339. [Google Scholar] [CrossRef]
- Gouzy, A.; Poquet, Y.; Neyrolles, O. Nitrogen metabolism in Mycobacterium tuberculosis physiology and virulence. Nat. Rev. Microbiol. 2014, 12, 729–737. [Google Scholar] [CrossRef]
- Krysenko, S.; Matthews, A.; Busche, T.; Bera, A.; Wohlleben, W. Poly- and Monoamine Metabolism in Streptomyces coelicolor: The New Role of Glutamine Synthetase-Like Enzymes in the Survival Under Environmental Stress. Microb. Physiol. 2021, 31, 233–247. [Google Scholar] [CrossRef]
- Rexer, H.; Schäberle, T.; Wohlleben, W.; Engels, A. Investigation of the functional properties and regulation of three glutamine synthetase-like genes in Streptomyces coelicolor A3(2). Arch. Microbiol. 2006, 186, 447–458. [Google Scholar] [CrossRef]
- Krysenko, S.; Emani, C.S.; Bäuerle, M.; Oswald, M.; Kulik, A.; Meyners, C.; Hillemann, D.; Merker, M.; Prosser, G.; Wohlers, I.; et al. GlnA3Mt is able to glutamylate spermine but it is not essential for the detoxification of spermine in Mycobacterium tuberculosis. J. Bacteriol. 2025, 207, e0043924. [Google Scholar] [CrossRef] [PubMed]
- Purder, S.; Okoniewski, N.; Nentwich, M.; Matthews, A.; Bäuerle, M.; Zinser, A.; Busche, T.; Kulik, A.; Gursch, S.; Kemeny, A. A second gamma-Glutamylpolyamine synthetase, GlnA2, is involved in polyamine catabolism in Streptomyces coelicolor. Int. J. Mol. Sci. 2022, 23, 3752. [Google Scholar]
- Krysenko, S.; Matthews, A.; Okoniewski, N.; Kulik, A.; Girbas, M.G.; Tsypik, O.; Meyners, C.S.; Hausch, F.; Wohlleben, W.; Bera, A. Initial metabolic step of a novel ethanolamine utilization pathway and its regulation in Streptomyces coelicolor M145. mBio 2019, 10, e00326-19. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef]
- Hooft, R.W.; Sander, C.; Vriend, G. Objectively judging the quality of a protein structure from a Ramachandran plot. Bioinformatics 1997, 13, 425–430. [Google Scholar] [CrossRef]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Ladner, J.E.; Atanasova, V.; Dolezelova, Z.; Parsons, J.F. Structure and activity of PA5508, a hexameric glutamine synthetase homologue. Biochemistry 2012, 51, 10121–10123. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Evans, C.G.T.; Herbert, D.; Tempest, D.W. Chapter XIII The Continuous Cultivation of Micro-Organisms: 2. Construction of a Chemostat. Meth. Microbiol. 1970, 2, 277–327. [Google Scholar]
- Gust, B.; Challis, G.L.; Fowler, K.; KIeser, T.; Chater, K.F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 2003, 100, 1541–1546. [Google Scholar] [CrossRef]
- Gawronski, J.D.; Benson, D.R. Microtiter assay for glutamine synthetase biosynthetic activity using inorganic phosphate detection. Anal. Biochem. 2004, 327, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Suzuki, H. (Eds.) Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Davis, R.H.; Ristow, J.L. Polyamine toxicity in Neurospora crassa: Protective role of the vacuole. Arch. Biochem. Biophys. 1991, 285, 306–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paulin, L.G.; Brander, E.E.; Pösö, H.J. Specific inhibition of spermidine synthesis in Mycobacteria spp. by the dextro isomer of ethambutol. Antimicrob. Agents Chemother. 1985, 28, 157–159. [Google Scholar] [CrossRef]
- Jain, A.; Tyagi, A.K. Role of polyamines in the synthesis of RNA in mycobacteria. Mol. Cell. Biochem. 1987, 78, 3–8. [Google Scholar] [CrossRef]
- Hamana, K.; Matsuzaki, S. Distribution of polyamines in actinomycetes. FEMS Microbiol. Lett. 1987, 41, 211–215. [Google Scholar] [CrossRef]
