1. Introduction
Ampakines are a class of orally bioavailable small molecules that bind allosterically to the AMPA-glutamate receptor (AMPAR) and enhance the actions of the endogenous ligand, glutamate [
1,
2,
3,
4]. AMPARs mediate the majority of fast excitatory neurotransmission in the CNS [
5,
6,
7] and thus play pivotal roles in the regulation of central nervous system (CNS) function at multiple levels. As such, ampakines have demonstrated efficacy across a number of preclinical models of neurological and neuropsychiatric diseases where excitatory synaptic transmission is compromised [
8,
9,
10,
11,
12,
13,
14]. In preclinical studies, ampakines have been shown to be effective in treating symptoms of Alzheimer’s, stroke, Huntington’s, Rett syndrome, and autism [
15,
16,
17,
18]. In the clinical setting, ampakines have been shown to reverse opioid-induced respiratory depression (OIRD) without eliminating the analgesic effects of opioids [
19] and have also been shown to reduce symptoms of ADHD [
20].
While this myriad of positive preclinical results looks promising, some ampakines have unfortunately demonstrated epileptic activity at therapeutic doses [
21,
22], a side effect which has drastically slowed their clinical translation. In 2002, Arai and colleagues discovered two novel benzoylpiperidine ampakines (CX516 and CX546) that demonstrated significantly different physiological properties even though they share a very similar chemical structure [
1]. CX546 preferentially binds the agonist-bound non-desensitized receptor and acts by destabilizing desensitized receptor conformation and by stabilizing open-channel receptor conformation [
1]. CX516 accelerates channel opening but exerts little effect on receptor desensitization [
1]. As such, CX546 and CX516 maximally enhanced AMPA-mediated currents in hippocampal excised patches by 250% and 25%, respectively [
1]. CX516 did not fully occupy the canonical cyclothiazide binding site [
23] and did not produce unwanted side effects observed with predecessor ampakines. As such, CX516 was characterized as a subclass of ampakines termed “low-impact” ampakines. However, CX516 did not produce positive results in several clinical trials, an outcome generally attributed to its low potency and short half-life [
24].
RespireRx Pharmaceuticals (previously Cortex) has designed and developed low-impact ampakines that may be used to treat OIRD and other respiratory impairments such as those induced by spinal cord injury [
25]. Ampakines act by augmenting glutamatergic currents in prebotzinger neurons, a pool of neurons in the brainstem that control inspiratory breathing rhythms [
26]. Additional work has suggested the utility of ampakines to treat symptoms of major depressive disorder, for which development is ongoing in the pharmaceutical industry [
27]. Current work on these compounds has been focused on respiratory insufficiency due to opioids [
28,
29] and to spinal cord injury and on the impairment in bladder function after spinal cord injury [
30,
31]. RespireRx Pharmaceuticals is actively engaged in developing these ampakine assets for these and other neurological and psychiatric disorders. One goal of the present research report is to provide the previously undisclosed preclinical findings on CX1739 to the research community as this compound moves ahead into further clinical studies.
Since the low-impact ampakine CX1739 is in clinical development (NCT02735629), we describe here the preclinical pharmacology of this novel low-impact ampakine, CX1739 (
Figure 1). We examine the ability of this ampakine to enhance long-term potentiation (LTP) in the hippocampi of rats, a cellular process often thought to be the substrate of learning and memory. Given the positive findings of CX1739 as an enhancer of LTP in vivo, we then tested the potential pro-cognitive effects of CX1739 in a novel object recognition test and the win shift radial arm maze test. CX1739 was tested in the five-choice serial reaction time task (5-CSRTT), an assay used to screen for compounds with the ability to treat impulsivity associated with ADHD. Additionally, we examined the ability of CX1739 to attenuate the hyperactivity induced by amphetamine, a drug currently used in the clinic to treat ADHD. Further, we gauge the ability of CX1739 to rapidly reverse opioid-induced respiratory depression. Finally, the ability of high doses of CX1739 to produce side-effects, including seizures, was evaluated.
2. Materials and Methods
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with protocols approved by the Institutional Animal Care and Use Committee of the University of California at Irvine (Irvine, CA, USA). The 5CSRTT studies complied with all local and national ethical requirements in accordance with the Animals (Experimental Procedures) Act, 1986, under license from the UK Home Office. Efforts were made to minimize animal suffering and the number of animals used was reduced to a minimum needed for statistical power. Animals were monitored by experimenters and veterinary staff for any signs of distress and all animals were rapidly euthanized after the LTP and toxicity studies to further mitigate distress. Animal species and specific strains were selected based upon previous characterizations in these animal models that have been disclosed in the scientific literature as described below.
2.1. CX1739 Solubilization
CX1739 (N-Methyl-N-(tetrahydro-2H-pyran-4-yl)-2,1,3-benzoxadiazole-5-carboxamide) (
Figure 1) was synthesized by RespireRx Pharmaceuticals Inc. The compound was finely suspended in 0.9% NaCl (saline) with the addition of 33% hydroxypropyl-P-cyclodextrin, HPCD) by bath sonication.
2.2. In Vivo LTP Experiments
Male Long–Evans rats (250–350 g) were used in these studies as previously reported [
32,
33]. For these experiments, rats were anesthetized (pentobarbital Na, 60 mg/kg, IP) before surgical preparation. For blood sampling and drug administration, two catheters made of polyethylene tubing (PE10) were inserted into the femoral artery and vein, respectively. Anesthesia was maintained in rats by infusing pentobarbital at a rate of 2–4 mg/kg/h. Sterotoxic surgery enabled small holes to be drilled into the skull above the left hemisphere. A monopolar stainless-steel stimulating electrode (175 μm, insulated with formvar) targeted the perforant path while a platinum/iridium recording electrode (75 μm) was placed into the hilus of the hippocampal dentate gyrus. Stimulating electrodes were placed at −7.8 to −8.1 from bregma, 4.2 to 4.4 lateral to midline; a recording electrode was positioned at −3.0 to −3.3 from bregma, 1.6 to 2.2 lateral to midline.
