Recovery from AMPA Receptor Potentiation by Ampakines
Abstract
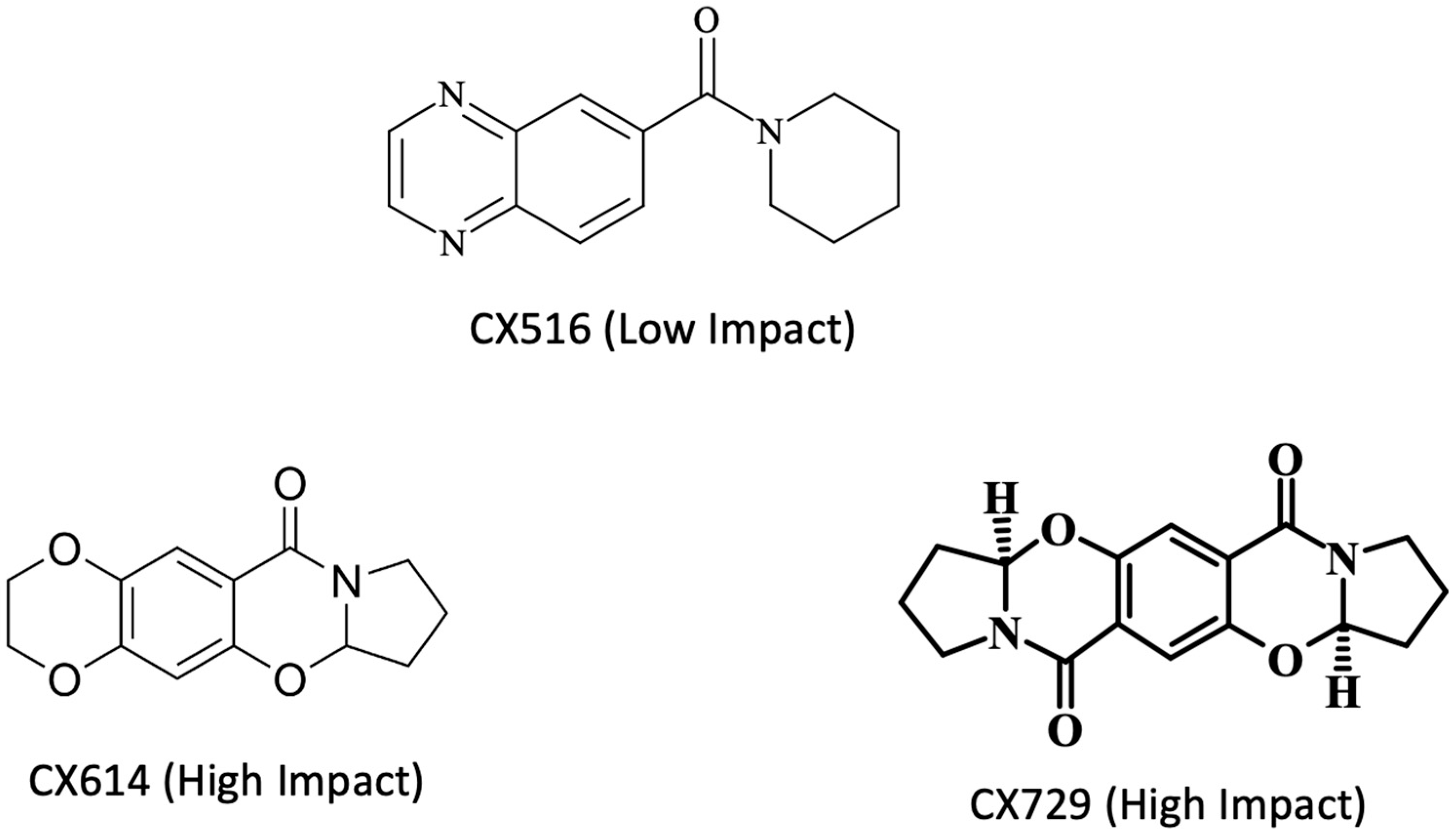
1. Introduction
2. Material and Methods
2.1. Cortical Neuron Cell Culture and Whole Cell Recording
2.2. Data Analysis
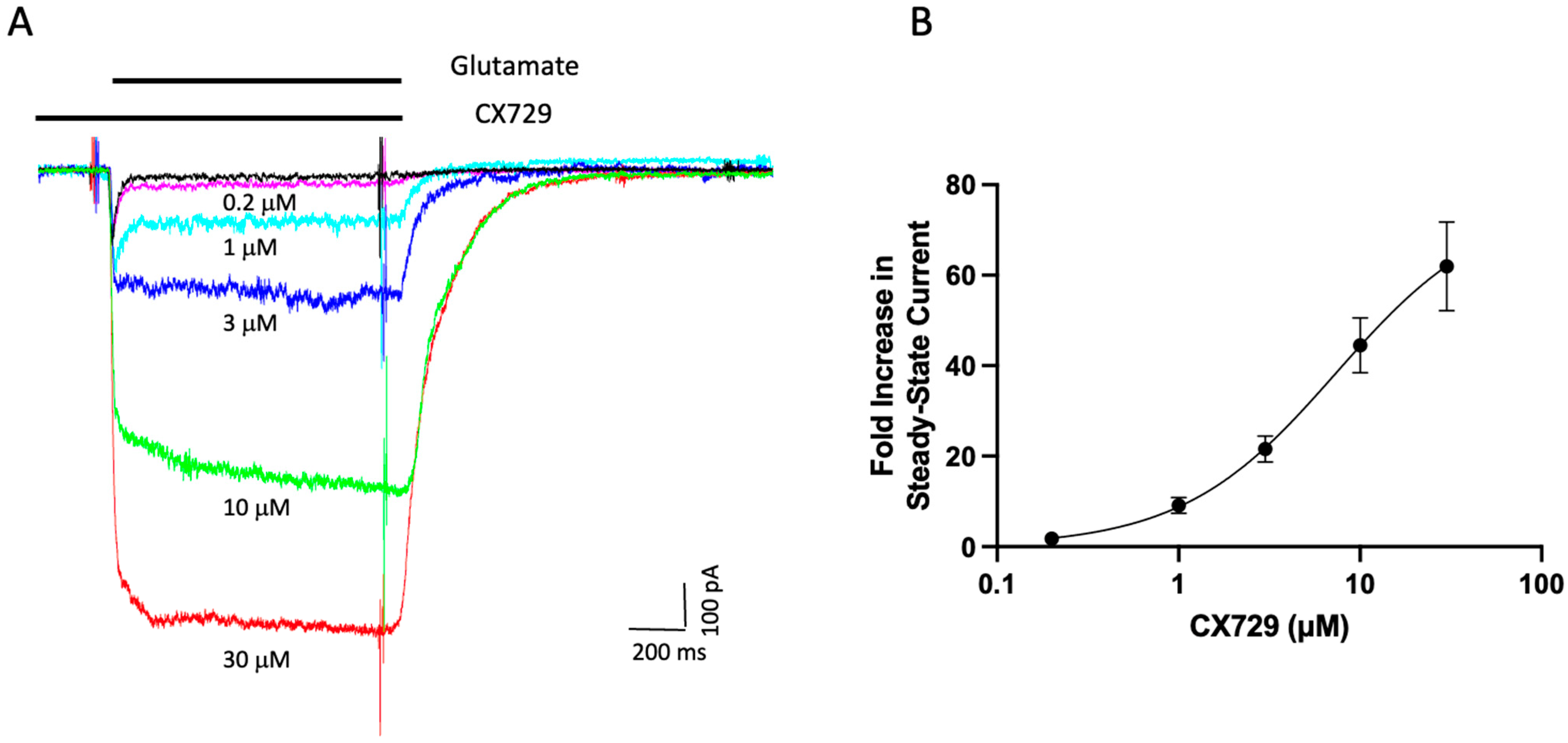
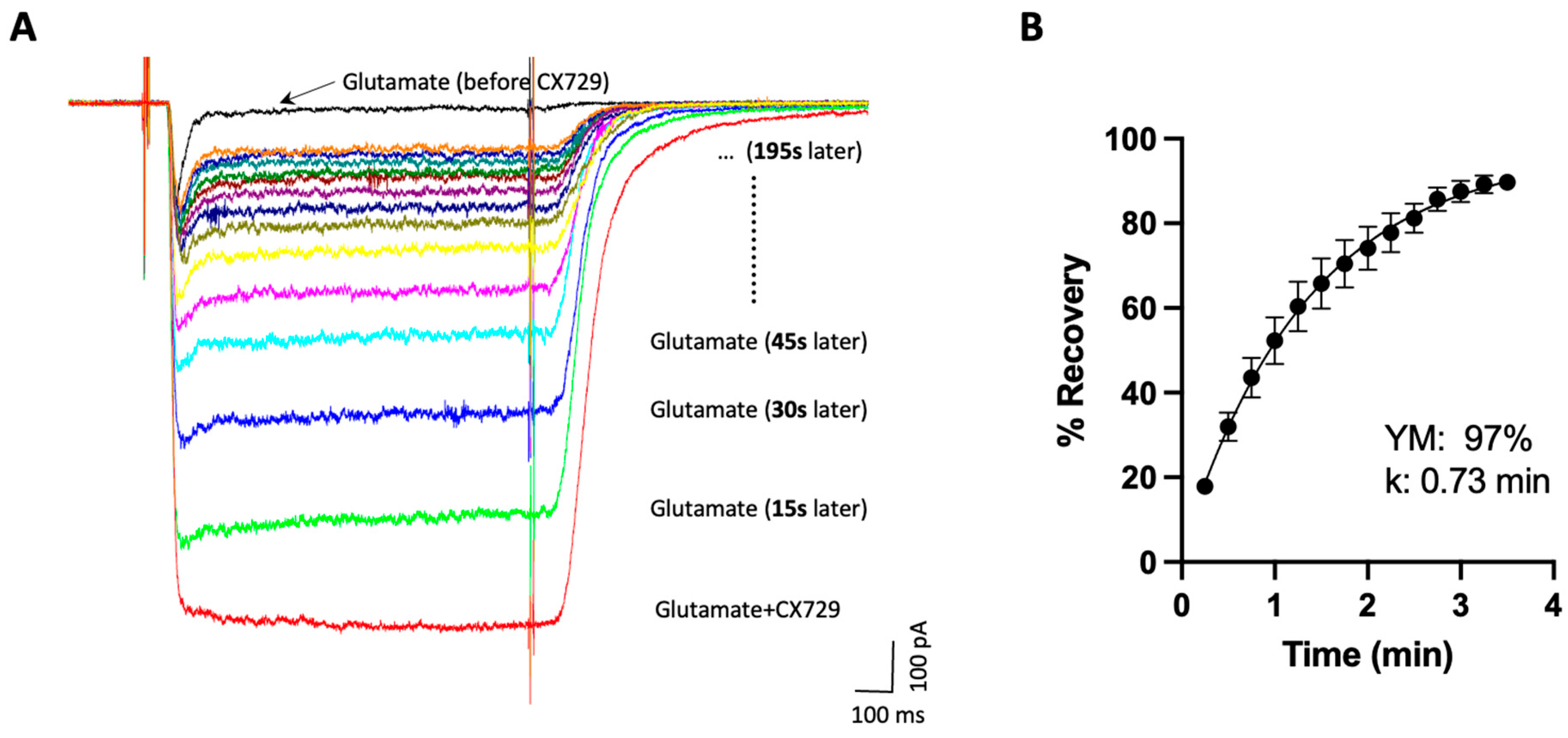
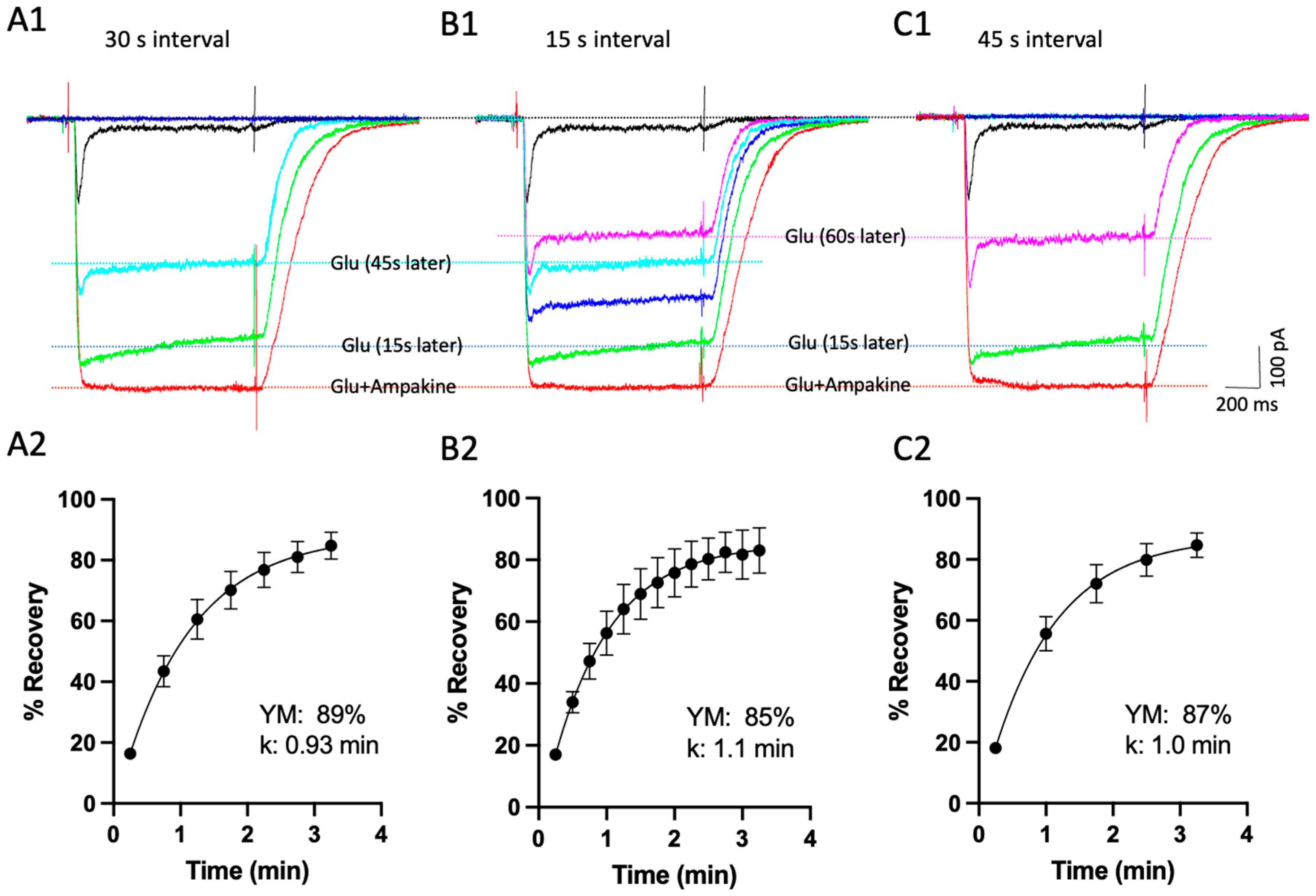
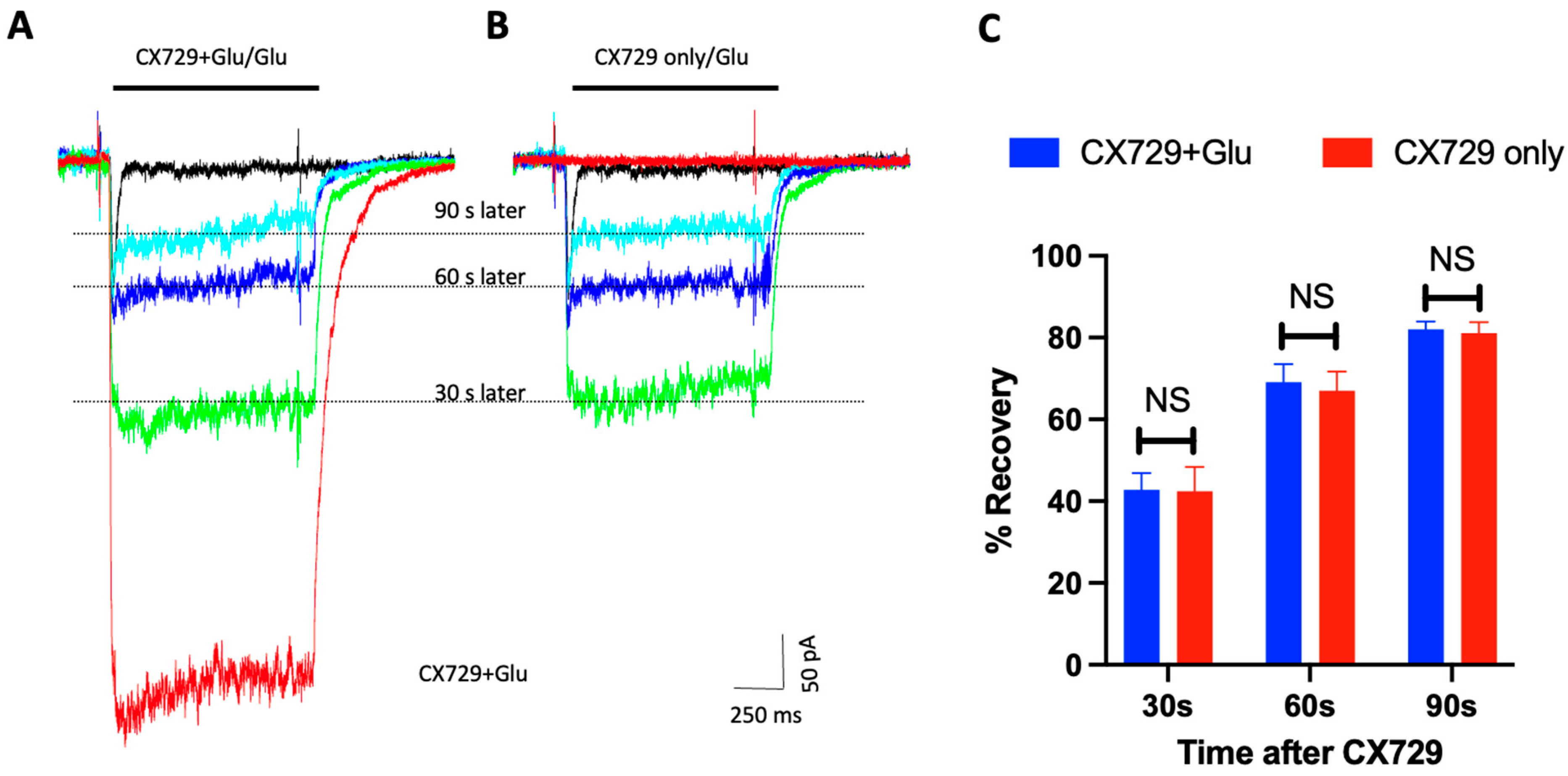
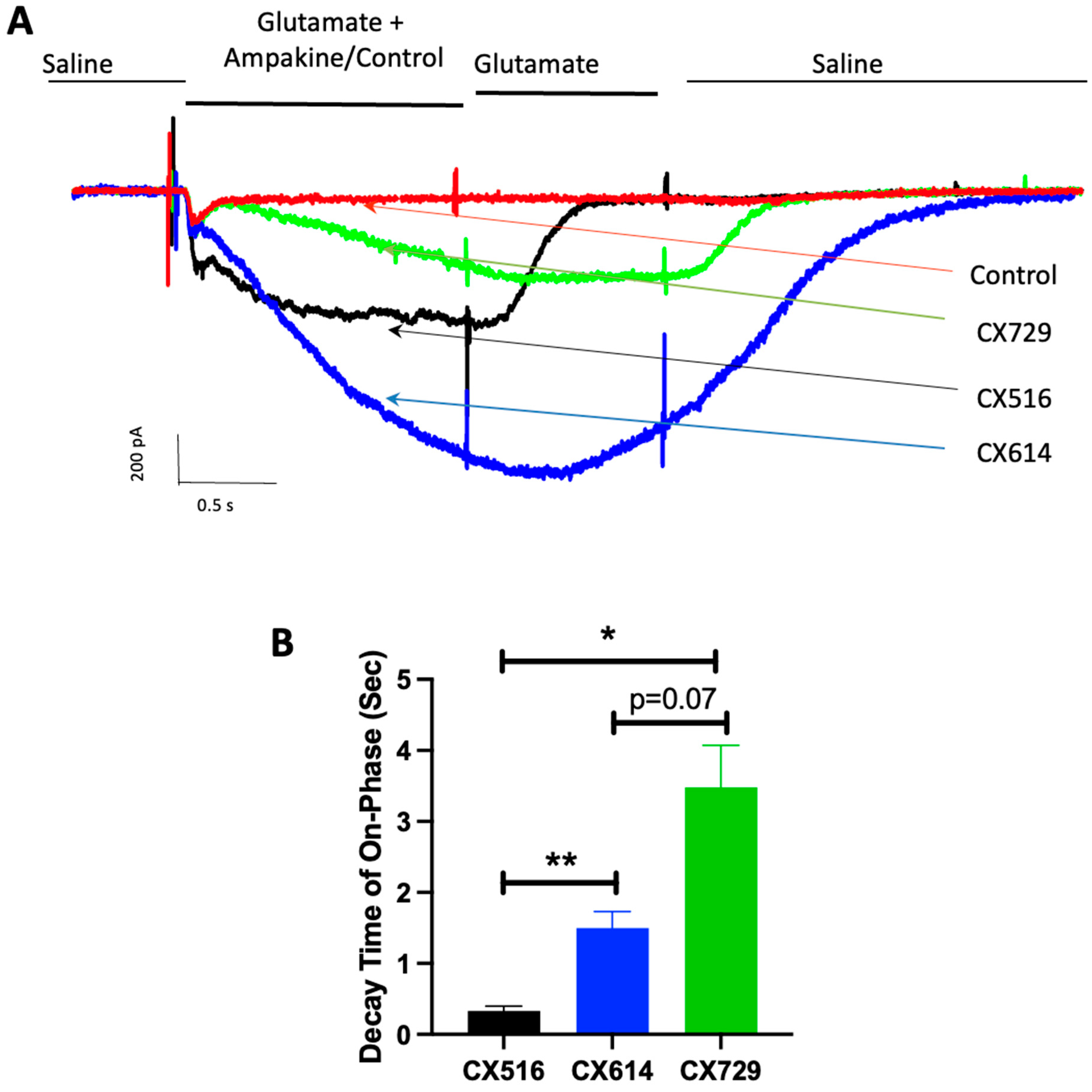
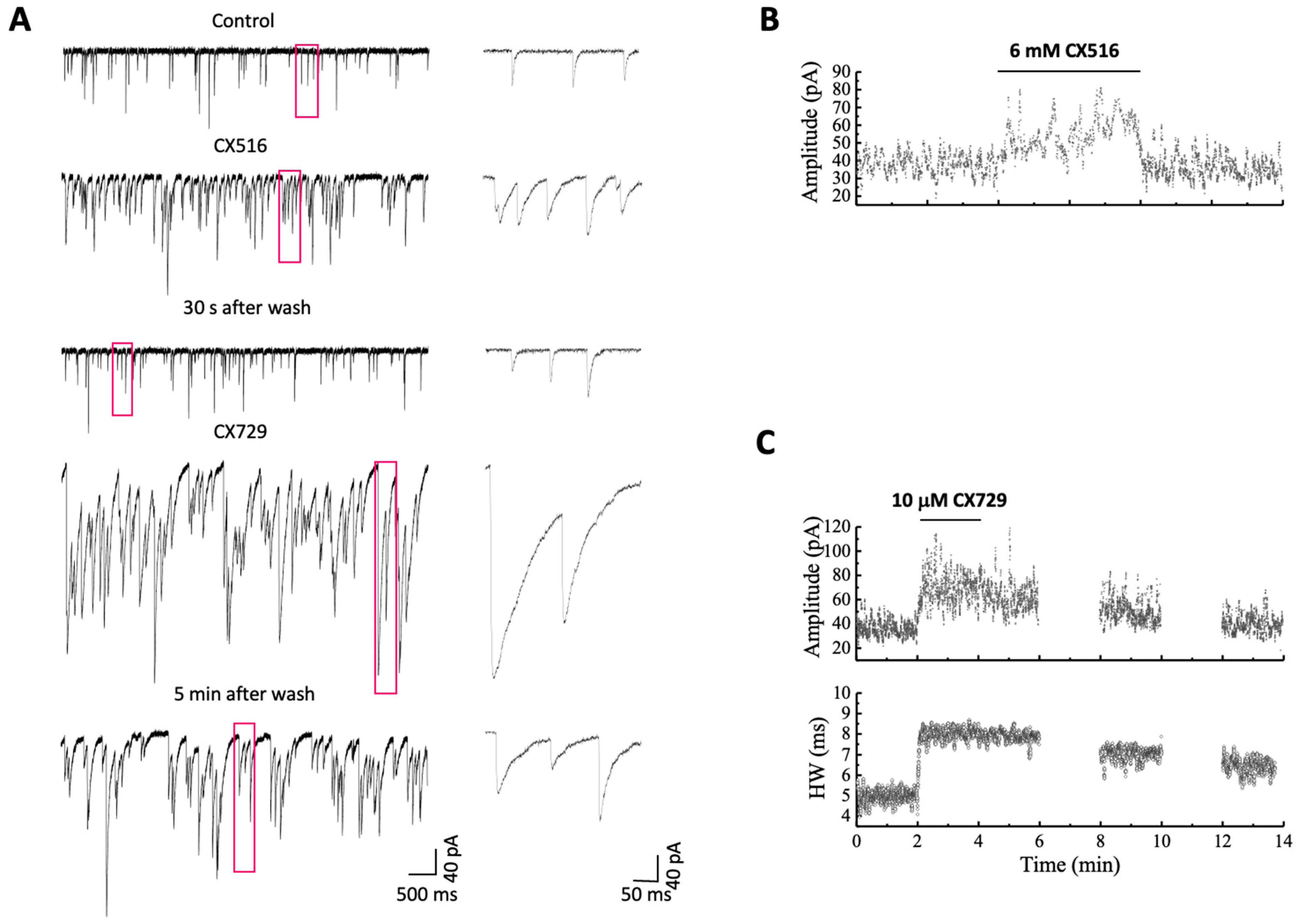
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, M.J.; Murray, T.K.; Whalley, K.; Ward, M.A.; Hicks, C.A.; Woodhouse, S.; Osborne, D.J.; Skolnick, P. Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson’s disease. Eur. J. Pharmacol. 2004, 486, 163–174. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.J.; Murray, T.K.; Clay, M.P.; Lindstrom, T.; Yang, C.R.; Nisenbaum, E.S. LY503430: Pharmacology, pharmacokinetics, and effects in rodent models of Parkinson’s disease. CNS Drug Rev. 2005, 11, 77–96. [Google Scholar] [CrossRef]
- Jourdi, H.; Hamo, L.; Oka, T.; Seegan, A.; Baudry, M. BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology 2009, 56, 876–885. [Google Scholar] [CrossRef]
- Simmons, D.A.; Rex, C.S.; Palmer, L.; Pandyarajan, V.; Fedulov, V.; Gall, C.M.; Lynch, G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc. Natl. Acad. Sci. USA 2009, 106, 4906–4911. [Google Scholar] [CrossRef]