- Chanphai, P.; Thomas, T.; Tajmir-Riahi, H. Conjugation of biogenic and synthetic polyamines with serum proteins: A comprehensive review. Int. J. Biol. Macromol. 2016, 92, 515–522. [Google Scholar] [CrossRef]
- Michael, A.J. Polyamine function in archaea and bacteria. J. Biol. Chem. 2018, 293, 18693–18701. [Google Scholar] [CrossRef]
- Tome, E.M.; Fliser, M.S.; Payne, M.C.; Gerner, W.E. Excess putrescine accumulation inhibits the formation of modified eukaryotic initiation factor 5A (eIF-5A) and induces apoptosis. Biochem. J. 1997, 328, 847–854. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of Polyamines and Their Metabolic Products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Lasbury, M.E.; Merali, S.; Durant, P.J.; Tschang, D.; Ray, C.A.; Lee, C.H. Polyamine-mediated apoptosis of alveolar macrophages during Pneumocystis pneumonia. J. Biol. Chem. 2007, 282, 11009–11020. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.S.; Spontak, J.S.; Klapper, D.G.; Richardson, A.R. Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol. Microbiol. 2011, 82, 9–20. [Google Scholar] [CrossRef]
- Norris, V.; Reusch, R.N.; Igarashi, K.; Root-Bernstein, R. Molecular complementarity between simple, universal molecules and ions limited phenotype space in the precursors of cells. Biol. Direct. 2015, 10, 28. [Google Scholar] [CrossRef][Green Version]
- Bullock, W.O.; Fernandez, J.M.; Short, J.M. XL1-blue: A high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 1987, 5, 376. [Google Scholar]
- Kurihara, S.; Oda, S.; Tsuboi, Y.; Kim, H.G.; Oshida, M.; Kumagai, H.; Suzuki, H. gamma-Glutamylputrescine synthetase in the putrescine utilization pathway of Escherichia coli K-12. J. Biol. Chem. 2008, 283, 19981–19990. [Google Scholar] [CrossRef]
- Yao, X.; He, W.; Lu, C.D. Functional characterization of seven gamma-Glutamylpolyamine synthetase genes and the bauRABCD locus for polyamine and beta-Alanine utilization in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2011, 193, 3923–3930. [Google Scholar] [CrossRef]
- Schneider, B.L.; Reitzer, L. Pathway and enzyme redundancy in putrescine catabolism in Escherichia coli. J. Bacteriol. 2012, 194, 4080–4088. [Google Scholar] [CrossRef]
- Forouhar, F.; Lee, I.-S.; Vujcic, J.; Vujcic, S.; Shen, J.; Vorobiev, S.M.; Xiao, R.; Acton, T.B.; Montelione, G.T.; Porter, C.W. Structural and functional evidence for Bacillus subtilis PaiA as a novel N1-spermidine/spermine acetyltransferase. J. Biol. Chem. 2005, 280, 40328–40336. [Google Scholar] [CrossRef]
- Foster, A.; Barnes, N.; Speight, R.; Keane, M.A. Genomic organisation, activity and distribution analysis of the microbial putrescine oxidase degradation pathway. Syst. Appl. Microbiol. 2013, 36, 457–466. [Google Scholar] [CrossRef]
- Campilongo, R.; Di Martino, M.L.; Marcocci, L.; Pietrangeli, P.; Leuzzi, A.; Grossi, M.; Casalino, M.; Nicoletti, M.; Micheli, G.; Colonna, B. Molecular and functional profiling of the polyamine content in enteroinvasive E. coli: Looking into the gap between commensal E. coli and harmful Shigella. PLoS ONE 2014, 9, e106589. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S.; Okoniewski, N.; Kulik, A.; Matthews, A.; Grimpo, J.; Wohlleben, W.; Bera, A. Gamma-Glutamylpolyamine Synthetase GlnA3 is Involved in the First Step of Polyamine Degradation Pathway in Streptomyces coelicolor M145. Front. Microbiol. 2017, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Sao Emani, C.; Reiling, N. Spermine enhances the activity of anti-tuberculosis drugs. Microbiol. Spectr. 2024, 12, e0356823. [Google Scholar] [CrossRef]