In the LTP studies, single-pulse electrical stimulation was delivered to the perforant path (one pulse per 20 s) and evoked excitatory post-synaptic field potentials (EPSPs) were recorded. Current levels for driving EPSPs were adjusted so as to elicit a response of 50–60% of the maximal spike-free amplitude. Peak amplitudes were detected with the use of standard data acquisition and analysis software (NAC and NACSHOW, Irvine, CA, USA). LTP studies were initiated after a stable baseline of EPSPs was established. Then, baseline EPSPs were recorded for 10 min and followed by an IP injection of either CX1739 (1 or 3 mg/kg) or vehicle. Fifteen minutes after injection, a tetanic stimulation protocol (20 trains at 400 Hz of 30 ms duration with current intensity adjusted to evoke 80% of maximal response) was used to produce LTP. EPSPs were recorded for a subsequent period of 95 mi under the same electrical stimulation parameters.
LTP was quantified by assessing peak amplitude EPSPs (as a percent of baseline) (means ± SEM) as recorded 20 min after delivery of the electrical stimulation [
32,
34].
2.3. Novel Object Recognition (NOR)
Adult male Wistar rats (2–3 months and ~230–320 g were used in this study due to their extensive characterization in this task, as described previously with this strain and others [
35]. This model is based on the tendency for rats to preferentially explore a novel object versus a previously explored or familiar object [
36]. A test apparatus containing an open-field arena in a sound-attenuating room with dim lighting was used for this experiment. A digital camera was used to capture rat exploration activity, which was monitored in an adjoining room. Rats were trained and tested individually, and objects were cleaned with alcohol between trials and rats.
The procedure [
36] was performed as follows. After a 5 min habituation period, the animals were put in the test area, which contained two identical objects (plastic shapes). The time spent actively exploring the two objects was then recorded over a 5 min test period (T1). The rats were then returned to their home cages. After 24 h, each rat was exposed to the test arena for 5 min (T2) in the presence of one of the familiar objects and one of the novel objects. Times for exploration of novel and familiar objects were recorded (sec). The position of the objects and their order of presentation was randomized. The preference for objects and the ratio of the time spent exploring both objects (during retention session T2) were used as the measure of cognitive function.
Rats were treated with the novel test compounds 20 min prior to the test period (T1) and (T2) via the IP route of administration. Rats were also treated with vehicle, saline, and the reference compound galantamine (3 mg/kg) via IP injection 20 min prior to T1. Eight rats were initially tested in each treatment group.
Data from the novel object recognition experiment were analyzed by analysis of variance (ANOVA). F values of p < 0.05 were considered to be significant. A Fisher’s LSD post hoc test was utilized for further analyses if there was a significant effect detected by ANOVA. Animals that failed to show any exploration for either one or both of the objects were removed from analysis. The Recognition Index was determined as follows: time exploring novel/time exploring familiar + time exploring novel × 100%.
2.4. Radial Arm Maze
Male Sprague-Dawley rats at about 3 mo of age and between 310 and 345 g (Harlan Sprague-Dawley, Indianapolis, IN, USA) were used in this assay due to their use in this test in prior works [
37,
38]. They were trained to perform the win shift assay on the radial arm maze for approximately 10 weeks prior to the study. On each day of training and testing, each rat completed one trial, split into a sample phase and a test phase. During the sample phase, the rat was placed on a 12-arm radial arm maze surrounded by visual cues. Food wells at the end of each arm were baited with sucrose pellets (each 45 mg), but access to three arms, selected randomly, was blocked with a clear plastic divider at the arm entrance. The rat was allowed 5 min to search the maze and collect the available food pellets before being removed from the maze. Fifteen minutes later, the rat was returned to the maze for the test phase of the trial. During the test phase, the rat was allowed access to all 12 arms of the maze but only the arms that had been blocked previously were baited with sucrose pellets. The rat was allowed 3 min to collect the available food pellets before being removed from the maze. On test days, rats were injected with CX1739 (0.03–1.0 mg/kg, IP) or vehicle immediately following the sample phase of the study. The primary dependent measure was the cumulative number of arm entries prior to entering the first, second, and third baited arm during the test phase.
2.5. Five-Choice Serial Reaction Time Test (5CSRTT)
Male hooded Lister rats (250–310 g at the start of training) (Charles River Labs, Kent, UK) were used in these studies. They were trained as previously described by Hahn et al. [
39]. Light flashes (1 s duration) were presented randomly in one of the nose holes after an inter-trial interval (ITI) of 15 s. Nose pokes into the illuminated hole or within 5 s after the light had been extinguished produced a 45 mg food pellet delivered into a food tray; these responses were counted as correct responses. Nose pokes into the non-illuminated hole were recorded as incorrect responses and these incorrect responses produced a 2 s timeout period during which the experimental chamber was dark and responses had no scheduled consequences. Failing to respond until the end of the limited hold was marked as an omission error. Responses during inter-trial intervals were recorded as premature responses and were expressed as percentage premature responses calculated as described by Robinson et al. [
40]. All subjects had to meet a stable performance criterion of <20% omissions and >70% correct responses on these task parameters before tests with compounds took place. Groups of 16 rats were used in each study and repeated tests with graded doses of compounds were conducted using a randomized sequence in which all rats received vehicle and all doses of the test substance.
The experiments were conducted in operant experimental chambers (Med-Associates, Fairfax, VT, USA). The chambers were contained within sound-attenuating enclosures. On the curved back wall of the chambers were positioned five square (2.5 cm) openings 5 cm above the grid floor and 5 cm deep. The holes had infra-red photocell beam detectors monitoring their entrance and a green light at the back. The tray into which food pellets could be delivered was on the opposite wall, equidistant from the rear wall openings. Chambers were lit from the ceiling.
CX1739 was injected in a volume of 1 mL/kg. The reference compound, atomoxetine, was dissolved in saline. A pretreatment time of 20 min was utilized for both drugs, and both drugs were administered IP. Test sessions in the 5CSRTT lasted 30 min.
For each measure of attentional performance, a one-way ANOVA was performed to ascertain significant changes between groups, followed by a post hoc Holm–Sidak multiple comparisons test to determine difference from the vehicle group.
