Abstract
Background and Aim: Neuropathic pain leads to a significant deterioration in health-related quality of life (HRQOL). Treating neuromusculoskeletal pain is especially important to prevent and improve physical frailty and the locomotive syndrome. Varied pharmacotherapies could be applicable for neuropathic pain patients, but evidence has been limited for a wide range of neuropathic pain conditions with different etiologies. The aim of this review was to highlight mirogabalin, a novel calcium channel α2δ ligand which was first approved in Japan, and which is effective for various types of neuropathic pain diseases. Methods: We conducted a narrative review of the recent evidence that mirogabalin has significant analgesic potency for varied types of neuropathic pain conditions. Futher, this review highlighted specific advantages over other calcium channel ligands. Results: Analgesic potency of mirogabalin could cover peripheral neuropathic pain conditions including post-herpetic neuralgia, diabetic peripheral neuropathy, cauda equina syndrome caused by lumbar spinal stenosis, radiculopathy caused by cervical spondylosis, and also central neuropathic pain conditions like spinal cord injury. Mirogabalin consistently demonstrated daytime sleepiness and dizziness as adverse effects, but most of these were mild. Conclusions: Mirogabalin is recommended as the first-line drug against most molecular mechanisms that cause neuropathic pain regardless of whether they have a peripheral or central origin. Mirogabalin demonstrates relatively less daytime sleepiness, making it age-friendly in the current global situation where population aging is accelerated. Considering the epidemic of ‘opiophobia’ in Japan and other countries, pharmacotherapy using mirogabalin could treat neuropathic pain associated with cancer and its treatment (e.g., chemotherapy-induced peripheral neuropathy), as well as non-cancer etiologies worldwide.
1. Introduction
Neuropathic pain is defined as “pain caused by lesions or diseases of the somatosensory nervous system.” Several lines of basic investigations have been reported. For example, in the phase of pain transmission, voltage-gated calcium channels (VGCCs) play a crucial role in regulating the secretion of excitatory neurotransmitters from the pre-synaptic terminal. Neuronal injury could increase the α2δ subunit of VGCC, which modifies the property of VGCC, and lead to enhanced pain transmission [1].
Approximately 7% of the general population is thought to suffer from neuropathic pain. Among the several kinds of pain disorders, neuropathic pain is generally severe and has a long disease duration of years, resulting in a significant deterioration in health-related quality of life (HRQOL) [2,3]. Neuromusculoskeletal pain usually impairs exercise habits and activities of daily living (ADLs). Furthermore, although the aging of the population is accelerating all over the world, including in Japan, the prevalence of neuropathic pain among the middle-aged and the elderly is as high as 14% [4]. In Japan, where a super-aged society continues to grow, the treatment of neuromusculoskeletal pain is important to prevent and improve physical frailty and the locomotive syndrome in the middle-aged and elderly population [5,6].
Among the treatments for neuropathic pain, pharmacotherapy is the most confirmed evidence-based treatment strategy. In several developed countries including Japan, several lines of pharmacotherapy guidelines for neuropathic pain have been suggested, including gabapentinoids, anti-depressants and opioid analgesics [7,8] (Figure 1). Based on these guidelines and recommendations, an optimized pharmacotherapeutic treatment strategy for neuropathic pain is expected to improve treatment outcomes. Non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, which do not indicate any effective evidence of neuropathic pain and hence are not recommended in any treatment guidelines for neuropathic pain, have still been mainly prescribed in primary care practices [9]. Neuronal inflammation seems to hardly contribute to the development and maintenance of neuropathic pain, and therefore NSAIDs are ineffective. Although the definite analgesic mechanism(s) of acetaminophen does not still be delineated, there is not any evidence that acetaminophen can modulate VGCC and suppress pain transmission. Opioid analgesics consistently demonstrate a practical usefulness in treating neuropathic pain; however, in some cases, the long-term use of opioid analgesics is practically required. Long-term use of opioid analgesics might contribute to developing metal dependence, with an excessive increase in opioid doses. Therefore, opioid analgesics are usually positioned as the third (or even the forth)-line drug in neuropathic pain pharmacotherapy guidelines [7,8].
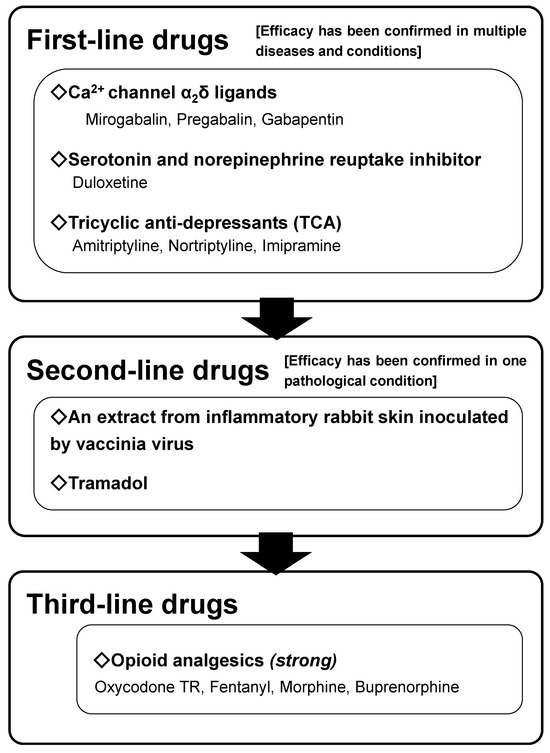
Figure 1.