- Kruh, N.A.; Troudt, J.; Izzo, A.; Prenni, J.; Dobos, K.M. Portrait of a pathogen: The Mycobacterium tuberculosis proteome in vivo. PLoS ONE 2010, 5, e13938. [Google Scholar] [CrossRef]
- Cole, S.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., III; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–540. [Google Scholar] [CrossRef]
- Bosch, B.; DeJesus, M.A.; Poulton, N.C.; Zhang, W.; Engelhart, C.A.; Zaveri, A.; Lavalette, S.; Ruecker, N.; Trujillo, C.; Wallach, J.B.; et al. Genome-wide gene expression tuning reveals diverse vulnerabilities of M. tubercolosis. Cell 2021, 184, 4579–4592.e24. [Google Scholar] [CrossRef]
- Hirsch, J.G.; Dubos, R.J. The effect of spermine on tubercle bacilli. J. Exp. Med. 1952, 95, 191–208. [Google Scholar] [CrossRef]
- Bhat, Z.S.; Rather, M.A.; Maqbool, M.; Ahmad, Z. Drug targets exploited in Mycobacterium tuberculosis: Pitfalls and promises on the horizon. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 103, 1733–1747. [Google Scholar] [CrossRef]
- Shetye, G.S.; Franzblau, S.G.; Cho, S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Transl. Res. J. Lab. Clin. Med. 2020, 220, 68–97. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.; Qi, Y.; Yan, X.; Qi, G.; Peng, Q. The progress of Mycobacterium tuberculosis drug targets. Front. Med. 2024, 11, 1455715. [Google Scholar] [CrossRef]
- Silver, R.F.; Myers, A.J.; Jarvela, J.; Flynn, J.; Rutledge, T.; Bonfield, T.; Lin, P.L. Diversity of Human and Macaque Airway Immune Cells at Baseline and during Tuberculosis Infection. Am. J. Respir. Cell Mol. Biol. 2016, 55, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, M.K.N.; Mohd Tazizi, M.H.D.; Ahmad, R.; Noh, M.A.A.; Bakhtiar, A.; Wahab, H.A.; Mohd Gazzali, A. Antituberculosis Targeted Drug Delivery as a Potential Future Treatment Approach. Antibiotics 2021, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, E.; Arrigo, P.; Bellinzoni, M.; Silva, P.E.A.; Martin, C.; Ainsa, J.A.; Guglierame, P.; Riccardi, G. The multidrug transporters belonging to major facilitator superfamily (MFS) in Mycobacterium tuberculosis. Mol. Med. 2002, 8, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Balganesh, M.; Dinesh, N.; Sharma, S.; Kuruppath, S.; Nair, A.V.; Sharma, U. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob. Agents Chemother. 2012, 56, 2643–2651. [Google Scholar] [CrossRef]
- Dutta, N.K.; Mehra, S.; Kaushal, D. A Mycobacterium tuberculosis sigma factor network responds to cell-envelope damage by the promising anti-mycobacterial thioridazine. PLoS ONE 2010, 5, e10069. [Google Scholar] [CrossRef]
- Rodrigues, L.; Villellas, C.; Bailo, R.; Viveiros, M.; Ainsa, J.A. Role of the Mmr efflux pump in drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 751–757. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary metabolites and biodiversity of actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Sakharkar, K.R.; Sakharkar, M.K.; Chow, V.T. Biocomputational strategies for microbial drug target identification. Methods Mol. Med. 2008, 142, 1–9. [Google Scholar] [CrossRef]
- Rodríguez-Bustamante, E.; Gómez-Manzo, S.; Valle, A.D.O.F.d.; Arreguín-Espinosa, R.; Espitia-Pinzón, C.; Rodríguez-Flores, E. New Alternatives in the Fight Against Tuberculosis: Possible Targets for Resistant Mycobacteria. Processes 2023, 11, 2793. [Google Scholar] [CrossRef]
- Parish, T.; Stoker, N.G. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 2000, 146, 1969–1975. [Google Scholar] [CrossRef]