2.6. Amphetamine-Induced Locomotor Activity
The aim of this test was to determine the effect of CX1739 on amphetamine-induced locomotor activity (LMA) in adult male 2–3-month-old CD1 mice (29–36 g, Charles River Labs) [
41]. Mice experienced a 12:12 h light/dark cycle with lights on at 6:00 a.m. Each of the test cages (standard polycarbonate animal cage; 26 cm × 48 cm × 20 cm; W × L × H) was surrounded by two photobeam arrays, which were placed to detect locomotor activity with a lower array and rearing behavior with an upper array. Locomotor activity was continuously monitored and recorded by a computer for all test cages (Photobeam Activity System, San Diego Instruments, San Diego, CA, USA). The test period was chosen as the 1st 2 h of the dark period of the light/dark cycle. Approximately 15 min before the start of the dark cycle, the mice were removed from the housing facility and transported to the testing room. Mice were placed in the locomotor chamber for 20 min to habituate, removed, injected with either vehicle or CX1739 IP (5.6–30 mg/kg), and 5 min later injected with 2 mg/kg IP amphetamine. Mice were returned to the locomotor chamber 10 min later, and their activity was measured for a further 15 min.
2.7. In Vivo Plethysmography Studies
One day prior to the experiment, male Sprague-Dawley rats (295–330 g) (Harlan Sprague-Dawley) underwent surgery to have two PE30 cannulas inserted into the right jugular vein. These cannulas are required to minimize handling of the animals during the experiment and to allow for remote intravenous administration of drugs. On the day of testing, rats were placed into a rodent-sized whole-body plethysmograph (Buxco, St. Paul, MN, USA) chamber for 1–2 h to acclimatize. Throughout the course of this study, several different respiratory parameters were measured. Once a stable baseline was obtained over a 20 min period, the opioid alfentanil was infused via one of the cannulas using an infusion pump calibrated to administer 250 μg/kg/20 min for 60 min, a dose targeted to produce an approximately 50% depression of breathing. After 20 min of infusion, a bolus injection of CX1739 (5, 10, or 20 mg/kg) was administered through the second cannula. After the alfentanil infusion was stopped, animals were followed for a further 20 min.
Data were analyzed by software incorporated within the plethysmograph system. For these studies, the minute volume (mL/min) of the rats’ respiration was calculated based on the rate of breathing (breaths/min) multiplied by the tidal volume (in mL). Baseline minute volumes were normalized to 100%, and the change in minute volume was expressed as a percent of the animal’s own baseline. Statistical analysis was performed using a one-way ANOVA and Dunnett’s multiple comparison tests.
2.8. Single-Dose Toxicity in Adult Rats
Small groups (n = 2–3) of young adult male Sprague-Dawley rats (250–350 g) (Harlan Sprague-Dawley) were administered CX1739 by oral gavage and placed singly into cages for observation. They were continuously observed for seizure and death for 2 h post dosage and then returned to the animal facility. On each of the subsequent 7 days, all rats were observed for 10 min. CX1739 oral dose groups were vehicle, 750 mg/kg and 1500 mg/kg (using a 100 mg/mL suspension), and 2000 and 3000 mg/kg (in a 250 mg/mL suspension). Seizure rating was by visual observation, as follows: (0) no seizure: animal activity is consistent with that of naïve uninjected animals; (1) mild-to-moderate tonic: mild rigidity, minor tail arch; (2) moderate-to-severe tonic: moderate rigidity, major tail arch, splayed paws; (3) moderate-to-severe tonic with clonic: moderate rigidity, major tail arch, splayed paws, minor spasm and jerking; (4) moderate-to-severe tonic with persistent clonic: severe rigidity, major tail-arch, splayed paws, maximal tonic spasm of all body musculature and major jerking; (5) severe seizure leading to death. As no mice experienced a seizure during the single-dose toxicology studies, a score of 0 was given to all.
3. Results
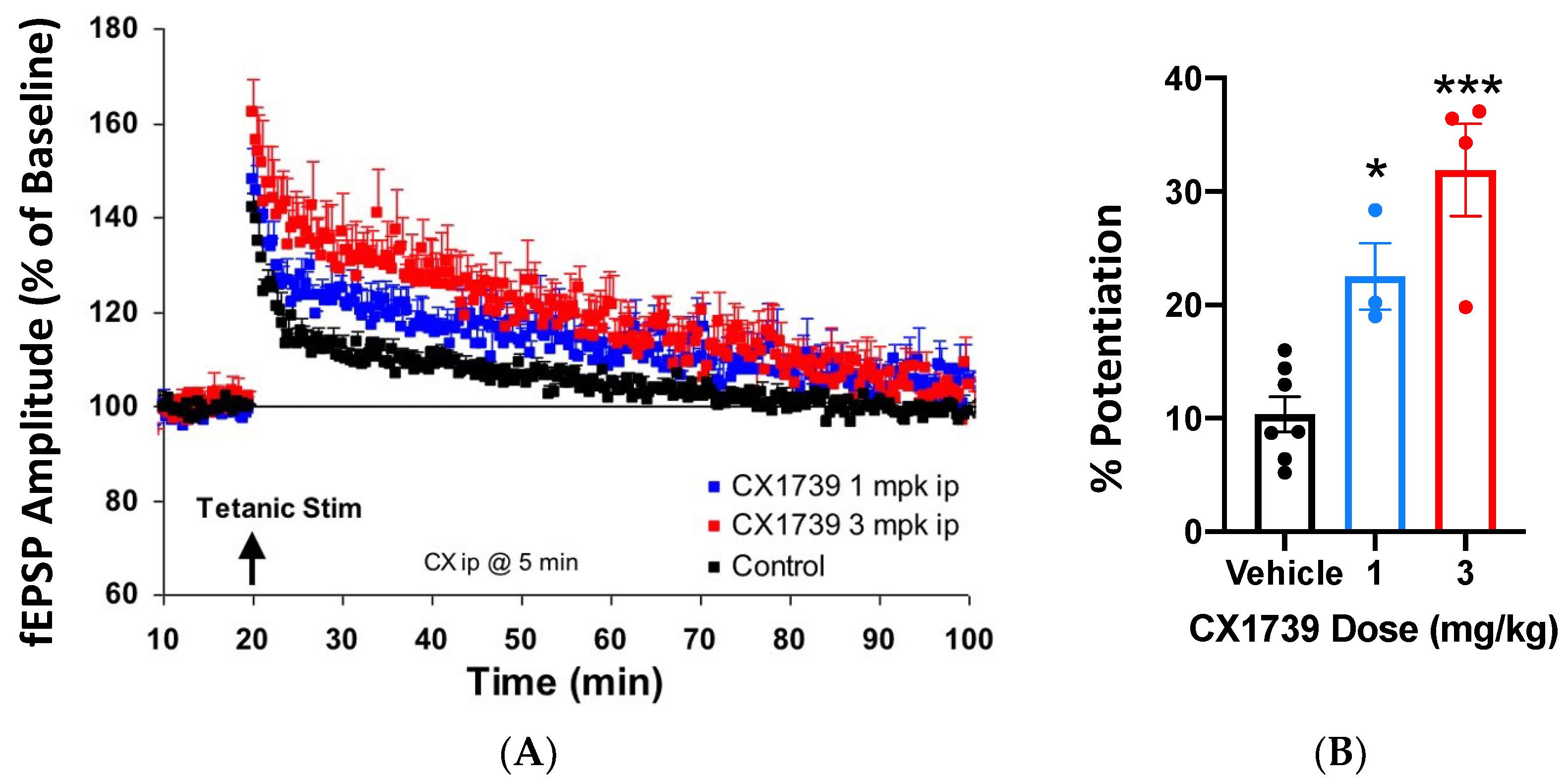
Ampakines enhance the induction of LTP [
32], which may explain their ability to augment memory. CX1739 (
Figure 1) facilitated the amplitude of evoked potentials after tetanic stimulation in vivo.
