Lipid Composition-, Medium pH-, and Drug-Concentration-Dependent Membrane Interactions of Ibuprofen, Diclofenac, and Celecoxib: Hypothetical Association with Their Analgesic and Gastrointestinal Toxic Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Biomimetic Membranes
2.3. Determination of Membrane Interactivity
2.4. Statistical Analysis
3. Results
3.1. Interactions with Neuro-Mimetic Membranes
3.2. Interactions with DPPC Membranes
3.3. Membrane Interactivity Depending on Lipid Composition, Medium pH, and Drug Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, A.; Buvanendran, A. Recent advances in multimodal analgesia. Anesthesiol. Clin. 2012, 30, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Kendall, L.; Reina, E. Multimodal analgesia: A systematic review of local NSAIDs for non-ophthalmologic postoperative pain management. Int. J. Surg. 2016, 32, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef] [PubMed]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Chapurlat, R.; Al-Daghri, N.; Herrero-Beaumont, G.; Bruyère, O.; Rannou, F.; Roth, R.; Uebelhart, D.; Reginster, J.Y. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: What does the literature say? Drugs Aging 2019, 36 (Suppl. S1), 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Lofrumento, D.D.; Vitale, P.; De Nuccio, F.; La Pesa, V.; Panella, A.; Calvello, R.; Cianciulli, A.; Panaro, M.A.; Scilimati, A. Selective cyclooxygenase-1 inhibition by p6 and gastrotoxicity: Preliminary investigation. Pharmacology 2015, 95, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; McKnight, W.; Reuter, B.K.; Vergnolle, N. NSAID-induced gastric damage in rats: Requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 2000, 119, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Dial, E.J.; Lichtenberger, L.M. Advent of novel phosphatidylcholine-associated nonsteroidal anti-inflammatory drugs with improved gastrointestinal safety. Gut Liver 2013, 7, 7–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bjarnason, I.; Scarpignato, C.; Holmgren, E.; Olszewski, M.; Rainsford, K.D.; Lanas, A. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology 2018, 154, 500–514. [Google Scholar] [CrossRef]
- Wagner, K.; Vito, S.; Inceoglu, B.; Hammock, B.D. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014, 113–115, 2–12. [Google Scholar] [CrossRef]
- Matta, J.A.; Miyares, R.L.; Ahern, G.P. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 2007, 578 Pt 2, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V.; González-Ros, J.M.; Goñi, F.M.; Kinnunen, P.K.; Vigh, L.; Sánchez-Magraner, L.; Fernández, A.M.; Busquets, X.; Horváth, I.; Barceló-Coblijn, G. Membranes: A meeting point for lipids, proteins and therapies. J. Cell. Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.H.; Qin, S.; Tatulian, S.A. Membrane fluidity is a key modulator of membrane binding, insertion, and activity of 5-lipoxygenase. Biophys. J. 2005, 88, 4084–4094. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.M.; Zhou, Y.; Jayaraman, V.; Doyen, J.R.; O’Neil, R.G.; Dial, E.J.; Volk, D.E.; Gorenstein, D.G.; Boggara, M.B.; Krishnamoorti, R. Insight into NSAID-induced membrane alterations, pathogenesis and therapeutics: Characterization of interaction of NSAIDs with phosphatidylcholine. Biochim. Biophys. Acta 2012, 1821, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leite, C.; Nunes, C.; Reis, S. Interaction of nonsteroidal anti-inflammatory drugs with membranes: In vitro assessment and relevance for their biological actions. Prog. Lipid Res. 2013, 52, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Mizogami, M. Membrane interactivity of non-steroidal anti-inflammatory drugs: A literature review. J. Adv. Med. Med. Res. 2019, 31, 1–30. [Google Scholar] [CrossRef]
- Giraud, M.N.; Motta, C.; Romero, J.J.; Bommelaer, G.; Lichtenberger, L.M. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: A possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs). Biochem. Pharmacol. 1999, 57, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Suwalsky, M.; Manrique, M.; Villena, F.; Sotomayor, C.P. Structural effects in vitro of the anti-inflammatory drug diclofenac on human erythrocytes and molecular models of cell membranes. Biophys. Chem. 2009, 141, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Mizogami, M. Discrimination of stereoisomers by their enantioselective interactions with chiral cholesterol-containing membranes. Molecules 2017, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Lopes, D.; Pinheiro, M.; Pereira-Leite, C.; Reis, S. In vitro assessment of NSAIDs-membrane interactions: Significance for pharmacological actions. Pharm. Res. 2013, 30, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Moreno, M.; Heinbockel, L.; Suwalsky, M.; Garidel, P.; Brandenburg, K. Biophysical study of the non-steroidal anti-inflammatory drugs (NSAID) ibuprofen, naproxen and diclofenac with phosphatidylserine bilayer membranes. Biochim. Biophys. Acta 2016, 1858, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Soares, T.B.; Gonçalves, H.; Bernstorff, S.; Real Oliveira, M.E.C.D.; Lopes, C.M.; Lúcio, M. A molecular biophysical approach to diclofenac topical gastrointestinal damage. Int. J. Mol. Sci. 2018, 19, 3411. [Google Scholar] [CrossRef] [PubMed]