- Sao Emani, C.; Williams, M.J.; Van Helden, P.D.; Taylor, M.J.C.; Carolis, C.; Wiid, I.J.; Baker, B. Generation and characterization of thiol-deficient Mycobacterium tuberculosis mutants. Sci. Data 2018, 5, 180184. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, E.; Branzoni, M.; Cantoni, R.; Milano, A.; Riccardi, G.; Ciferri, O. Mmr, a Mycobacterium tuberculosis gene conferring resistance to small cationic dyes and inhibitors. J. Bacteriol. 1998, 180, 6068–6071. [Google Scholar] [CrossRef] [PubMed]
- Zelmer, A.; Carroll, P.; Andreu, N.; Hagens, K.; Mahlo, J.; Redinger, N.; Robertson, B.D.; Wiles, S.; Ward, T.H.; Parish, T.; et al. A new in vivo model to test anti-tuberculosis drugs using fluorescence imaging. J. Antimicrob. Chemother. 2012, 67, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Carroll, P.; Schreuder, L.J.; Muwanguzi-Karugaba, J.; Wiles, S.; Robertson, B.D.; Ripoll, J.; Ward, T.H.; Bancroft, G.J.; Schaible, U.E.; Parish, T. Sensitive detection of gene expression in mycobacteria under replicating and non-replicating conditions using optimized far-red reporters. PLoS ONE 2010, 5, e9823. [Google Scholar] [CrossRef]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef]
- Brötz-Oesterhelt, H.; Brunner, N.A. How many modes of action should an antibiotic have? Curr. Opin. Pharmacol. 2008, 8, 564–573. [Google Scholar] [CrossRef]
- Jumde, R.P.; Guardigni, M.; Gierse, R.M.; Alhayek, A.; Zhu, D.; Hamid, Z.; Johannsen, S.; Elgaher, W.A.; Neusens, P.J.; Nehls, C. Hit-optimization using target-directed dynamic combinatorial chemistry: Development of inhibitors of the anti-infective target 1-deoxy-d-xylulose-5-phosphate synthase. Chem. Sci. 2021, 12, 7775–7785. [Google Scholar] [CrossRef]
- Wallace, R.J.; Nash, D.R.; Steele, L.C.; Steingrube, V. Susceptibility Testing of Slowly Growing Mycobacteria by a Microdilution MIC Method with 7H9 Broth. J. Clin. Microbiol. 1986, 24, 976–981. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef]
- Merker, M.; Kohl, T.A.; Roetzer, A.; Truebe, L.; Richter, E.; Rusch-Gerdes, S.; Fattorini, L.; Oggioni, M.R.; Cox, H.; Varaine, F.; et al. Whole genome sequencing reveals complex evolution patterns of multidrug-resistant Mycobacterium tuberculosis Beijing strains in patients. PLoS ONE 2013, 8, e82551. [Google Scholar] [CrossRef]
- Andreu, N.; Zelmer, A.; Fletcher, T.; Elkington, P.T.; Ward, T.H.; Ripoll, J.; Parish, T.; Bancroft, G.J.; Schaible, U.; Robertson, B.D.; et al. Optimisation of bioluminescent reporters for use with mycobacteria. PLoS ONE 2010, 5, e10777. [Google Scholar] [CrossRef]
- Radkov, A.D.; Hsu, Y.P.; Booher, G.; VanNieuwenhze, M.S. Imaging Bacterial Cell Wall Biosynthesis. Annu. Rev. Biochem. 2018, 87, 991–1014. [Google Scholar] [CrossRef]
- Boutte, C.C.; Baer, C.E.; Papavinasasundaram, K.; Liu, W.; Chase, M.R.; Meniche, X.; Fortune, S.M.; Sassetti, C.M.; Ioerger, T.R.; Rubin, E.J. A cytoplasmic peptidoglycan amidase homologue controls mycobacterial cell wall synthesis. eLife 2016, 5, e14590. [Google Scholar] [CrossRef]
- Capela, R.; Félix, R.; Clariano, M.; Nunes, D.; Perry, M.d.J.; Lopes, F. Target Identification in Anti-Tuberculosis Drug Discovery. Int. J. Mol. Sci. 2023, 24, 10482. [Google Scholar] [CrossRef]
- Stec, J.; Onajole, O.K.; Lun, S.; Guo, H.; Merenbloom, B.; Vistoli, G.; Bishai, W.R.; Kozikowski, A.P. Indole-2-Carboxamide-Based MmpL3 Inhibitors Show Exceptional Antitubercular Activity in an Animal Model of Tuberculosis Infection. J. Med. Chem. 2016, 59, 6232–6247. [Google Scholar] [CrossRef]