Figure 2A shows the EPSP amplitude of responses over time, while
Figure 2B summarizes the amplitude change of LTP for each treatment. Tetanizing electrical stimulation produced the typical pattern of LTP. A near immediate rise in potentiation of EPSPs was followed by a rapid decline over the first 5 min, with a more gradual decline over the next 80 min. Twenty minutes after stimulation, the mean peak amplitude of EPSPs was still about 10% greater than baseline (
Figure 2). Administration of CX1739 produced an enhanced mean peak of the EPSP amplitude over the entire recording period. At 20 min post stimulation, the mean peak EPSP amplitudes for 1 and 3 mg/kg of CX1739 were approximately 12% (
p < 0.05) and 22% (
p < 0.001) greater than those produced by vehicle injection, respectively (
Figure 2). CX1739 did not significantly augment EPSPs before electrical stimulation. These data demonstrate that 1 and 3 mg/kg CX1739 significantly facilitate the induction of LTP in vivo.
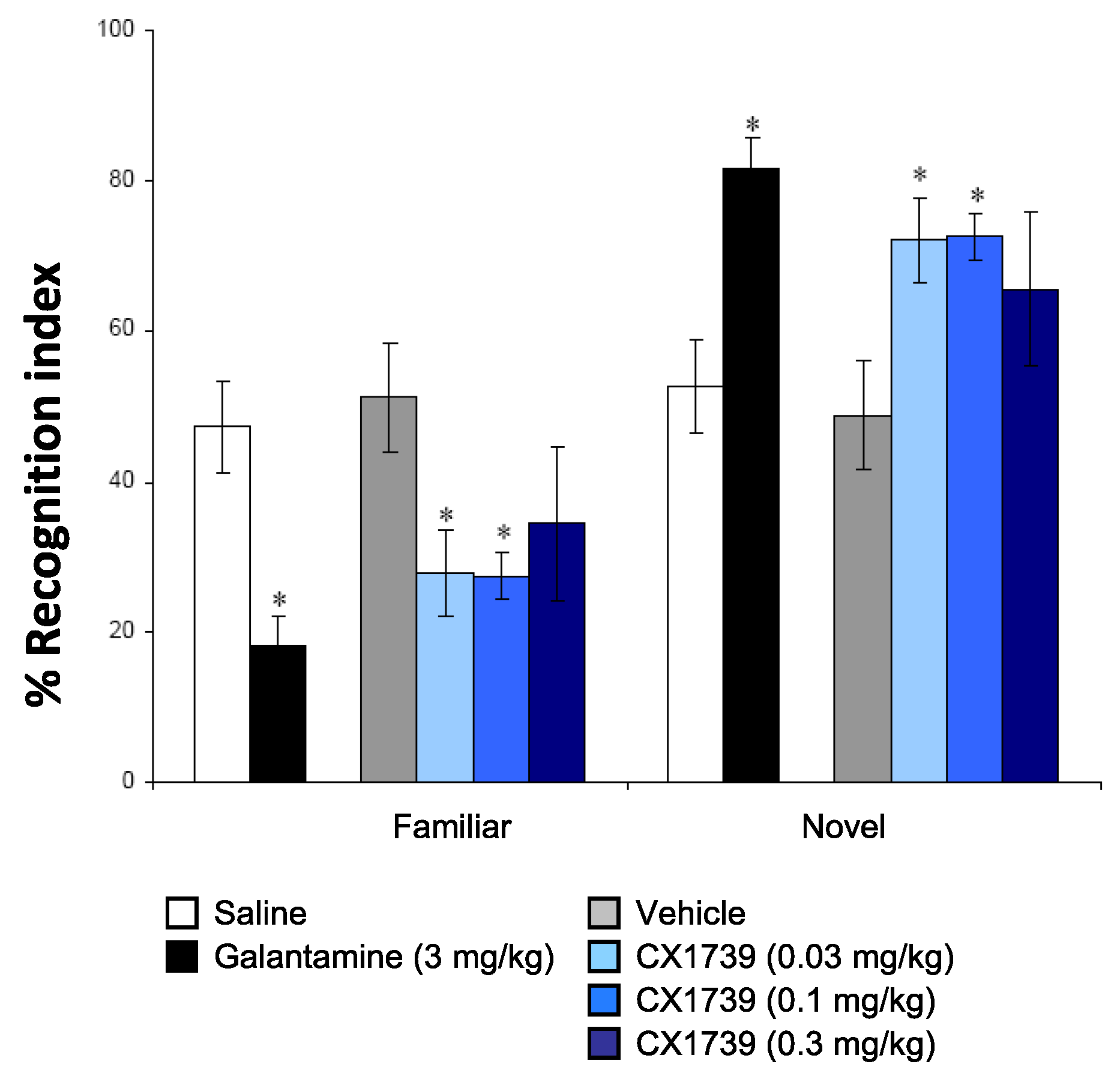
We also characterized the experimental ampakine in some tests of cognition to determine the types of memory/cognition the drug tends to alter in vivo. The NOR is a rodent model of recognition learning memory retrieval and takes advantage of the spontaneous behavior of rodents to investigate a novel object, compared with a familiar object [
36]. This paradigm has been used widely to test for cognitive enhancing capabilities of novel chemical compounds. The effects of saline, vehicle, the cholinergic galantamine, and CX1739 are shown in
Figure 3. Post hoc analysis indicated that CX1739 significantly increased NOR memory at 0.03 and 0.1 mg/kg (
p < 0.05). A dose of 0.3 mg/kg tended to increase novel object recognition memory, but the increase was not significant. The positive reference compound galantamine, at 3 mg/kg, also significantly increased the recognition index when compared to vehicle (
p < 0.05).