- Tomisato, W.; Tanaka, K.; Katsu, T.; Kakuta, H.; Sasaki, K.; Tsutsumi, S.; Hoshino, T.; Aburaya, M.; Li, D.; Tsuchiya, T.; et al. Membrane permeabilization by non-steroidal anti-inflammatory drugs. Biochem. Biophys. Res. Commun. 2004, 323, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Lúcio, M.; Lima, J.L.; Matos, C.; Reis, S. Effects of diclofenac on EPC liposome membrane properties. Anal. Bioanal. Chem. 2005, 382, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kishi, Y.; Ishigami, T.; Suga, K.; Umakoshi, H. Chiral selective adsorption of ibuprofen on a liposome membrane. J. Phys. Chem. B 2016, 120, 2790–2795. [Google Scholar] [CrossRef] [PubMed]
- Sade, A.; Tunçay, S.; Cimen, I.; Severcan, F.; Banerjee, S. Celecoxib reduces fluidity and decreases metastatic potential of colon cancer cell lines irrespective of COX-2 expression. Biosci. Rep. 2012, 32, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Gamerdinger, M.; Clement, A.B.; Behl, C. Cholesterol-like effects of selective cyclooxygenase inhibitors and fibrates on cellular membranes and amyloid-beta production. Mol. Pharmacol. 2007, 72, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Nunes, C.; Lúcio, M.; Ferreira, H.; Lima, J.L.; Tavares, J.; Cordeiro-da-Silva, A.; Reis, S. Effect of nonsteroidal anti-inflammatory drugs on the cellular membrane fluidity. J. Pharm. Sci. 2008, 97, 3195–3206. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Brezesinski, G.; Lopes, D.; Lima, J.L.; Reis, S.; Lúcio, M. Lipid-drug interaction: Biophysical effects of tolmetin on membrane mimetic systems of different dimensionality. J. Phys. Chem. B 2011, 115, 12615–12623. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leite, C.; Nunes, C.; Lima, J.L.; Reis, S.; Lúcio, M. Interaction of celecoxib with membranes: The role of membrane biophysics on its therapeutic and toxic effects. J. Phys. Chem. B 2012, 116, 13608–13617. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.; Sakr, O.S.; Nasr, M.; Sammour, O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102174. [Google Scholar] [CrossRef]
- Mizogami, M.; Tsuchiya, H. Acetaminophen has lipid composition-dependent membrane interactivity that could be related to nephrotoxicity but not to analgesic activity and hepatotoxicity. Med. Princ. Pract. 2022, 31, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ehehalt, R.; Wagenblast, J.; Erben, G.; Lehmann, W.D.; Hinz, U.; Merle, U.; Stremmel, W. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoElectrospray-tandem mass spectrometry. Scand. J. Gastroenterol. 2004, 39, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S.; Gardouh, A.R.; Ghorab, M.M. Factors affecting liposomes particle size prepared by ethanol injection method. Res. Pharm. Sci. 2017, 12, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Effects of green tea catechins on membrane fluidity. Pharmacology 1999, 59, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Structure-specific membrane-fluidizing effect of propofol. Clin. Exp. Pharmacol. Physiol. 2001, 28, 292–299. [Google Scholar] [CrossRef]