- Simmons, D.A.; Mehta, R.A.; Lauterborn, J.C.; Gall, C.M.; Lynch, G. Brief ampakine treatments slow the progression of Huntington’s disease phenotypes in R6/2 mice. Neurobiol. Dis. 2011, 41, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, N.; Shamsizadeh, A.; Fatemi, I.; Allahtavakoli, M.; Moghadam-Ahmadi, A.; Kaviani, E.; Kaeidi, A. CX691, as an AMPA receptor positive modulator, improves the learning and memory in a rat model of Alzheimer’s disease. Iran. J. Basic. Med. Sci. 2018, 21, 724–730. [Google Scholar] [CrossRef]
- Adler, L.A.; Kroon, R.A.; Stein, M.; Shahid, M.; Tarazi, F.I.; Szegedi, A.; Schipper, J.; Cazorla, P. A translational approach to evaluate the efficacy and safety of the novel AMPA receptor positive allosteric modulator org 26576 in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry 2012, 72, 971–977. [Google Scholar] [CrossRef]
- Knapp, R.J.; Goldenberg, R.; Shuck, C.; Cecil, A.; Watkins, J.; Miller, C.; Crites, G.; Malatynska, E. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur. J. Pharmacol. 2002, 440, 27–35. [Google Scholar] [CrossRef]