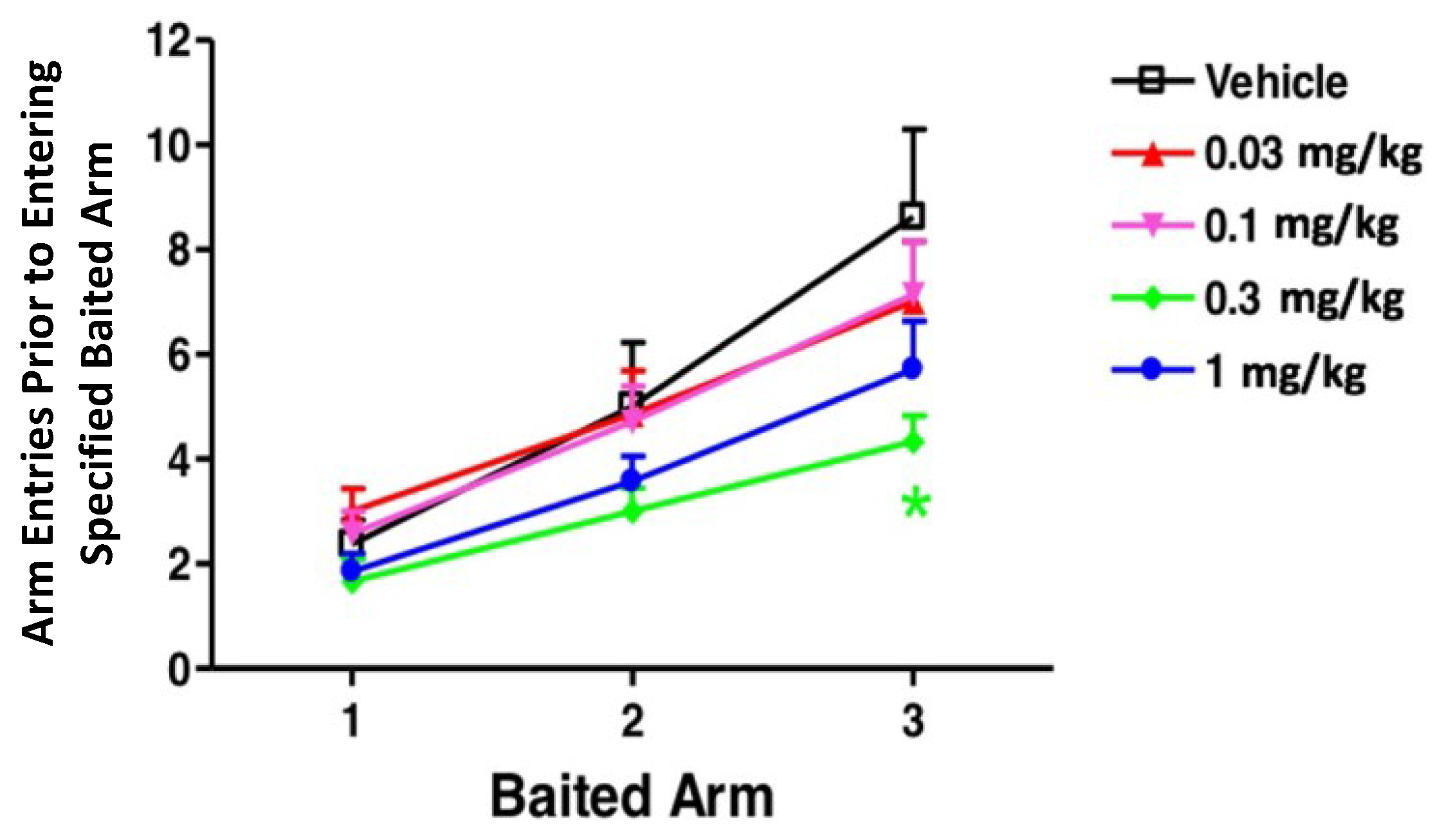
The win shift radial arm maze is a test that assesses the reference and working memory of rats. A good reference memory is associated with the ability of a rat to only enter arms that contain rewards, while working memory is assessed by whether a rat enters each arm once or re-enters an arm of the maze a second time. The latter observation is known as a working memory error. Here, the primary measure was the cumulative number of arm entries before entering the first, second, and third baited arm during the testing phase, shown in
Figure 4. CX1739 reduced the number of arm entries required to collect the three food rewards. An ANOVA indicated significant main effects of dose, F (4, 93) = 2.67,
p < 0.05, and baited arm, F (2, 93) = 29.44,
p < 0.0001, but no significant interaction. Bonferroni-corrected post hoc tests indicated that rats treated with 0.3 mg/kg CX1739 made significantly fewer entries before finding the third baited arm. No other comparisons were significant.