- Heine, P.; Witt, G.; Gilardi, A.; Gribbon, P.; Kummer, L.; Plückthun, A. High-throughput fluorescence polarization assay to identify ligands using purified G protein-coupled receptor. SLAS Discov. 2019, 24, 915–927. [Google Scholar] [CrossRef]
- Iyamu, I.D.; Huang, R. Development of fluorescence polarization-based competition assay for nicotinamide N-methyltransferase. Anal. Biochem. 2020, 604, 113833. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, H.; Tanaka, K.; Takeda, M.; Katsu, T.; Mima, S.; Mizushima, T. Geranylgeranylacetone protects membranes against nonsteroidal anti-inflammatory drugs. Mol. Pharmacol. 2005, 68, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Chiricozzi, E.; Mauri, L.; Sonnino, S.; Prinetti, A. Sphingolipids and neuronal degeneration in lysosomal storage disorders. J. Neurochem. 2019, 148, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Sántha, P.; Dobos, I.; Kis, G.; Jancsó, G. Role of gangliosides in peripheral pain mechanisms. Int. J. Mol. Sci. 2020, 21, 1005. [Google Scholar] [CrossRef] [PubMed]
- Alsop, R.J.; Armstrong, C.L.; Maqbool, A.; Toppozini, L.; Dies, H.; Rheinstädter, M.C. Cholesterol expels ibuprofen from the hydrophobic membrane core and stabilizes lamellar phases in lipid membranes containing ibuprofen. Soft Matter 2015, 11, 4756–4767. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S.; Voss, B. Pharmacokinetics of intravenous ibuprofen: Implications of time of infusion in the treatment of pain and fever. Drugs 2012, 72, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef] [PubMed]
- Cebrecos, J.; Carlson, J.D.; Encina, G.; Lahjou, M.; Sans, A.; Sust, M.; Vaqué, A.; Morte, A.; Gascón, N.; Plata-Salamán, C. Celecoxib-tramadol co-crystal: A randomized 4-way crossover comparative bioavailability study. Clin. Ther. 2021, 43, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.A.; Ernst, C.C.; Kramer, W.G.; Madden, D.; Lang, E.; Liao, E.; Lacouture, P.G.; Ramaiya, A.; Carr, D.B. Pharmacokinetics of diclofenac and hydroxypropyl-β-cyclodextrin (HPβCD) following administration of injectable HPβCD-diclofenac in subjects with mild to moderate renal insufficiency or mild hepatic impairment. Clin. Pharmacol. Drug Dev. 2018, 7, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, M.; Ferreira, H.; Lima, J.L.; Reis, S. Interactions between oxicams and membrane bilayers: An explanation for their different COX selectivity. Med. Chem. 2006, 2, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Sigthorsson, G.; Simpson, R.J.; Walley, M.; Anthony, A.; Foster, R.; Hotz-Behoftsitz, C.; Palizban, A.; Pombo, J.; Watts, J.; Morham, S.G.; et al. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 2002, 122, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; Scarpignato, C.; Takeuchi, K.; Rainsford, K.D. Determinants of the short-term gastric damage caused by NSAIDs in man. Aliment. Pharmacol. Ther. 2007, 26, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.M.; Barron, M.; Marathi, U. Association of phosphatidylcholine and NSAIDs as a novel strategy to reduce gastrointestinal toxicity. Drugs Today 2009, 45, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Koenigsknecht, M.J.; Baker, J.R.; Wen, B.; Frances, A.; Zhang, H.; Yu, A.; Zhao, T.; Tsume, Y.; Pai, M.P.; Bleske, B.E.; et al. In vivo dissolution and systemic absorption of immediate release ibuprofen in human gastrointestinal tract under fed and fasted conditions. Mol. Pharm. 2017, 14, 4295–4304. [Google Scholar] [CrossRef]
- Hens, B.; Tsume, Y.; Bermejo, M.; Paixao, P.; Koenigsknecht, M.J.; Baker, J.R.; Hasler, W.L.; Lionberger, R.; Fan, J.; Dickens, J.; et al. Low buffer capacity and alternating motility along the human gastrointestinal tract: Implications for in vivo dissolution and absorption of ionizable drugs. Mol. Pharm. 2017, 14, 4281–4294. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leite, C.; Jamal, S.K.; Almeida, J.P.; Coutinho, A.; Prieto, M.; Cuccovia, I.M.; Nunes, C.; Reis, S. Neutral diclofenac causes remarkable changes in phosphatidylcholine bilayers: Relevance for gastric toxicity mechanisms. Mol. Pharmacol. 2020, 97, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.; Lim, L.L.; Garcia Rodriguez, L.A.; Perez Gutthann, S.; Carson, J.L.; Griffin, M.; Savage, R.; Logan, R.; Moride, Y.; Hawkey, C.; et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: Results of a collaborative meta-analysis. BMJ 1996, 312, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leite, C.; Figueiredo, M.; Burdach, K.; Nunes, C.; Reis, S. Unraveling the role of drug-lipid interactions in NSAIDs-induced cardiotoxicity. Membranes 2021, 11, 24. [Google Scholar] [CrossRef] [PubMed]

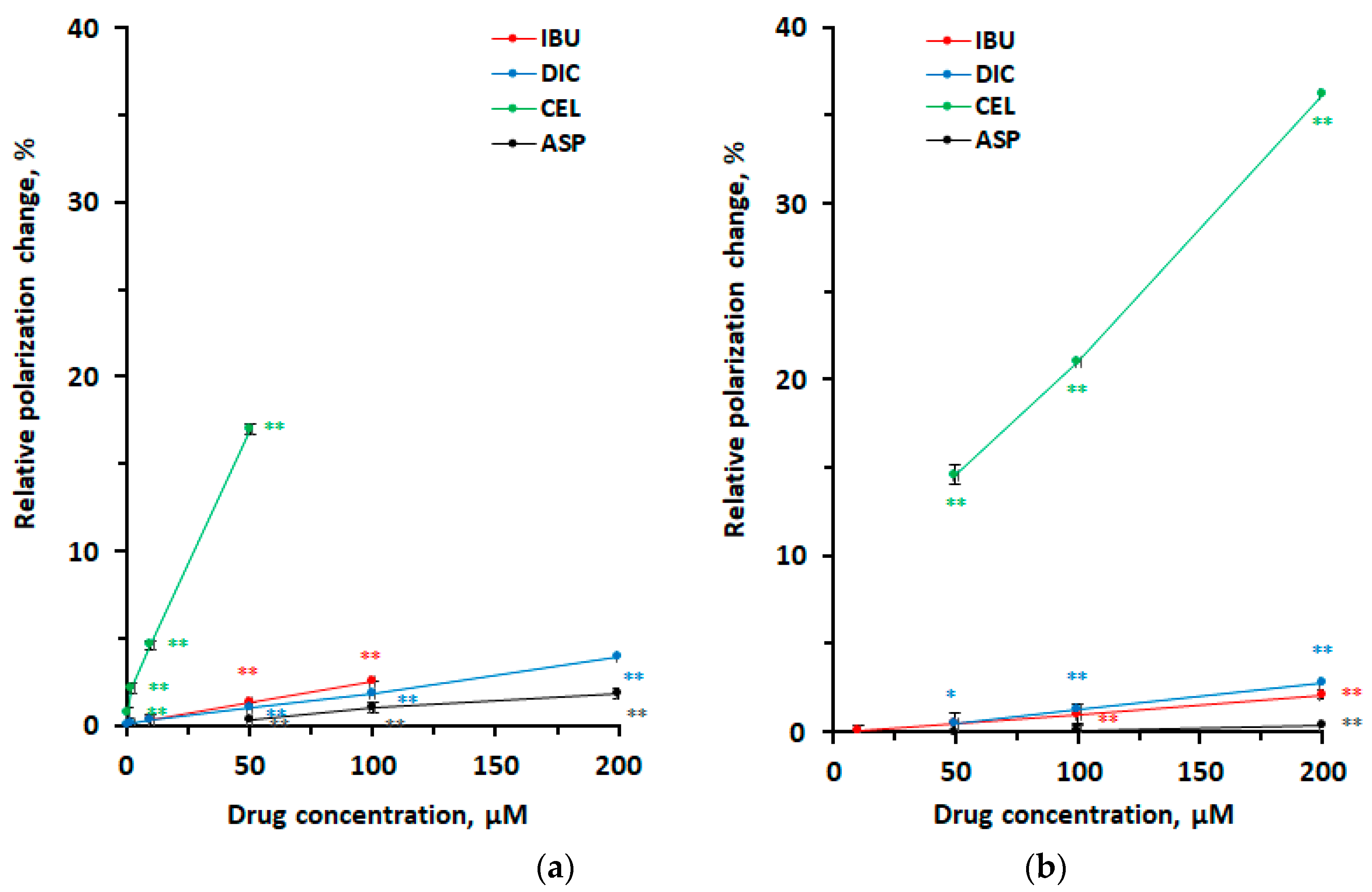

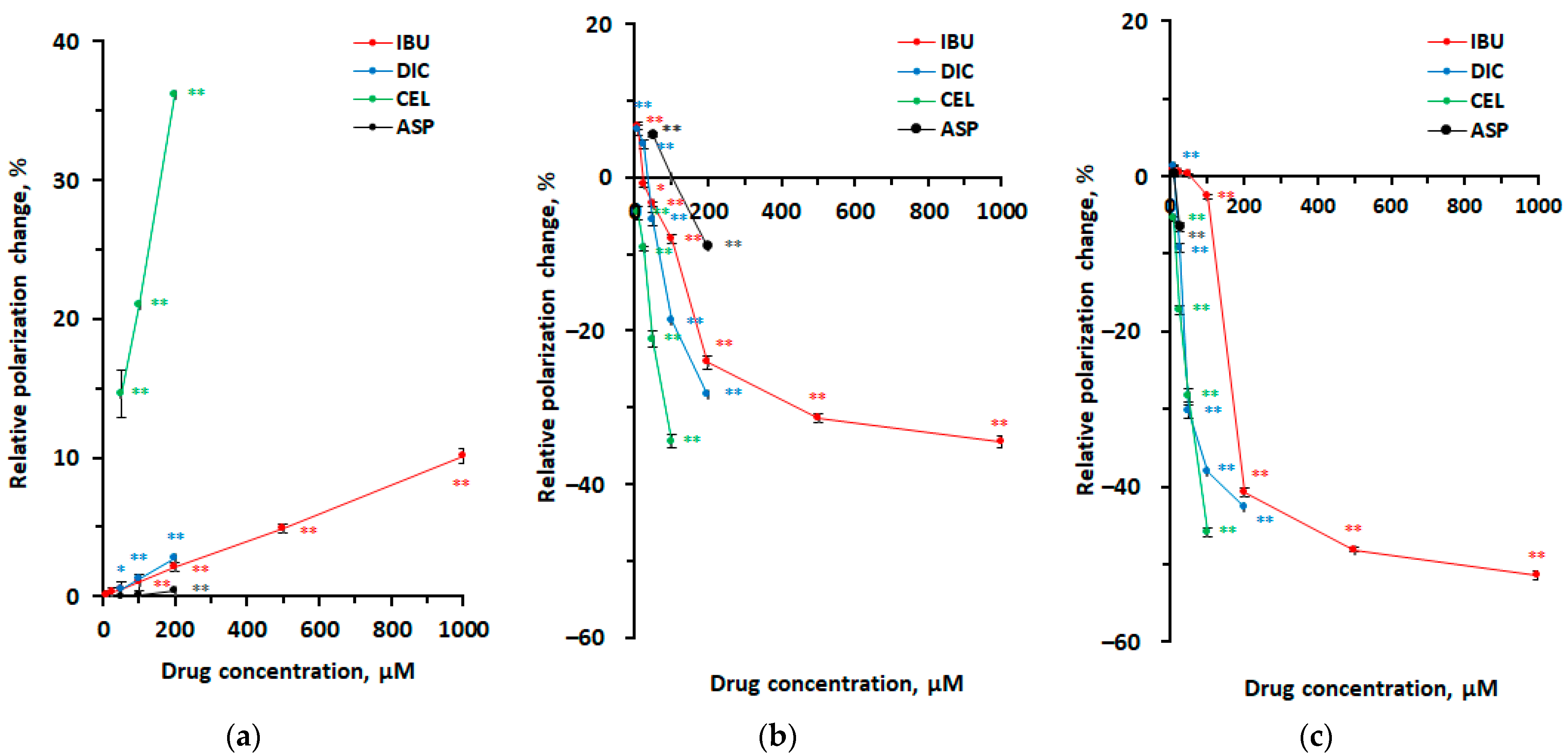
| Polarization Change | ||||
|---|---|---|---|---|
| Drug Concentration | Membrane Interaction at pH 7.4 | |||
| IBU | DIC | CEL | ASP | |
| 0.5 μM | 0.0000 ± 0.0008 | 0.0000 ± 0.0006 | 0.0018 ± 0.0006 * | |
| 2 μM | 0.0002 ± 0.0006 | 0.0002 ± 0.0011 | 0.0054 ± 0.0011 ** | |
| 10 μM | 0.0009 ± 0.0003 | 0.0006 ± 0.0003 | 0.0121 ± 0.0006 ** | |
| 50 μM | 0.0034 ± 0.0000 ** | 0.0027 ± 0.0003 ** | 0.0445 ± 0.0008 ** | 0.0009 ± 0.0000 ** |
| 100 μM | 0.0065 ± 0.0008 ** | 0.0047 ± 0.0000 ** | 0.0026 ± 0.0000 ** | |
| 200 μM | 0.0102 ± 0.0006 ** | 0.0046 ± 0.0003 ** | ||
| Membrane Interaction at pH 4.0 | ||||
| IBU | ||||
| 50 μM | 0.0098 ± 0.0008 ** | |||
| 100 μM | −0.0067 ± 0.0006 ** | |||
| 500 μM | −0.0270 ± 0.0006 ** | |||
| Polarization Change | ||||