The neuropathic pain pharmacotherapy algorithm of the Japanese Society of Pain Clinicians from 2019 onward. This algorithm is applicable for non-cancer neuropathic pain. A novel calcium channel α2δ ligand, mirogabalin, is added to the first-line drugs based on the evidence of its efficacy. (Reproduced from [8], Springer Nature, 2018).
An optimal pharmacotherapy treatment strategy for neuropathic pain, which could be applicable worldwide, should be disseminated expeditiously [4]. We summarized the present clinical situations surrounding neuropathic pain, reviewed the analgesic potency and safety of microgabalin, a novel calcium channel α2δ ligand, and finally suggested an expert opinion of the clinical usefulness and positioning of mirogabalin in the pharmacotherapy treatment strategy for neuropathic pain.
2. Methods
This was a narrative review, not a systematic review, conducted after an extensive and thorough review of the available literature on the topics from a variety of sources. We did not set any inclusion/exclusion criteria of the literature, time frames for the literature search, or conduct data synthesis. The topics of this narrative review were as follows: First, we focused on the clinical significance of neuropathic pain as a healthcare burden. Second, we reassured the present pharmacotherapy treatment strategy for neuropathic pain in Japan. And finally, we conducted a narrative review of the recent evidence from clinical trials, in which mirogabalin has the significant analgesic potency for varied types of neuropathic pain conditions. Further, this review highlighted specific advantages of mirogabalin over other calcium channel ligands. We conclusively assembled the topics and suggested our expert opinion on an optimal pharmacotherapy treatment strategy for neuropathic pain, which could be applicable worldwide.
3. The Clinical Significance of Neuropathic Pain as a Healthcare Burden
Focusing on the rapidly growing super-aged society worldwide, the prevalence of neuropathic pain is over 25% in patients with the locomotive syndrome [10]. Moreover, neuropathic pain conditions of neuromusculoskeletal disorders are regarded as one of the inhibitory factors of gait stability in the elderly [10,11]. Because a super-aged society is growing in Japan before the rest of the world, lessons from Japanese clinical situations surrounding neuropathic pain as a healthcare burden would be valuable for the prevention and improvement of physical frailty and the locomotive syndrome in the middle-aged and elderly population [5,6]. Furthermore, neuromusculoskeletal disorders, including physical frailty, the locomotive syndrome, neuromusculoskeletal pain, and the accompanying psychological distress known as decreased self-efficacy (self-confidence in one’s own body), can lead to decreased motivation and a depressed mood for exercise habits and maintaining ADL. Decreased self-efficacy caused maladaptive effects on outcomes with pharmacotherapy in neuropathic pain patients [12]. Psychological and psychiatric conditions such as depression, anxiety, sleep disorder and chronic pain including neuropathic pain are highly comorbid [13], and such conditions are significantly associated with pronounced changes in behavior, decreased activity, social withdrawal and avoiding behavior [14]. Further, sleep disorders, which are psychological and psychiatric conditions, and pain impair reciprocally, as shown in longitudinal studies [15]. These can result in excessive and unnecessary resting and actualized avoidance behaviors for exercise habits and ADL to avoid inducing and/or exaggerating the pain. Consequently, disuse associated with neuromusculoskeletal disorders and pain worsen. Thus, a vicious loop is established in patients with neuromusculoskeletal disorders and pain, resulting in a decline in HRQOL (Figure 2) [16].
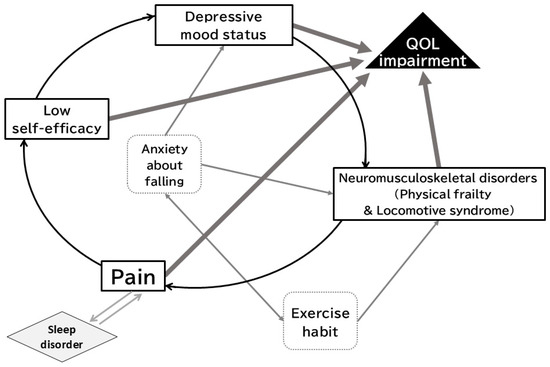
Figure 2.
A vicious cycle model of chronic pain with several factors, leading to impairment of quality of life. Neuromusculoskeletal disorders, pain, and psychological factors form a vicious cycle. Exercise habits contribute to the prevention and improvement of neuromusculoskeletal disorders and anxiety related to falls. (Reproduced from [16], Japan Medical Association, 2019).