- Gordillo-Salas, M.; Pascual-Anton, R.; Ren, J.; Greer, J.; Adell, A. Antidepressant-Like Effects of CX717, a Positive Allosteric Modulator of AMPA Receptors. Mol. Neurobiol. 2020, 57, 3498–3507. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Overman, J.J.; Zhong, S.; Mueller, R.; Lynch, G.; Carmichael, S.T. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J. Neurosci. 2011, 31, 3766–3775. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Parker, K.; Nilsson, M.; Walker, F.R.; Gowing, E.K. Combined ampakine and BDNF treatments enhance poststroke functional recovery in aged mice via AKT-CREB signaling. J. Cereb. Blood Flow. Metab. 2015, 35, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Alom, F.; Martinez, R.C.; Fuller, D.D.; Mickle, A.D. Acute ampakines increase voiding function and coordination in a rat model of SCI. eLife 2024, 12. [Google Scholar] [CrossRef]
- Wollman, L.B.; Streeter, K.A.; Fusco, A.F.; Gonzalez-Rothi, E.J.; Sandhu, M.S.; Greer, J.J.; Fuller, D.D. Ampakines stimulate phrenic motor output after cervical spinal cord injury. Exp. Neurol. 2020, 334, 113465. [Google Scholar] [CrossRef]
- Rana, S.; Sunshine, M.D.; Greer, J.J.; Fuller, D.D. Ampakines Stimulate Diaphragm Activity after Spinal Cord Injury. J. Neurotrauma 2021, 38, 3467–3482. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Fusco, A.F.; Witkin, J.M.; Radin, D.P.; Cerne, R.; Lippa, A.; Fuller, D.D. Pharmacological modulation of respiratory control: Ampakines as a therapeutic strategy. Pharmacol. Ther. 2024, 265, 108744. [Google Scholar] [CrossRef]