|---|---|---|---|---|
| Drug Concentration | Membrane Interaction at pH 7.4 | |||
| IBU | DIC | CEL | ASP | |
| 50 μM | 0.0010 ± 0.0006 | 0.0010 ± 0.0000 * | 0.0283 ± 0.0008 ** | 0.0000 ± 0.0003 |
| 100 μM | 0.0018 ± 0.0008 ** | 0.0025 ± 0.0006 ** | 0.0407 ± 0.0025 ** | 0.0002 ± 0.0003 |
| 200 μM | 0.0040 ± 0.0006 ** | 0.0054 ± 0.0008 ** | 0.0703 ± 0.0006 ** | 0.0008 ± 0.0000 ** |
| Membrane Interaction atpH 4.0 | ||||
| IBU | DIC | CEL | ASP | |
| 25 μM | −0.0017 ± 0.0006 * | 0.0074 ± 0.0014 ** | −0.0154 ± 0.0006 ** | |
| 50 μM | −0.0059 ± 0.0006 ** | −0.0095 ± 0.0014 ** | −0.0350 ± 0.0020 ** | 0.0098 ± 0.0008 ** |
| 100 μM | −0.0134 ± 0.0011 ** | −0.0310 ± 0.0023 ** | −0.0570 ± 0.0014 ** | |
| 200 μM | −0.0398 ± 0.0011 ** | −0.0469 ± 0.0006 ** | −0.0150 ± 0.0006 ** | |
| 500 μM | −0.0518 ± 0.0011 ** | |||
| 1 mM | −0.0562 ± 0.0014 ** | |||
| Membrane Interaction atpH 2.5 | ||||
| IBU | DIC | CEL | ASP | |
| 10 μM | 0.0010 ± 0.0008 | 0.0033 ± 0.0011 ** | −0.0123 ± 0.0011 ** | 0.0010 ± 0.0008 |
| 25 μM | 0.0009 ± 0.0008 | −0.0252 ± 0.0011 ** | −0.0369 ± 0.0011 ** | −0.0163 ± 0.0017 ** |
| 50 μM | 0.0006 ± 0.0000 | −0.0832 ± 0.0011 ** | −0.0634 ± 0.0017 ** | |
| 100 μM | −0.0059 ± 0.0008 ** | −0.0920 ± 0.0011 ** | −0.1028 ± 0.0014 ** | |
| 200 μM | −0.0916 ± 0.0014 ** | −0.1029 ± 0.0017 ** | ||
| 500 μM | −0.1081 ± 0.0008 ** | |||
| 1 mM | −0.1119 ± 0.0014 ** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizogami, M.; Tsuchiya, H. Lipid Composition-, Medium pH-, and Drug-Concentration-Dependent Membrane Interactions of Ibuprofen, Diclofenac, and Celecoxib: Hypothetical Association with Their Analgesic and Gastrointestinal Toxic Effects. Future Pharmacol. 2024, 4, 437-448. https://doi.org/10.3390/futurepharmacol4020024
Mizogami M, Tsuchiya H. Lipid Composition-, Medium pH-, and Drug-Concentration-Dependent Membrane Interactions of Ibuprofen, Diclofenac, and Celecoxib: Hypothetical Association with Their Analgesic and Gastrointestinal Toxic Effects. Future Pharmacology. 2024; 4(2):437-448. https://doi.org/10.3390/futurepharmacol4020024
Chicago/Turabian StyleMizogami, Maki, and Hironori Tsuchiya. 2024. "Lipid Composition-, Medium pH-, and Drug-Concentration-Dependent Membrane Interactions of Ibuprofen, Diclofenac, and Celecoxib: Hypothetical Association with Their Analgesic and Gastrointestinal Toxic Effects" Future Pharmacology 4, no. 2: 437-448. https://doi.org/10.3390/futurepharmacol4020024
APA StyleMizogami, M., & Tsuchiya, H. (2024). Lipid Composition-, Medium pH-, and Drug-Concentration-Dependent Membrane Interactions of Ibuprofen, Diclofenac, and Celecoxib: Hypothetical Association with Their Analgesic and Gastrointestinal Toxic Effects. Future Pharmacology, 4(2), 437-448. https://doi.org/10.3390/futurepharmacol4020024