Most patients with neuropathic pain have symptoms other than pain, such as sleep disorders, daytime sleepiness, decreased motivation, depressed mood, anxiety, and loss of appetite [17]. Among patients with chronic pain visiting Japanese interdisciplinary pain centers in tertiary medical districts, we revealed that neuropathic pain, psychological distress related to pain, sleep disorders, and severe pain intensity contribute to particularly impaired physical HRQOL. Generally speaking, in clinical practice, active screening for neuropathic pain and its management are important for improving HRQOL and preventing its decline.
4. Pharmacotherapy for Neuropathic Pain in Japan
The pharmacotherapy guidelines of the Japan Society of Pain Clinicians determine the level of recommendation based on clinical usefulness (balancing effectiveness and safety) and the number of neuropathic pain diseases for which such evidence has been obtained [8]. Basic appraisals and concepts of the Japanese guidelines, based on several lines of evidence of pharmacotherapy treatment strategies, resemble the recommendations by the special interest group on neuropathic pain (NeuPSIG) of the International Association for the Study of Pain [7]. Neuropathic pain can develop and be maintained by various etiologies (i.e., diseases and lesions) of the peripheral and central somatosensory nervous systems. Even if the etiologies differ, symptoms such as spontaneous pain, allodynia, and hyperalgesia generally emerge as phenotypes of hypersensitivity and sustained excitement of the somatosensory nervous system. Such molecular mechanisms of neuropathic pain are commonly observed among various etiologies in various animal studies. Based on these notions, if evidence of analgesic application is given for multiple (at least two or more) etiologies of neuropathic pain, it can be expected to be effective against neuropathic pain caused by other etiologies. In clinical terms, the majority of high-quality randomized controlled trials (Phase III clinical trials) conducted worldwide, in conformity with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, have targeted diabetic polyneuropathy (DPN) and post-herpetic neuralgia (PHN). Since these conditions are relatively common in the general population, Phase III clinical trials can gather a certain number of relatively homogeneous DPN or PHN participants and ensure acceptable feasibility. In clinical practice, pharmacotherapy is required for neuropathic pain conditions other than DPN and PHN. However, neuropathic pain conditions are highly variable in general, and their prevalence rates are limited. Hence, Phase III pharmacological trials are not feasible. Therefore, the European Medicines Agency of the European Union has adopted this rule for the regulation and approval of neuropathic pain medicines in the health insurance system. This concept has been officially adopted by the Japanese Ministry of Health, Labor, and Welfare. In concrete terms, when the Phase III RCTs of one pharmacotherapy have been successfully demonstrated as useful for two or more peripheral neuropathic pain diseases (e.g., DPN and PHN), their recommended use is expanded from the respective diseases toward any type of peripheral neuropathic pain diseases. Therefore, all peripheral neuropathic pain conditions, such as chemotherapy-induced peripheral neuropathy, cauda equina syndrome caused by lumbar spinal canal stenosis, and traumatic nerve injury, are covered by health insurance. Furthermore, pharmacotherapy successfully demonstrates its application in one or more central neuropathic pain disease [for example, post-spinal cord injury pain (SCI pain) and post-stroke central pain], and the indication is expanded from peripheral neuropathic pain to overall “neuropathic pain,” which consists of all types of both peripheral and central neuropathic pain conditions. In such cases, health insurance coverage is approved for all neuropathic pain diseases, regardless of whether they are peripheral or central.
From another perspective on pharmacotherapy for neuropathic pain in cancer patients (for example, chemotherapy-induced peripheral neuropathy (CIPN)), opioid analgesics are recommended as the third-line drug [7,8]. However, in Japan, the use of opioid analgesics for cancer-related pain (i.e., pain caused by cancer itself in patients with advanced cancer condition and pain caused by cancer treatment like CIPN) is far below the World Health Organization (WHO)-defined standards [18,19]. A third-line recommendation unfortunately would not be provided as a workable system even in cancer patients. The exaggerated fear of the risks associated with opioid analgesics (recently called opiophobia) has been rampant. Such inappropriate avoidance of opioid use, which potentially reflects opiophobia, is recognized as a global health issue and has been called the second opioid crisis as patients suffering from pain lose the opportunity to realize appropriate pain relief [20]. In the WHO guidelines updated in 2018, either anticonvulsants or anti-depressants were labeled as “no recommendation for or against” for cancer-related neuropathic pain, including CIPN, due to a lack of sufficient evidence [21]. Essentially, the purpose of guidelines is to assist practitioners and patients in making decisions regarding appropriate healthcare for specific clinical circumstances and to optimize the quality of patient care. However, in such situations in Japan, undertreatment of cancer-related neuropathic with potentially appropriate pharmacotherapy might be concerned.