- Shaffer, C.L.; Hurst, R.S.; Scialis, R.J.; Osgood, S.M.; Bryce, D.K.; Hoffmann, W.E.; Lazzaro, J.T.; Hanks, A.N.; Lotarski, S.; Weber, M.L.; et al. Positive allosteric modulation of AMPA receptors from efficacy to toxicity: The interspecies exposure-response continuum of the novel potentiator PF-4778574. J. Pharmacol. Exp. Ther. 2013, 347, 212–224. [Google Scholar] [CrossRef]
- Kunugi, A.; Tanaka, M.; Suzuki, A.; Tajima, Y.; Suzuki, N.; Suzuki, M.; Nakamura, S.; Kuno, H.; Yokota, A.; Sogabe, S.; et al. TAK-137, an AMPA-R potentiator with little agonistic effect, has a wide therapeutic window. Neuropsychopharmacology 2019, 44, 961–970. [Google Scholar] [CrossRef]
- Arai, A.C.; Xia, Y.F.; Rogers, G.; Lynch, G.; Kessler, M. Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J. Pharmacol. Exp. Ther. 2002, 303, 1075–1085. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Cerne, R.; Witkin, J.M.; Lippa, A. High Impact AMPAkines Induce a Gq-Protein Coupled Endoplasmic Calcium Release in Cortical Neurons: A Possible Mechanism for Explaining the Toxicity of High Impact AMPAkines. Synapse 2024, 78, e22310. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Cerne, R.; Smith, J.L.; Witkin, J.M.; Lippa, A. Preclinical Pharmacology of the Low-Impact Ampakine CX717. Future Pharmacol. 2024, 4, 494–509. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Cerne, R.; Shoaib, M.; Witkin, J.M.; Lippa, A. Low-Impact Ampakine CX1739 Exerts Pro-Cognitive Effects and Reverses Opiate-Induced Respiratory Depression in Rodents. Future Pharmacol. 2024, 4, 173–187. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Cerne, R.; Shoaib, M.; Witkin, J.M.; Lippa, A. Preclinical characterization of a water-soluble low-impact ampakine prodrug, CX1942 and its active moiety, CX1763. Future Med. Chem. 2024, 16, 2325–2336. [Google Scholar] [CrossRef]
- Arai, A.C.; Kessler, M.; Rogers, G.; Lynch, G. Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: Interactions with cyclothiazide and GYKI 52466. Mol. Pharmacol. 2000, 58, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Radin, D.P.; Rogers, G.A.; Hewitt, K.E.; Purcell, R.; Lippa, A. Ampakines Attenuate Staurosporine-induced Cell Death in Primary Cortical Neurons: Implications in the ‘Chemo-Brain’ Phenomenon. Anticancer. Res. 2018, 38, 3461–3465. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Clark, S.; Weeks, A.M.; Dudman, J.T.; Gouaux, E.; Partin, K.M. Mechanism of positive allosteric modulators acting on AMPA receptors. J. Neurosci. 2005, 25, 9027–9036. [Google Scholar] [CrossRef]