- Banerjee, A.; Dubnau, E.; Quemard, A.; Balasubramanian, V.; Um, K.S.; Wilson, T.; Collins, D.; de Lisle, G.; Jacobs, W.R., Jr. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 1994, 263, 227–230. [Google Scholar] [CrossRef]
- Culp, E.; Wright, G.D. Bacterial proteases, untapped antimicrobial drug targets. J. Antibiot. 2017, 70, 366–377. [Google Scholar] [CrossRef]
- Schmitz, K.R.; Sauer, R.T. Substrate delivery by the AAA+ ClpX and ClpC1 unfoldases activates the mycobacterial ClpP1P2 peptidase. Mol. Microbiol. 2014, 93, 617–628. [Google Scholar] [CrossRef]
- Haagsma, A.C.; Abdillahi-Ibrahim, R.; Wagner, M.J.; Krab, K.; Vergauwen, K.; Guillemont, J.; Andries, K.; Lill, H.; Koul, A.; Bald, D. Selectivity of TMC207 towards mycobacterial ATP synthase compared with that towards the eukaryotic homologue. Antimicrob. Agents Chemother. 2009, 53, 1290–1292. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Eisenberg, D.; Gill, H.S.; Pfluegl, G.M.; Rotstein, S.H. Structure-function relationships of glutamine synthetases. Biochim. Biophys. Acta 2000, 1477, 122–145. [Google Scholar] [CrossRef]
- Gill, H.S.; Pfluegl, G.M.; Eisenberg, D. Multicopy crystallographic refinement of a relaxed glutamine synthetase from Mycobacterium tuberculosis highlights flexible loops in the enzymatic mechanism and its regulation. Biochemistry 2002, 41, 9863–9872. [Google Scholar] [CrossRef]
- Nilsson, M.T.; Mowbray, S.L. Crystal structure of Mycobacterium tuberculosis glutamine synthethase in complex with imidazopyridine inhibitor ((4-(6-Bromo-3-(Butylamino)imidazo(1,2-A)Pyridin-2-YL)Phenoxy) Acetic acid) and L-Methionine-S-Sulfoximine phosphate. Med. Chem. Comm. 2012, 3, 620. [Google Scholar]
- Nilsson, M.T.; Mowbray, S.L. Crystal structure of Mycobacterium tuberculosis Glutamine Synthetase in complex with tri-substituted imidazole inhibitor (4-(2-tert-butyl- 4-(6-methoxynaphthalen-2-yl)-1H-imidazol-5-yl)pyridin-2-amine) and L-methionine-S-sulfoximine phosphate. J. Med. Chem. 2012, 55, 2894. [Google Scholar]
- Rhee, K.Y.; de Carvalho, L.P.; Bryk, R.; Ehrt, S.; Marrero, J.; Park, S.W.; Schnappinger, D.; Venugopal, A.; Nathan, C. Central carbon metabolism in Mycobacterium tuberculosis: An unexpected frontier. Trends Microbiol. 2011, 19, 307–314. [Google Scholar] [CrossRef]
- Bryk, R.; Arango, N.; Venugopal, A.; Warren, J.D.; Park, Y.H.; Patel, M.S.; Lima, C.D.; Nathan, C. Triazaspirodimethoxybenzoyls as selective inhibitors of mycobacterial lipoamide dehydrogenase. Biochemistry 2010, 49, 1616–1627. [Google Scholar] [CrossRef]
- Mougous, J.D.; Green, R.E.; Williams, S.J.; Brenner, S.E.; Bertozzi, C.R. Sulfotransferases and sulfatases in mycobacteria. Chem. Biol. 2002, 9, 767–776. [Google Scholar] [CrossRef]
- Paritala, H.; Carroll, K.S. New targets and inhibitors of mycobacterial sulfur metabolism. Infect. Disord. Drug Targets 2013, 13, 85–115. [Google Scholar] [CrossRef]
- Hatzios, S.K.; Bertozzi, C.R. The regulation of sulfur metabolism in Mycobacterium tuberculosis. PLoS Pathog 2011, 7, e1002036. [Google Scholar] [CrossRef]
- Sassetti, C.M.; Boyd, D.H.; Rubin, E.J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003, 48, 77–84. [Google Scholar] [CrossRef]
- Rath, V.L.; Verdugo, D.; Hemmerich, S. Sulfotransferase structural biology and inhibitor discovery. Drug Discov. Today 2004, 9, 1003–1011. [Google Scholar] [CrossRef]
- Bhave, D.P.; Muse, W.B., 3rd; Carroll, K.S. Drug targets in mycobacterial sulfur metabolism. Infect. Disord. Drug Targets 2007, 7, 140–158. [Google Scholar] [CrossRef]