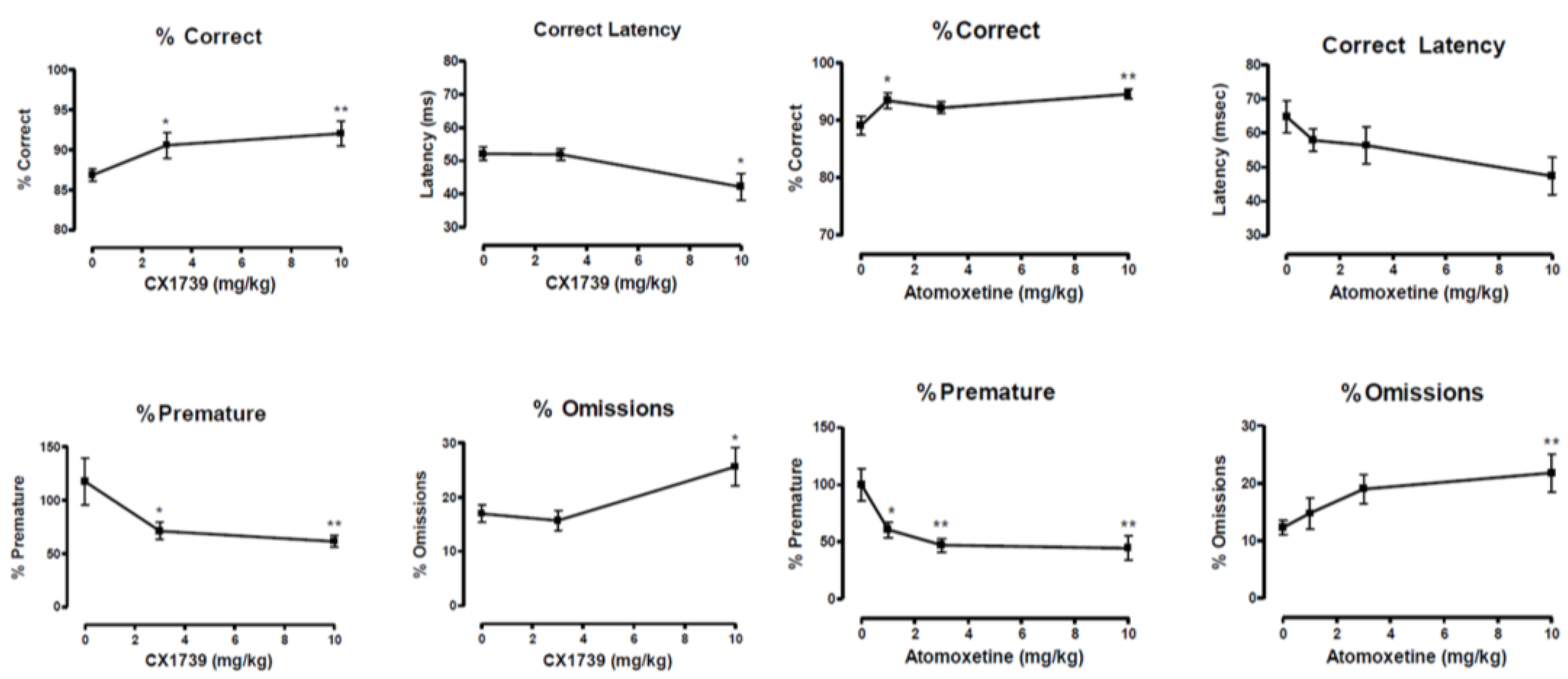
In the 5CSRTT, both CX1739 and the ADHD treatment drug atomoxetine produced significant cognitive augmentation with similar behavioral changes in the four measures of attentional performance (
Figure 5). The figure illustrates the four key measures of attentional performance. The indices mainly affected were the latency to correctly respond and the percentage of premature responses, a measure used to assess impulsive behavior. It should be noted that baseline levels of premature responses were low, but since the ITI was altered from 5 s to 15 s during the test, rats emitted far more premature responses during the test, an increase of around 300%. This is significantly greater than those reported by Robinson et al. [
40] in their study investigating atomoxetine.
Low-impact ampakines have been shown to enhance attention functioning in rodents, which supports the idea that they may treat attentional deficits of ADHD in the clinical setting [
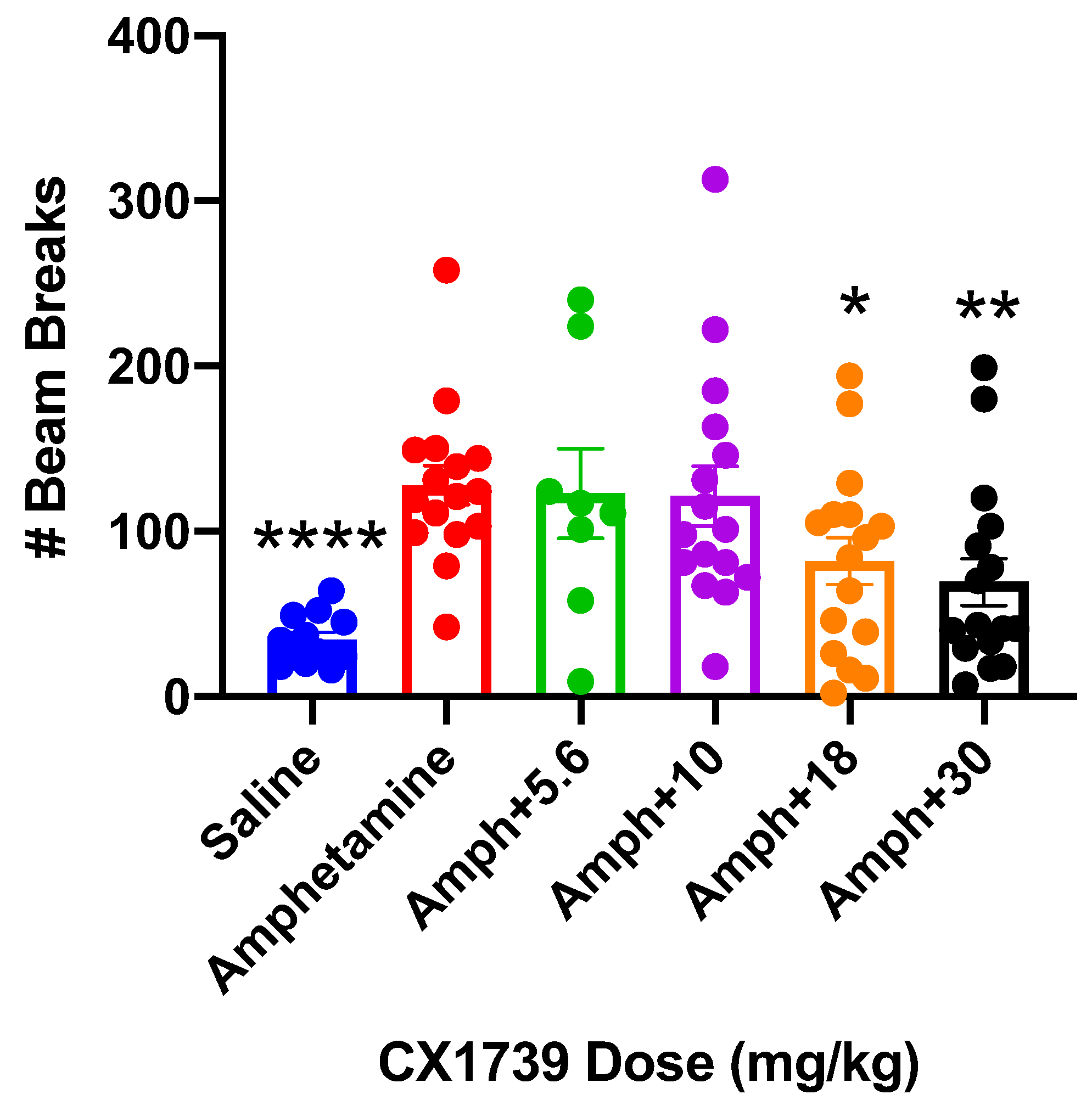
20]. As potential novel treatments for ADHD, we reasoned it would be pertinent to interrogate how low-impact ampakines like CX1739 interact with stimulants, the standard of care for ADHD. To that end, we treated mice with amphetamine to stimulate hyperactivity and treated these mice with the addition of increasing doses of CX1739. Amphetamine tripled the beam breaks compared to saline treatment (
Figure 6). CX1739 dose-dependently reduced the hyperactivity associated with acute amphetamine treatment (
Figure 6). Both 18 mg/kg and 30 mg/kg produced statistically significant reductions in amphetamine-induced LMA in this model.
CX1739 was also tested for its ability to reverse opioid-induced respiratory depression in rats. The efficacy and timing of action of CX1739 were examined in vivo using adult plethysmographic recordings of breathing in rats.
Figure 7 shows data for alfentanil-induced suppression of minute volume and the subsequent antagonism of this impairment by CX1739, which was dose-dependent. Additional population data for changes in respiratory frequency and minute volume induced by increasing doses of CX1739 are shown in
Figure 7. A dose of 20 mg/kg produced a full reversal of alfentanil-induced respiratory depression, with data normalized to minute volume prior to alfentanil.
Finally, we performed a single-dose toxicity study by orally treating rats with increasing doses of CX1739 (750–3000 mg/kg) (