5. Mirogabalin: A Novel Calcium Channel α2δ Ligand
Mirogabalin, a potent selective ligand of the α2δ subunit of voltage-gated calcium channels, has been approved in Japan for the treatment of neuropathic pain. Mirogabalin was developed in Japan. The chemical name of mirogabalin is [(1R,5S,6S)-6-(Aminomethyl)-3-ethylbicyclo [3.2.0]hept-3-en-6-yl]acetic acid monobenzenesulfonate. Mirogabalin demonstrates a different chemical structure from those of pregabalin and gabapentin, and residues in the hydrophobic interaction region, in addition to several amino-binding motif residues around the amino and carboxyl groups of mirogabalin, are critical for mirogabalin binding to the α2δ subunit [22].
Mirogabalin is quickly absorbed, with a mean time to maximum plasma concentration (Tmax) of one hour after both single and multiple ascending doses; the peak plasma concentration and concentration–time curve increase in a dose-dependent manner. The mean half-life ranged from 2.96 to 3.37 h in a single ascending dose or from 3.58 to 4.55 h in multiple ascending doses, in subjects with normal renal function [23]. Phase III mirogabalin trials were conducted in Asia, including in Japan, initially for the treatment of DPN and PHN. In addition to its safety and analgesic efficacy in the management of peripheral neuropathic pain diseases, it is also safe and effective for SCI pain, which is a central neuropathic pain condition. All of these Phase III studies were randomized, double-blind, and placebo-controlled. Participants took medicines for three months, and the primary endpoint was the difference in an 11-point numerical rating scale of average pain intensity at the cessation of medication between mirogabalin and placebo [24,25,26]. Therefore, it has been approved as a first-line drug in the pharmacotherapy guidelines for neuropathic pain by the Japanese Society of Pain Clinicians (Figure 1). Moreover, mirogabalin has been approved for both central and peripheral neuropathic pain in the health insurance system [24,25,26]. Its clinical use has expanded rapidly since its marketing approval in Japan [27], and in January 2024, it was approved for marketing in Korea and Thailand in addition to Japan.
Mirogabalin is administered at a dose of 5 mg twice a day, starting with a daily dose of 10 mg, gradually increasing to 20 mg/day, and reaching a maximum dose of 30 mg/day every week for patients with normal renal function. Similar to other calcium channel α2δ ligands, dose reduction is required for patients suffering renal impairment. Patients with chronic pain generally demonstrate low HRQOL; therefore, improving HRQOL is important in addition to pain control when setting treatment goals. In general, a 30% improvement in pain severity is expected to improve HRQOL [28]. In addition to the assessment of pain intensity as a metric, the proportion of patients who demonstrate a ≥30% pain reduction is considered an alternative indicator of the efficacy of the analgesics. The proportion of patients with 30% pain reduction with mirogabalin was slightly less than half of the patients at the dosages of 20 mg/day and 30 mg/day [25], suggesting that mirogabalin is effective in improving HRQOL in patients with neuropathic pain. Further, the percentage of patients with 50% or more pain reduction is clinically considered to have a significantly potent efficacy. This critical outcome was demonstrated in 25–30% of patients receiving 20 or 30 mg/day of mirogabalin. These responder proportions were almost comparable to other first-line drugs for neuropathic pain, although there were no head-to-head study data of mirogabalin and other analgesics [29]. Therefore, mirogabalin can be recognized as a drug that reliably provides analgesic effects against neuropathic pain, although neuropathic pain is generally considered resistant to several kinds of treatments. At moderate-to-high doses, mirogabalin has an additional effect on improving sleep disorders caused by neuropathic pain; however, adverse effects on daily sleepiness can also occur. In Phase III RCTs, only a few patients complained of moderate sleepiness, but most adverse symptoms were mild. The safety of mirogabalin for long-term use was confirmed in two observational studies for both peripheral and central neuropathic conditions [26,30]. In a study evaluating the safety of calcium channel α2δ ligands, average doses of pregabalin (approved maximum doses = 600 mg/day in Japan) were 134.2 +/− 77.9 mg/day in patients with almost normal renal function (i.e., creatinine clearance of 60 mL/min or higher). This indicates that most patients could not tolerate a gradual increase in pregabalin to analgesic doses. In contrast, many patients complained of mild drowsiness after switching from pregabalin to mirogabalin (approved maximum dose = 30 mg/day); however, more than half of the patients were able to receive mirogabalin at a dose of 30 mg/day with a gradual increase. Finally, mirogabalin demonstrated a more potent analgesic effect than pregabalin did [31]. In our clinical experience, we have the impression that patients with neuropathic pain, including the elderly and/or those with renal impairment, sometimes experience relatively mild sleepiness but can adhere to a gradual dose increase in mirogabalin toward moderate to maximum doses. Calcium channel α2δ subunits in the nervous system consist of α2δ-1 and α2δ-2 subunits. The α2δ-1 subunit is mainly involved in an analgesic effect, and the α2δ-2 subunit mainly contributes to the adverse effect on the central nervous system (somnolence, dizziness, etc.) [32,33]. In an in vivo study, mirogabalin and pregabalin were found to have similar binding selectivity to α2δ-1 and α2δ-2 subunits; however, mirogabalin was found to dissociate from the α2δ-2 subunit relatively quickly. In contrast, mirogabalin kept binding to the α2δ-1 subunit for a relatively longer time [34]. Such a binding property of mirogabalin would enable more patients to demonstrate better tolerability than pregabalin. And consequently, behavioral assessments demonstrated that mirogabalin had more potent and longer-lasting analgesic effects and reduced sleepiness. To our knowledge, there were no precise data about a difference in binding properties for the α2δ-1 and α2δ-2 subunits between mirogabalin and gabapentin.