- Chapman, E.; Ding, S.; Schultz, P.G.; Wong, C.H. A potent and highly selective sulfotransferase inhibitor. J. Am. Chem. Soc. 2002, 124, 14524–14525. [Google Scholar] [CrossRef]
- Cook, G.M.; Hards, K.; Dunn, E.; Heikal, A.; Nakatani, Y.; Greening, C.; Crick, D.C.; Fontes, F.L.; Pethe, K.; Hasenoehrl, E.; et al. Oxidative Phosphorylation as a Target Space for Tuberculosis: Success, Caution, and Future Directions. Microbiol. Spectr. 2017, 5, 295–316. [Google Scholar] [CrossRef]
- Bald, D.; Villellas, C.; Lu, P.; Koul, A. Targeting Energy Metabolism in Mycobacterium tuberculosis, a New Paradigm in Antimycobacterial Drug Discovery. mBio 2017, 8, e00272-17. [Google Scholar] [CrossRef]
- Purder, P.L.; Meyners, C.; Krysenko, S.; Funk, J.; Wohlleben, W.; Hausch, F. Mechanism-Based Design of the First GlnA4-Specific Inhibitors. Chembiochem 2022, 23, e202200312. [Google Scholar] [CrossRef]
- Krysenko, S.; Lopez, M.; Meyners, C.; Purder, P.L.; Zinser, A.; Hausch, F.; Wohlleben, W. A novel synthetic inhibitor of polyamine utilization in Streptomyces coelicolor. FEMS Microbiol. Lett. 2023, 370, fnad096. [Google Scholar] [CrossRef]
- Krysenko, S.; Purder, P.; Hausch, F.; Wohlleben, W. Neuartige synthetische Wirkstoffe zur Tuberkulosebekämpfung. Biospektrum 2024, 30, 819–821. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef]
- Krajewski, W.W.; Jones, T.A.; Mowbray, S.L. Structure of Mycobacterium tuberculosis glutamine synthetase in complex with a transition-state mimic provides functional insights. Proc. Natl. Acad. Sci USA 2005, 102, 10499–10504. [Google Scholar] [CrossRef]
- Bayer, E.; Gugel, K.H.; Hägele, K.; Hagenmaier, H.; Jessipow, S.; König, W.A.; Zähner, H. Stoffwechselprodukte von Mikroorganismen. 98. Phosphinothricin und Phosphinothricyl-Alanyl-Alanin [Metabolic products of microorganisms. 98. Phosphinothricin and phosphinothricyl-alanyl-analine]. Helv. Chim. Acta 1972, 55, 224–239. [Google Scholar] [CrossRef]
- Patrick, G.J.; Fang, L.; Schaefer, J.; Singh, S.; Bowman, G.R.; Wencewicz, T.A. Mechanistic Basis for ATP-Dependent Inhibition of Glutamine Synthetase by Tabtoxinine-β-lactam. Biochemistry 2018, 57, 117–135. [Google Scholar] [CrossRef]
- León, J.; Castillo, M.C.; Coego, A.; Lozano-Juste, J.; Mir, R. Diverse functional interactions between nitric oxide and abscisic acid in plant development and responses to stress. J. Exp. Bot. 2014, 65, 907–921. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef]
- Shastri, M.D.; Shukla, S.D.; Chong, W.C.; Dua, K.; Peterson, G.M.; Patel, R.P.; Hansbro, P.M.; Eri, R.; O’Toole, R.F. Role of Oxidative Stress in the Pathology and Management of Human Tuberculosis. Oxidative Med. Cell. Longev. 2018, 2018, 7695364. [Google Scholar] [CrossRef]
- Bajeli, S.; Baid, N.; Kaur, M.; Pawar, G.P.; Chaudhari VDKumar, A. Terminal Respiratory Oxidases: A Targetable Vulnerability of Mycobacterial Bioenergetics? Front. Cell. Infect. Microbiol. 2020, 10, 589318. [Google Scholar] [CrossRef]
- Gunjan, S.; Sharma, T.; Yadav, K.; Chauhan, B.S.; Singh, S.K.; Siddiqi MITripathi, R. Artemisinin Derivatives and Synthetic Trioxane Trigger Apoptotic Cell Death in Asexual Stages of Plasmodium. Front. Cell. Infect. Microbiol. 2018, 8, 256. [Google Scholar] [CrossRef]
- Lievens, L.; Pollier, J.; Goossens, A.; Beyaert, R.; Staal, J. Abscisic Acid as Pathogen Effector and Immune Regulator. Front. Plant Sci. 2017, 8, 587. [Google Scholar] [CrossRef]
- Xu, J.; Lu, X.; Liu, Y.; Lan, W.; Wei, Z.; Yu, W.; Li, C. Interaction between ABA and NO in plants under abiotic stresses and its regulatory mechanisms. Front. Plant Sci. 2024, 15, 1330948. [Google Scholar] [CrossRef]