Table 1). From 750 mg/kg to 2000 mg/kg, we only observed reduced activity a few hours after CX1739 administration. The minimum lethal dose in rats was greater than 2000 mg/kg but less than 3000 mg/kg, the dose at which 2 of 2 animals died. The minimum lethal dose for IV or IP routes in rats was not determined because of limited solubility of CX1739 in a vehicle acceptable for parenteral administration. These data demonstrate that CX1739 has a favorable therapeutic ratio and does not produce seizures or lethality at therapeutic concentrations.
4. Discussion
It has long been known that amplification of AMPA receptor function by ampakines can have a broad range of effects on the CNS that can yield therapeutic benefits [
10,
11,
12,
13,
14]. CX1739 is a potent ampakine that acts as a positive allosteric potentiator of the endogenous agonist glutamate. CX1739 exhibits distinct effects from high-impact ampakines like cyclothiazide on AMPARs and has been termed a low-impact ampakine [
42]. CX1739 is currently undergoing clinical development for ADHD, respiratory depression, and symptoms of spinal cord injury such as respiratory and bladder insufficiency. The present study was undertaken to characterize the preclinical pharmacology of the CX1739.
In addition to enhancing glutamatergic neurotransmission, which will tend to normalize network function, ampakines also facilitate the induction and increase the extent of a synaptic strengthening process known as LTP (
Figure 2). This process is widely considered to be of crucial importance in memory formation and sustained cognition [
32,
43,
44]. In the NOR test, which assesses working memory in rodents, CX1739 was active at 30 μg/kg, a dose far lower than 1 mg/kg, the lowest dose to augment LTP in vivo. A dose of 0.3 mg/kg was pro-cognitive in the win shift test (spatial memory) and impulsivity reduction was observed at 3 mg/kg in the 5CSRTT. These comparative potency data suggest that enhancing AMPAergic signaling independent of acute LTP augmentation may be sufficient to augment memory and cognition in patients with neurodegenerative diseases. Thus, low doses of CX1739 might be used in the treatment of dementias associated with neurodegenerative disorders like Alzheimer’s disease, most likely by restoring AMPAergic functionality in brain areas that experience age-related reductions in AMPAR protein production [
45,
46,
47] or reduced insertion into synapses [
48]. The potential neurotrophic and cognition-augmenting effects of chronic CX1739 administration, as opposed to the acute single dosing used in the studies herein, serves as an avenue of future research.