All calcium channel α2δ ligands have been confirmed to have their analgesic effect in a dose-dependent manner, and therefore it is necessary to increase the dose while balancing the tolerability of adverse effects. Mirogabalin was found to show relatively high tolerability to sleepiness in the majority of patients with neuropathic pain, including those with renal impairment, and could be gradually increased toward effective analgesic doses. Consequently, mirogabalin can achieve clinically meaningful pain relief [35]. In another view of the properties of calcium channel α2δ ligands, pregabalin was not found to have an analgesic effect compared to the placebo for neuropathic pain caused by degenerative lumbar spine diseases, which are common in the elderly [36]. However, in a two-group comparison of NSAIDs alone or a combination of NSAIDs and mirogabalin for patients with lumbar spinal canal stenosis, mirogabalin showed a significant analgesic effect in the group receiving mirogabalin [37].
Neuropathic pain has been considered to have different responses to treatments depending on the characteristics of the pain, and these characteristics are thought to reflect phenotypes of different molecular biological mechanisms [38,39]. In addition to demonstrating significant analgesic effects on spinal cord injury (SCI) pain intensity compared to placebo, mirogabalin also demonstrated significant analgesic effects on several pain characteristics, such as spontaneous pain, paroxysmal pain attacks, evoked pain, paresthesia, and dysesthesia [26]. Therefore, it is plausible that mirogabalin is effective against most molecular mechanisms that cause neuropathic pain.
Among middle-aged and elderly individuals, accidental falls are the most common cause of SCI [40]. Cervical spine decompression surgery during the acute period after injury could accelerate the recovery of motor function; however, its effectiveness for neuropathic pain after SCI has not been observed [41]. Therefore, for the global aging society, mirogabalin is expected to play an important role in the treatment of neuropathic pain.
6. Expert Opinion
Neuropathic pain is generally severe and has a long disease duration of years, resulting in a significant deterioration in health-related quality of life (HRQOL). The prevalence of neuropathic pain is increasing accordance with the global situation where the aging of the population is accelerating. The aim of this review was to highlight how mirogabalin, a novel calcium channel α2δ ligand, is effective for various types of neuropathic pain diseases in the super-aged society.
Mirogabalin successfully demonstrated analgesic efficacy for both peripheral and central neuropathic pain diseases. Different pain characteristics in clinical practice have been considered to be derived from different molecular pathophysiological mechanisms. The analgesic potency of mirogabalin could cover all types of neuropathic pain characteristics, suggesting that mirogabalin is effective against most molecular mechanisms that cause neuropathic pain. Sleep disorder and pain impair reciprocally. Mirogabalin could consistently improve sleep disorder caused by neuropathic pain. This would contribute to the improvement of HRQOL. Another example of the clinical usefulness of mirogabalin is balancing its analgesic potency and achieving relatively less sleepiness. Daytime sleepiness occasionally encompasses accidental falls. Anxiety about falling is specified as the accelerator of decreased motivation and depressed mood for exercise habits and maintaining ADL, which keeps the vicious loop running (Figure 2). Further, accidental falls are the most common cause of fractures and cervical SCI, especially in middle-aged and elderly individuals, both of which make the injured individuals become bedridden and increase mortality. Considering these, minimizing daytime sleepiness by pharmacotherapies is required for the global aging society and mirogabalin can function as an example of balancing the analgesic potency and demand for relatively less sleepiness. Compared to pregabalin, the proportion of neuropathic pain patients who could increase their daily doses of mirogabalin until the desired analgesic dose were larger. And changing from pregabalin to mirogablin could attain a further improvement in neuropathic pain [25].
These findings should suggest that mirogabalin is recommended as the realistic first-line drug for neuropathic pain with all etiologies in today’s global-scale super-aged society. The availability of mirogabalin in the world is still limited, and it would be desired that mirogabalin spreads across the entire globe.
Mirogabalin demonstrated its efficacy against CIPN; in particular, severe CIPN improved more, although this was an observational study [42]. For patients with cancer, appropriate prescription of opioid analgesics and complementing the present difficult “opiophobia” situation with non-opioid analgesics are crucial where cancer-related pain is not adequately controlled. It is necessary to develop treatment strategies that protect patients from long-term opioid use (Table 1) [43,44]. In this connection, mirogabalin would be suitable for all types of cancer-related neuropathic pain, but especially for the long-term pain sequelae of cancer treatments like CIPN in cancer survivors.