- Makhoba, X.H. Two sides of the same coin: Heat shock proteins as biomarkers and therapeutic targets for some complex diseases. Front. Mol. Biosci. 2025, 12, 1491227. [Google Scholar] [CrossRef]
- Magwenyane, A.M.; Ugbaja, S.C.; Amoako, D.G.; Somboro, A.M.; Khan, R.B.; Kumalo, H.M. Heat Shock Protein 90 (HSP90) Inhibitors as Anticancer Medicines: A Review on the Computer-Aided Drug Discovery Approaches over the Past Five Years. Comput. Math. Methods Med. 2022, 2022, 2147763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Massey, A.J.; Williamson, D.S.; Browne, H.; Murray, J.B.; Dokurno, P.; Shaw, T.; Macias, A.T.; Daniels, Z.; Geoffroy, S.; Dopson, M.; et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother. Pharmacol. 2010, 66, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.M.; Lee, H.; Zagal, D.; Nam, J.W.; Oh, D.C.; Lee, H.; Suh, J.W.; Pauli, G.F.; Cho, S.; Abad-Zapatero, C. Structure of the N-terminal domain of ClpC1 in complex with the antituberculosis natural product ecumicin reveals unique binding interactions. Acta Crystallogr. D Struct. Biol. 2020, 76, 458–471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasegawa, T.; Yoshida, S.; Sugeno, N.; Kobayashi, J.; Aoki, M. DnaJ/Hsp40 Family and Parkinson’s Disease. Front. Neurosci. 2018, 11, 743. [Google Scholar] [CrossRef]
- Fournier, P.E.; Richet, H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 2006, 42, 692. [Google Scholar] [CrossRef]
- Lolans, K.; Rice, T.W.; Munoz-Price, L.S.; Quinn, J.P. Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40. Antimicrob. Agents Chemother. 2006, 50, 2941. [Google Scholar] [CrossRef]
- Sao Emani, C.; Reiling, N. The efflux pumps Rv1877 and Rv0191 play differential roles in the protection of Mycobacterium tuberculosis against chemical stress. Front. Microbiol. 2024, 15, 1359188. [Google Scholar] [CrossRef]
- Armengol, E.; Kragh, K.N.; Tolker-Nielsen, T.; Sierra, J.M.; Higazy, D.; Ciofu, O.; Viñas, M.; Høiby, N. Colistin Enhances Rifampicin’s Antimicrobial Action in Colistin-Resistant Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2023, 67, e0164122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshpande, D.; Magombedze, G.; Srivastava, S.; Bendet, P.; Lee, P.S.; Cirrincione, K.N.; Martin, K.R.; Dheda, K.; Gumbo, T. Once-a-week tigecycline for the treatment of drug-resistant TB. J. Antimicrob. Chemother. 2019, 74, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Patel, S.; Abboud, C.; Diep, J.; Ly, N.S.; Pogue, J.M.; Kaye, K.S.; Li, J.; Rao, G.G. Polymyxin B in combination with meropenem against carbapenemase-producing Klebsiella pneumoniae: Pharmacodynamics and morphological changes. Int. J. Antimicrob. Agents 2017, 49, 224–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clark, R.B.; Pakiz, C.B.; Hostetter, M.K. Synergistic activity of aminoglycoside-beta-lactam combinations against Pseudomonas aeruginosa with an unusual aminoglycoside antibiogram. Med. Microbiol. Immunol. 1990, 179, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zahari, N.I.N.; Engku Abd Rahman, E.N.S.; Irekeola, A.A.; Ahmed, N.; Rabaan, A.A.; Alotaibi, J.; Alqahtani, S.A.; Halawi, M.Y.; Alamri, I.A.; Almogbel, M.S.; et al. A Review of the Resistance Mechanisms for β-Lactams, Macrolides and Fluoroquinolones among Streptococcus pneumoniae. Medicina 2023, 59, 1927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewin, G.R.; Carlos, C.; Chevrette, M.G.; Horn, H.A.; McDonald, B.R.; Stankey, R.J.; Fox, B.G.; Currie, C.R. Evolution and Ecology of Actinobacteria and Their Bioenergy Applications. Annu. Rev. Microbiol. 2016, 70, 235–254. [Google Scholar] [CrossRef] [PubMed]