- Arai, A.; Kessler, M.; Rogers, G.; Lynch, G. Effects of a memory-enhancing drug on DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J. Pharmacol. Exp. Ther. 1996, 278, 627–638. [Google Scholar] [CrossRef]
- Arai, A.; Lynch, G. AMPA receptor desensitization modulates synaptic responses induced by repetitive afferent stimulation in hippocampal slices. Brain Res. 1998, 799, 235–242. [Google Scholar] [CrossRef]
- Ogier, M.; Wang, H.; Hong, E.; Wang, Q.; Greenberg, M.E.; Katz, D.M. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J. Neurosci. 2007, 27, 10912–10917. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Purcell, R.; Lippa, A. Acute ampakine treatment ameliorates age-related deficits in long-term potentiation. Biomed. Pharmacother. 2016, 84, 806–809. [Google Scholar] [CrossRef]
- Seese, R.R.; Le, A.A.; Wang, K.; Cox, C.D.; Lynch, G.; Gall, C.M. A TrkB agonist and ampakine rescue synaptic plasticity and multiple forms of memory in a mouse model of intellectual disability. Neurobiol. Dis. 2020, 134, 104604. [Google Scholar] [CrossRef]
- Kunugi, A.; Tajima, Y.; Kuno, H.; Sogabe, S.; Kimura, H. HBT1, a Novel AMPA Receptor Potentiator with Lower Agonistic Effect, Avoided Bell-Shaped Response in In Vitro BDNF Production. J. Pharmacol. Exp. Ther. 2018, 364, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Tajima, Y.; Kunugi, A.; Kimura, H. Electrophysiological characterization of a novel AMPA receptor potentiator, TAK-137, in rat hippocampal neurons. Neurosci. Lett. 2019, 712, 134488. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kunugi, A.; Tajima, Y.; Suzuki, N.; Suzuki, M.; Toyofuku, M.; Kuno, H.; Sogabe, S.; Kosugi, Y.; Awasaki, Y.; et al. Strictly regulated agonist-dependent activation of AMPA-R is the key characteristic of TAK-653 for robust synaptic responses and cognitive improvement. Sci. Rep. 2021, 11, 14532. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Suzuki, A.; Kunugi, A.; Tajima, Y.; Yamada, R.; Kimura, H. TAK-653, an AMPA receptor potentiator with minimal agonistic activity, produces an antidepressant-like effect with a favorable safety profile in rats. Pharmacol. Biochem. Behav. 2021, 211, 173289. [Google Scholar] [CrossRef]
- Neurocrine. Neurocrine Biosciences Reports Positive Phase 2 Data for NBI-1065845 in Adults with Major Depressive Disorder. 2024. Available online: https://neurocrine.gcs-web.com/news-releases/news-release-details/neurocrine-biosciences-reports-positive-phase-2-data-nbi-1065845 (accessed on 1 March 2025).