Table 1.
Expert opinion on the rationale use of opioid analgesics and non-opioid analgesics to address cancer-related pain according to cancer status. Cancer-related pain impairs patients with cancer and potentially affects adherence to cancer treatments such as chemotherapy. According to the cancer status, pharmacotherapy should be realized in view of adequate pain control by either one or both opioid and non-opioid analgesics and in view of the protection of patients from long-term opioid use. (Reproduced with permission from [43], John Wiley and Sons, 2020).
7. Conclusions
Neuropathic pain pharmacotherapy, such as with mirogabalin, is generally expected to be useful for treating neuropathic pain associated with cancer and cancer treatment, as well as non-cancer etiologies worldwide, where the elderly population continues to grow. Future studies, such as with global randomized controlled trials and validation studies on long-term safety, are required, but these could further highlight mirogabalin’s potential to reduce opioid reliance in diverse settings all over the world.
Author Contributions
Conceptualization: M.S. (Mizuho Sumitani) and M.S. (Masahiko Sumitani); Methodology: M.S. (Masahiko Sumitani); Writing—original draft preparation: M.S. (Mizuho Sumitani), T.K., R.T. and R.I.; Writing—review and editing: all authors; Visualization; R.I., H.A. and M.S. (Masahiko Sumitani); supervision: H.A. and M.S. (Masahiko Sumitani); Funding acquisition; M.S. (Masahiko Sumitani). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Masahiko Sumitani received funding from Shionogi & Co., Ltd., Nippon Zoki Pharmaceutical, Heartfelt Inc., Nipro Corporation, Eisai Co., Ltd., Freasu Co., Ltd., Pfizer Inc., Aiwa Hospital, and Yoshida Hospital, and received payments from Daiichi-Sankyo, Shionogi & Co., Ltd., Nippon Zoki Pharmaceutical, Freasu Co., Ltd., and GlaxoSmithKline K.K. Hiroaki Abe belongs to a collaborative research department of Shionogi & Co., Ltd., Nippon Zoki Pharmaceutical, Aiwa Hospital and Yoshida Hospital, and received payments from Daiichi-Sankyo. The funders/compamies (Shionogi & Co. Ltd., Nippon Zoki Pharmaceutical, Heartfelt Inc., Nipro Corporation, Eisai Co. Ltd., Freasu Co. Ltd., Pfizer Inc., Aiwa Hospital, Yoshida Hospital, Daiichi-Sankyo, GlaxoSmithKline K.K.) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bauer, C.S.; Rahman, W.; Tran-Van-Minh, A.; Lujan, R.; Dickerson, A.H.; Dolphin, A.C. The anti-allodynic alpha-2-delta ligand, pregabalin inhibits the trafficking of the calcium channel alpha-2-delta subunit to presynaptic terminals in vivo. Biochem. Soc. Trans. 2010, 38, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Lantéri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S. Pathophysiology of pain: From theory to clinical evidence. Eur. J. Pain 2008, 2, S13–S17. [Google Scholar] [CrossRef]
- Meisinger, C.; Bongaerts, B.W.C.; Heier, M.; Amann, U.; Kowall, B.; Herder, C.; Ruckert-Eheberg, I.M.; Rathmann, W.; Ziegler, D. Neuropathic pain is not adequately treated in the older general population: Results from the KORA F4 survey. Pharmacoepidemiol. Drug Saf. 2018, 27, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.; Sayer, A.A. Sarcopenia and frailty: New challenges for clinical practice. Clin. Med. 2015, 15 (Suppl. S6), s88–s891. [Google Scholar] [CrossRef]
- Nakamura, K.; Ogata, T. Locomotive syndrome: Definition and management. Clin. Rev. Bone Miner. Metab. 2016, 14, 56–67. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Sumitani, M.; Sakai, T.; Matsuda, Y.; Abe, H.; Yamaguchi, S.; Hosokawa, T.; Fukui, S. Executive summary of the Clinical Guidelines of Pharmacotherapy for Neuropathic Pain: Second edition by the Japanese Society of Pain Clinicians. J. Anesth. 2018, 32, 463–478. [Google Scholar] [CrossRef]
- Martinez, V.; Attal, N.; Vanzo, B.; Vicaut, E.; Gautier, J.M.; Bouhassira, D.; Lanteri-Minet, M. Adherence of French GPs to chronic neuropathic pain clinical guidelines: Results of a cross-sectional, randomized, “e” case-vignette survey. PLoS ONE 2014, 9, e93855. [Google Scholar] [CrossRef]
- Imagama, S.; Hasegawa, Y.; Ando, K.; Kobayashi, K.; Hida, T.; Ito, K.; Tsushima, M.; Nishida, Y.; Ishiguro, N. Staged decrease of physical ability on the locomotive syndrome risk test is related to neuropathic pain, nociceptive pain, shoulder complaints, and quality of life in middle-aged and elderly people—The utility of the locomotive syndrome risk test. Mod. Rheumatol. 2017, 27, 1051–1056. [Google Scholar] [CrossRef]