Our results demonstrate that low-impact ampakines have the potential to augment multiple aspects of cognition, though more extensive clinical testing is required to parcel out what aspects of memory may be most improved via ampakine treatment. One clinical study using the low-impact ampakine CX717 indicated that 1000 mg CX717 improved attention-based task performance in sleep-deprived adults undergoing acute sleep deprivation [
49]. Farampator, also known as CX691, improved short-term memory in healthy adults yet appeared to diminish episodic memory [
50]. One of the earliest clinical assessments of low-impact ampakine function was undertaken examining the effects of CX516 (Ampalex) on multiple aspects of memory. The results indicated that 300 mg CX516 augmented visual associations, odor recognition, acquisition of a visuospatial maze and location, and identity playing cards, yet did not increase scores in a task based on recalling verbal information [
51]. While these preliminary clinical results are encouraging, another clinical study of CX717 demonstrated CX717 did not reverse the deficits in performance and alertness associated with night shift work [
52]. The paucity of clinical data and some conflicting data indicate that more clinical studies are required to determine areas and situations in which ampakines can effectively enhance cognitive performance. Furthermore, higher doses of ampakines such as of CX717 or CX1739, possibly >1500 mg, may be required to exert therapeutic effects in certain neurological disease settings.
In addition to the pro-cognitive effects of CX1739 and the anti-impulsivity effects of CX1739, we assessed the utility of CX1739 to treat ADHD by examining its anti-hyperactive effects. The interaction of CX1739 with amphetamine, a stimulant frequently prescribed to patients with ADHD, showed that CX1739 reduced amphetamine-induced LMA with an AD
50 of 18 mg/kg (
Figure 6). In this regard, we suggest that CX1739 might be valuable either alone (as with CX717 in patients, [
53]) or in conjunction with a stimulant such as amphetamine. This combination therapy could be considered a possible augmentation therapeutic effect while antagonizing the unwanted side effects of the stimulant alone. This dual-drug regimen may prove to be a powerful tool in treating patients who are refractory to treatment with a stimulant alone.
We tested whether CX1739 could reverse alfentanil-induced respiratory depression in vivo. This is based on the fact that breathing is regulated by a balance between excitatory glutamatergic and inhibitory opioid input to respiratory control neurons in the brainstem [
26]. Ampakines have previously been shown to reverse opioid-induced respiratory depression in preclinical [
26] and clinical settings [
19], results that reflect appropriate target engagement. Our results demonstrate that CX1739 injection rapidly and dose-dependently reversed the respiratory depression produced by the opioid and that the minimum effective dose was 10 mg/kg. Furthermore, we observed a complete reversal of respiratory depression at 20 mg/kg. Our preclinical results, showing a partial reversal of respiratory depression at 10 mg/kg, correlate well with preliminary clinical data, in which we also observed a partial effect using a CX1739 dose of ~11 mg/kg [
29].
A preliminary single-dose toxicity study in rats was conducted to determine doses at which CX1739 would begin to produce adverse effects. CX1739 was without adverse events in doses up to 2000 mg/kg. It was established that 3000 mg/kg CX1739 was lethal in the rats tested, demonstrating a profound therapeutic ratio of this drug—with doses of 0.03 to 18 mg/kg being efficacious in the behavioral studies. Importantly, CX1739 was also devoid of convulsant activities in all in vivo paradigms examined. These data demonstrate that the low-impact ampakine CX1739 exhibits a more favorable therapeutic margin, in contrast to AMPAR modulators such as the high-impact ampakine cyclothiazide [
22] and the AMPAR potentiator PF-4778574 [
54]. This notable therapeutic ratio may be attributable to CX1739’s only modest alteration on AMPAergic steady-state currents, whereas cyclothiazide exhibits a profound effect on glutamate-induced inward currents [
42].
It is interesting to speculate that the widely variable potency of CX1739 in the studies described herein might be due to differences in AMPA receptor subunit expression [
55] and/or differential association with accessory proteins [
56,
57] in various brain regions. In the NOR task and the radial arm maze, CX1739 exhibited efficacy in the μg/kg range, while for other assays, higher doses were needed to observe an effect (respiratory depression, 5-CSRTT, amphetamine-induced LMA). While we cannot be certain why CX1739 was more potent in some assays over others, it is important to note that other low-impact ampakines like CX516 and CX691 increased neurotrophin levels in the rat brain to a greater extent in some brain regions than in others [
58]. This may be due to differences in AMPAR subunit expression and AMPAR heterogeneity in various brain regions. Therefore, in tasks associated with brain regions that express AMPARs for which CX1739 has a higher affinity, we may observe increased potency. In tasks associated with brain regions that express AMPARs for which CX1739 has a lower affinity, higher doses may be needed to observe an effect. Future receptor binding studies using protein homogenates from different brain regions will assist in answering this question. However, in two of the assays, amphetamine-induced LMA and opiate-induced respiratory depression, CX1739 had to overcome the pharmacological actions of another drug, making it difficult to compare potencies in these assays with potencies of CX1739 in other assays where drug interactions were not a factor. In these two assays, however, CX1739 exhibited similar potencies, an important observation for future clinical studies.
CX1739 may have uses in addition to those described here. When the cervical spine is injured, respiratory drive is diminished, which can be a substantial burden for the remainder of these patients’ lives. In an effort to increase respiratory neuroplasticity, those with cervical spinal cord injury are subjected to bouts of acute intermittent hypoxia [
25]. To enhance therapeutic gains, it has been described recently that low-impact ampakines can enhance respiratory neuroplasticity induced by acute intermittent hypoxia [
25]. Furthermore, also in the setting of spinal cord injury, ampakines have been shown to increase voiding function and coordination [
30]. It is becoming evident that in multiple neurological disease settings, AMPAR-positive allosteric modulation may yield significant benefits to patients with wide ranging symptomatologies.
In the work described here, we demonstrated that CX1739 has pro-cognitive effects in several preclinical models. CX1739 also mitigates the unwanted motor-activating effects of amphetamine, which could have robust implications for the treatment of ADHD in the clinical setting. We also demonstrate that CX1739 can rapidly reverse opiate-induced respiratory depression, all while failing to produce convulsant effects or adverse events in these preclinical models. Altogether, enhancement of glutamatergic neurotransmission by low-impact ampakines, including CX1739, may represent an alternative approach for symptomatic treatment of neurological/neuropsychiatric conditions in which excitatory neurosynaptic transmission is compromised. The ability of CX1739 to antagonize opioid-induced respiratory depression in humans in the absence of effects on analgesia [
29] strongly suggests that target engagement of AMPA receptors may be safely achieved in the absence of neurotoxicity and raises hope that the present preclinical results may be translated into therapeutic benefits for neurological and psychiatric disorders.