- Ahmed, A.H.; Oswald, R.E. Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J. Med. Chem. 2010, 53, 2197–2203. [Google Scholar] [CrossRef]
- Radin, D.P.; Li, Y.X.; Rogers, G.; Purcell, R.; Lippa, A. Stargazin differentially modulates ampakine gating kinetics and pharmacology. Biochem. Pharmacol. 2018, 148, 308–314. [Google Scholar] [CrossRef]
- Radin, D.P.; Li, Y.X.; Rogers, G.; Purcell, R.; Lippa, A. Tarps differentially affect the pharmacology of ampakines. Biochem. Pharmacol. 2018, 154, 446–451. [Google Scholar] [CrossRef]
- Ingvar, M.; Ambros-Ingerson, J.; Davis, M.; Granger, R.; Kessler, M.; Rogers, G.A.; Schehr, R.S.; Lynch, G. Enhancement by an ampakine of memory encoding in humans. Exp. Neurol. 1997, 146, 553–559. [Google Scholar] [CrossRef]
- Oertel, B.G.; Felden, L.; Tran, P.V.; Bradshaw, M.H.; Angst, M.S.; Schmidt, H.; Johnson, S.; Greer, J.J.; Geisslinger, G.; Varney, M.A.; et al. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin. Pharmacol. Ther. 2010, 87, 204–211. [Google Scholar] [CrossRef]
- Boyle, J.; Stanley, N.; James, L.M.; Wright, N.; Johnsen, S.; Arbon, E.L.; Dijk, D.J. Acute sleep deprivation: The effects of the AMPAKINE compound CX717 on human cognitive performance, alertness and recovery sleep. J. Psychopharmacol. 2012, 26, 1047–1057. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Cerne, R.; Shoaib, M.; Smith, J.L.; Witkin, J.; Lippa, A. Preclinical Pharmacology of CX1837, a High-Impact Ampakine with an Improved Safety Margin: Implications for Treating Alzheimer’s Disease and Ischemic Stroke. Curr. Alzheimer Res. 2024, 21, 745–754. [Google Scholar] [CrossRef]
- Radin, D.P.; Cerne, R.; Witkin, J.; Lippa, A. High-Impact AMPAkines Elevate Calcium Levels in Cortical Astrocytes by Mobilizing Endoplasmic Reticular Calcium Stores. Neuroglia 2024, 5, 344–355. [Google Scholar] [CrossRef]
- Radin, D.P.; Cerne, R.; Witkin, J.M.; Lippa, A. Safety, Tolerability, and Pharmacokinetic Profile of the Low-Impact Ampakine CX1739 in Young Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2025, 14, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Radin, D.P.; Cerne, R.; Smith, J.L.; Witkin, J.M.; Lippa, A. Safety, Tolerability and Pharmacokinetic Profile of the Low-Impact Ampakine CX717 in Young Healthy Male Subjects and Elderly Healthy Male and Female Subjects. Eur. J. Pharmacol. 2025, 993, 177317. [Google Scholar] [CrossRef] [PubMed]
- Radin, D.P.; Lippa, A.; Rana, S.; Fuller, D.D.; Smith, J.L.; Cerne, R.; Witkin, J.M. Amplification of the therapeutic potential of AMPA receptor potentiators from the nootropic era to today. Pharmacol. Biochem. Behav. 2025, 248, 173967. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Bleakman, D.; Zimmerman, D.M.; Nisenbaum, E.S. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord 2024, 3, 181–194. [Google Scholar] [CrossRef]
- Legutko, B.; Li, X.; Skolnick, P. Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology 2021, 40, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tizzano, J.P.; Griffey, K.; Clay, M.; Lindstrom, T.; Skolnick, P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098). Neuropharmacology 2021, 40, 1028–1033. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Dix, S. AMPA receptor potentiators as cognitive enhancers. IDrugs 2007, 10, 185–192. [Google Scholar]
- Jones, N.; Messenger, M.J.; O’Neill, M.J.; Oldershaw, A.; Gilmour, G.; Simmons, R.M.; Williams, S.C. AMPA receptor potentiation can prevent ethanol-induced intoxication. Neuropsychopharmacology 2008, 33, 1713–1723. [Google Scholar] [CrossRef]
- Lindholm, J.S.; Autio, H.; Vesa, L.; Antila, H.; Lindemann, L.; Hoener, M.C.; Castren, E. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf(+)/(-) heterozygous null mice. Neuropharmacology 2012, 62, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Su, X.W.; Li, X.Y.; Banasr, M.; Koo, J.W.; Shahid, M.; Henry, B.; Duman, R.S. Chronic treatment with AMPA receptor potentiator Org 26576 increases neuronal cell proliferation and survival in adult rodent hippocampus. Psychopharmacology 2009, 206, 215–222. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radin, D.P.; Cerne, R.; Smith, J.L.; Witkin, J.M.; Lippa, A. Recovery from AMPA Receptor Potentiation by Ampakines. Future Pharmacol. 2025, 5, 27. https://doi.org/10.3390/futurepharmacol5020027
Radin DP, Cerne R, Smith JL, Witkin JM, Lippa A. Recovery from AMPA Receptor Potentiation by Ampakines. Future Pharmacology. 2025; 5(2):27. https://doi.org/10.3390/futurepharmacol5020027
Chicago/Turabian StyleRadin, Daniel P., Rok Cerne, Jodi L. Smith, Jeffrey M. Witkin, and Arnold Lippa. 2025. "Recovery from AMPA Receptor Potentiation by Ampakines" Future Pharmacology 5, no. 2: 27. https://doi.org/10.3390/futurepharmacol5020027
APA StyleRadin, D. P., Cerne, R., Smith, J. L., Witkin, J. M., & Lippa, A. (2025). Recovery from AMPA Receptor Potentiation by Ampakines. Future Pharmacology, 5(2), 27. https://doi.org/10.3390/futurepharmacol5020027