- Hamacher, D.; Liebl, D.; Hödl, C.; HeBler, V.; Kniewasser, C.K.; Thonnessen, T.; Zech, A. Gait stability and its influencing factors in older adults. Front. Physiol. 2018, 9, 1955. [Google Scholar] [CrossRef]
- Toth, C.; Brady, S.; Hatfield, M. The importance of catastrophizing for successful pharmacological treatment of peripheral neuropathic pain. J. Pain Res. 2014, 7, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef]
- Bras, M.; Dordevic, V.; Gregurek, R.; Blajic, M. Neurobiological and clinical relationship between psychiatric disorders and chronic pain. Psychiatr. Danub. 2010, 22, 221–226. [Google Scholar] [PubMed]
- Andersen, M.L.; Araujo, P.; Frange, C.; Tufik, S. Sleep disturbance and pain—A tale of two common problems. Chest 2018, 154, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Ushio, M.; Sumitani, M.; Abe, H.; Mietani, K.; Hozumi, J.; Inoue, R.; Tsuchida, R.; Ushida, T.; Yamada, Y. Characteristics of locomotive syndrome in Japanese patients with chronic pain and results of a path analysis confirming the relevance of a vicious cycle involving locomotive syndrome, musculoskeletal pain and its psychological factors. JMA J. 2019, 2, 184–189. [Google Scholar] [CrossRef]
- Meyer-Rosberg, K.; Kvarnström, A.; Kinnman, E.; Gordh, T.; Nordfors, L.O.; Kristofferson, A. Peripheral neuropathic pain—A multidimensional burden for patients. Eur. J. Pain 2001, 5, 379–389. [Google Scholar] [CrossRef]
- Azuma, K.; Abe, H.; Hozumi, J.; Inoue, R.; Konishi, M.; Tsuchida, R.; Ando, M.; Saita, K.; Sumitani, M. Prefectural adequacy of opioid availability for cancer pain and its determinants in Japan: A preliminary study. JMA J. 2020, 3, 340–346. [Google Scholar]
- Hasegawa-Moriyama, M.; Morioka, Y.; Hiroi, S.; Naya, N.; Suzuki, Y.; Koretaka, Y.; Hara, E.; Abe, H.; Uchida, K.; Sumitani, M. High prevalence of severe pain is associated with low opioid availability in patients with advanced cancer: Combinated database study and nationwide questionnaire survey in Japan. Neuropsychopharmacol. Rep. 2024, 44, 502–511. [Google Scholar] [CrossRef]
- Marchetti Calônego, M.A.; Sikandar, S.; Ferris, F.D.; de Barros, G.A.M. Spread the Word: There are two opioid crises! Drugs 2020, 80, 1147–1154. [Google Scholar] [CrossRef]
- World Health Organization. WHO Health Organization Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organization: Geneve, Switzerland, 2018. [Google Scholar]
- Kozai, D.; Numoto, N.; Nishikawa, K.; Kamegawa, A.; Kawasaki, S.; Hiroaki, Y.; Irie, K.; Oshima, A.; Hanzawa, H.; Shimada, K.; et al. Recognition mechanism of a novel gabapentinoid drug, mirogabalin, for recombinant human α2δ1, a voltage-gated calcium channels subunit. J. Mol. Biol. 2023, 435, 168049. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Zhang, M.; Yu, S. Mirogabalin as a novel calcium channel alpha-2-delta ligand for the treatment of neuropathic pain: A review of clinical update. Front. Pharmacol. 2024, 15, 1491570. [Google Scholar] [CrossRef]
- Baba, M.; Matsui, N.; Kuroha, M.; Wasaki, Y.; Ohwada, S. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: A randomized, double-blind, placebo-controlled phase III study in Asia patients. J. Diabet. Investig. 2019, 10, 1299–1306. [Google Scholar] [CrossRef]
- Kato, J.; Matsui, N.; Kakehi, Y.; Murayama, E.; Ohwada, S.; Sugihara, M. Mirogabalin for the management of postherpetic neuralgia: A randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain 2019, 160, 1175–1185. [Google Scholar] [CrossRef]
- Ushida, T.; Katayama, Y.; Hiasa, Y.; Nishihara, M.; Tajima, F.; Katoh, S.; Tanaka, H.; Maeda, T.; Furusawa, K.; Richardson, M.; et al. Mirogabalin for central neuropathic pain after spinal cord injury: A randomized, double-blind, placebo-controlled, phase 3 study in Asia. Neurology 2023, 100, e1193–e1206. [Google Scholar] [CrossRef] [PubMed]