| Name | Mode of Action | Structure | References |
|---|---|---|---|
| Methionine sulfoximine (MetSox/MSO) | Potent, ATP-dependent inactivator of GS | 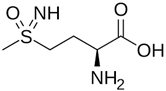 | [113] |
| Phosphinothricin (PPT) | Potent, ATP-dependent inactivator of GS that is produced as part of a tripeptide antibiotic by Streptomyces viridochromogenes. | 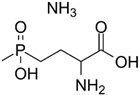 | [114] |
| Tabtoxinine β-lactam | Potent, ATP-dependent inactivator of GS produced by Pseudomonas pv. tabaci | 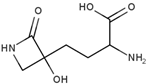 | [115] |
| Alanosine | Antibiotic produced by Streptomyces alanosinicus | 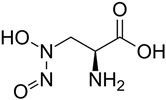 | [94] |
| Oxetin | Antibiotic produced by Streptomyces sp., inhibitor of GS | 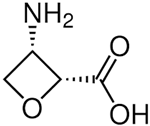 | [94] |
| 7b (PPU301) | Synthetic inactivator of the GS-like enzyme GlnA4 from Streptomyces coelicolor |  | [109] |
| PPU268 | Synthetic inactivator of the GS-like enzyme GlnA2 from Streptomyces coelicolor | 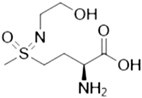 | [110] |
| Name | Roles | Action | Inhibitor | Structure | References |
|---|---|---|---|---|---|
| Hsp90 (Grp94) | Holdase | These compounds bind to the N-terminal ATP-binding domain of Hsp90, inhibiting its chaperone activity | Geldanamycin and its derivatives |  | [124] |
| Hsp70 (Dnak) | Foldase | A small molecule inhibitor that binds to the ATPase domain of Hsp70, inhibiting its activity | VER-155008 | 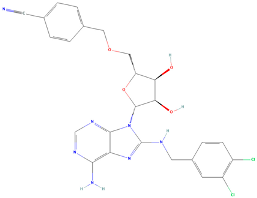 | [125] |
| ClpB | Holdase | A cyclic peptide that targets the ClpC1 ATPase component of the Clp protease complex, enhancing its ATPase activity but preventing proteolysis | Ecumicin | 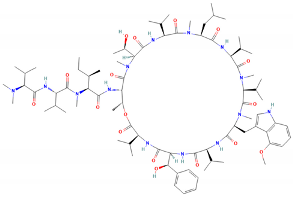 | [126] |
| Use Case | Mechanism | Combination | References |
|---|---|---|---|
| Multidrug-resistant strains | Synergistic effect | Colistin + Rifampicin | [131] |
| Severe infections | Enhanced efficacy | Tigecycline + Sulbactam | [132] |
| Carbapenem-resistant strains | Broad-spectrum activity | Meropenem + Polymyxin | [133] |
| General use in resistant infection | Synergistic effect | Beta-lactam + Aminoglycoside | [134] |
| Reducing resistance development | Targeting different pathways | Fluoroquinolone + Beta-lactam | [135] |
| General use in resistant infection | Enhancing effect of spermine on conventional drugs | Spermine + isoniazid, rifampicin, aminosalicylic acid, bedaquiline | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhoba, X.H.; Krysenko, S. Drug Target Validation in Polyamine Metabolism and Drug Discovery Advancements to Combat Tuberculosis. Future Pharmacol. 2025, 5, 32. https://doi.org/10.3390/futurepharmacol5030032
Makhoba XH, Krysenko S. Drug Target Validation in Polyamine Metabolism and Drug Discovery Advancements to Combat Tuberculosis. Future Pharmacology. 2025; 5(3):32. https://doi.org/10.3390/futurepharmacol5030032
Chicago/Turabian StyleMakhoba, Xolani H., and Sergii Krysenko. 2025. "Drug Target Validation in Polyamine Metabolism and Drug Discovery Advancements to Combat Tuberculosis" Future Pharmacology 5, no. 3: 32. https://doi.org/10.3390/futurepharmacol5030032
APA StyleMakhoba, X. H., & Krysenko, S. (2025). Drug Target Validation in Polyamine Metabolism and Drug Discovery Advancements to Combat Tuberculosis. Future Pharmacology, 5(3), 32. https://doi.org/10.3390/futurepharmacol5030032