- Ushida, T.; Yokoyama, M.; Shiosakai, K.; Saito, K.; Ibe, S.; Okuizumi, K. A large-scale database study for the prescription status of a new voltage-gated calcium channel α2δ ligand, mirogabalin, in Japan. Exp. Opin. Pharmacother. 2022, 23, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Young JPJr LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, A.A.; Gehr, N.L.; Finnerup, N.B. A systematic review and meta-analysis of randomized controlled head-to-head trials of recommended drugs for neuropathic pain. Pain Rep. 2024, 982, e1138. [Google Scholar] [CrossRef]
- Kato, J.; Matsui, N.; Kakehi, Y.; Murayama, E.; Ohwada, S. Long-term safety and efficacy of mirogabalin in Asian patients with postherpetic neuralgia. Medicine 2020, 99, e21976. [Google Scholar] [CrossRef]
- Kimura, Y.; Yamaguchi, S.; Suzuki, T.; Kato, J.; Chiba, S.; Hirakawa, N.; Yamaguchi, K.; Tanabe, Y.; Takatsuna, H.; Kenyoshi, Y.; et al. Switching from pregabalin to mirogabalin in patients with peripheral neuropathic pain: A multi-center, prospective, single-arm, open-label study (MIROP study). Pain Ther. 2021, 10, 711–727. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, X.L.; Matthews, E.A.; Li, K.W.; Kurwa, A. Calcium channel α2δ1 subunit mediates spinal hyperexcitability in pain modulation. Pain 2006, 125, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.; Balaguero, N.; Minone, M.; Ackerman, S.L.; Brodbeck, V.A.L.J.; Canti, C.; Meir, A.; Page, K.M.; Kusumi, K.; Perez-Reyes, E.; et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001, 21, 6095–6104. [Google Scholar] [CrossRef]
- Domon, Y.; Arakawa, N.; Inoue, T.; Matsuda, F.; Takahashi, M.; Yamamura, N.; Kai, K.; Kitano, Y. Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels. J. Pharmacol. Exp. Ther. 2018, 365, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Kodama, S.; Shiosakai, K.; Kimura, T. Relationship between the dose titration and adherence of mirogabalin in patients with peripheral neuropathic pain depending on renal function: A nationwide electronic medical record database study. Exp. Opin. Pharmacother. 2023, 24, 267–282. [Google Scholar] [CrossRef]
- Mathieson, S.; Maher, C.G.; McLachlan, A.J.; Latimer, J.; Koes, B.W.; Hancock, M.J.; Harris, I.; Day, R.O.; Billot, L.; Pik, J.; et al. Trial of pregabalin for acute and chronic sciatica. N. Engl. J. Med. 2017, 376, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, T.; Takatsuna, H.; Tabata, S.; Shiosakai, K.; Nakatani, T.; Konno, S. Efficacy and safety of add-on mirogabalin to NSAIDs in lumbar spinal canal stenosis with peripheral neuropathic pain: A randomized, open-label study. Pain Ther. 2022, 11, 1195–1214. [Google Scholar] [CrossRef]
- Attal, N.; Fermanian, C.; Fermanian, J.; Lanteri-Minet, M.; Alchaar, H.; Bouhassira, D. Neuropathic pain: Are there distinct subtypes depending on the aetiology or anatomical lesion? Pain 2008, 138, 343–353. [Google Scholar] [CrossRef]
- Sumitani, M.; Miyauchi, S.; McCabe, C.S.; Shibata, M.; Maeda, L.; Saitoh, Y.; Tashiro, T.; Mashimo, T. Mirror visual feedback alleviates deafferentation pain, depending on qualitative aspects of the pain: A preliminary report. Rheumatology 2008, 47, 1038–1043. [Google Scholar] [CrossRef]
- World Health Organization. International Perspectives on Spinal Cord Injury; World Health Organization: Geneve, Switzerland, 2013. [Google Scholar]
- OSCIS investigators; Chikuda, H.; Koyama, Y.; Matsubayashi, Y.; Ogata, T.; Ohtsu, H.; Sugita, S.; Sumitani, M.; Kadono, Y.; Miura, T.; et al. Effect of early vs. delayed surgical treatment on motor recovery in incomplete cervical spinal cord injury with preexisting cervical stenosis: A randomized clinical trial. JAMA Netw. Open 2021, 4, e2133604. [Google Scholar] [CrossRef]
- Misawa, S.; Denda, T.; Kodama, S.; Suzuki, T.; Naito, Y.; Kogawa, T.; Takada, M.; Suichi, T.; Shiosakai, K.; Kuwabara, S.; et al. Efficacy and safety of mirogabalin for chemotherapy-induced peripheral neuropathy: A prospective single-arm trial (MiroCIP study). BMC Cancer 2023, 23, 1098. [Google Scholar] [CrossRef]
- Sumitani, M.; Nishizawa, D.; Hozumi, J.; Ikeda, K. Genetic implications in quality palliative care and preventing opioid crisis in cancer-related pain management. J. Neurosci. Res. 2022, 100, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Sumitani, M. Time to act for reappraising the educational system for universal access to opioid analgesics, for quality palliative care and cancer-related pain relief in East Asian countries. Lancet Reg. Health West. Pac. 2021, 16, 100270. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).