Atazanavir/Ritonavir Increased Tizoxanide Exposure from Oral Nitazoxanide through Pharmacokinetic Interaction in Healthy Volunteers
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Sample Collection
2.3. Bioanalysis of Study Samples
2.4. Data Analysis
3. Results
3.1. Participant’s Disposition and Demographics
3.2. Method Validation
3.2.1. Linearity, Accuracy, and Precision of Plasma and DBS Assay
3.2.2. Recovery, Matrix Effect, and Extraction Proficiency
3.2.3. Pharmacokinetics of Tizoxanide and the Effects of Co-Administration of Atazanavir/Ritonavir
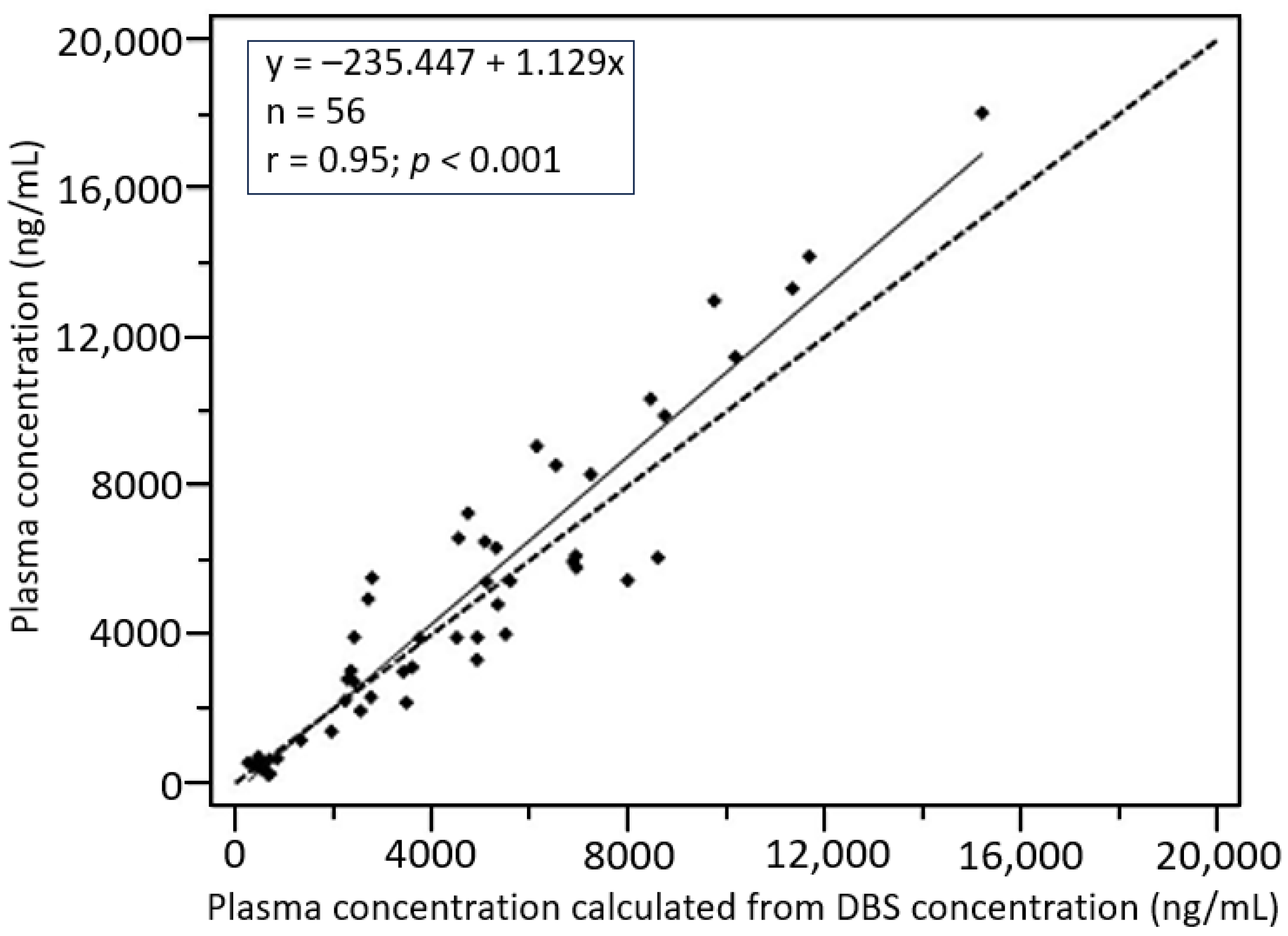
3.2.4. Plasma-DBS Cross-Validation
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Wang, R.; Wu, P.; Kong, D. Drug Repurposing Based on Drug–Drug Interaction. Chem. Biol. Drug Des. 2014, 85, 137–144. [Google Scholar] [CrossRef]
- Sahu, N.U.; Kharkar, P.S. Computational Drug Repositioning: A Lateral Approach to Traditional Drug Discovery? Curr. Top. Med. Chem. 2016, 16, 2069–2077. [Google Scholar] [CrossRef]
- Low, Z.Y.; Farouk, I.A.; Lal, S.K. Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak. Viruses 2020, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Ai, N.; Fan, X.; Ekins, S. In silico methods for predicting drug–drug interactions with cytochrome P-450s, transporters and beyond. Adv. Drug Deliv. Rev. 2015, 86, 46–60. [Google Scholar] [CrossRef] [PubMed]
- El-Fadeal, N.M.A.; Nafie, M.S.; El-Kherbetawy, M.K.; El-Mistekawy, A.; Mohammad, H.M.F.; Elbahaie, A.M.; Hashish, A.A.; Alomar, S.Y.; Aloyouni, S.Y.; El-Dosoky, M.; et al. Antitumor Activity of Nitazoxanide against Colon Cancers: Molecular Docking and Experimental Studies Based on Wnt/β-Catenin Signaling Inhibition. Int. J. Mol. Sci. 2021, 22, 5213. [Google Scholar] [CrossRef]
- Bolia, R. Nitazoxanide: Jack of All, Master of None? Indian J. Pediatr. 2019, 87, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, N.; Ehrisman, J. A functional perspective of nitazoxanide as a potential anticancer drug. Mutat. Res. Mol. Mech. Mutagen. 2014, 768, 16–21. [Google Scholar] [CrossRef]
- Lü, Z.; Li, X.; Li, K.; Wang, C.; Du, T.; Huang, W.; Ji, M.; Li, C.; Xu, F.; Xu, P.; et al. Structure–Activity Study of Nitazoxanide Derivatives as Novel STAT3 Pathway Inhibitors. ACS Med. Chem. Lett. 2021, 12, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Grayson, M.L.; Cosgrove, S.E.; Crowe, S.; Hope, W.; McCarthy, J.S.; Mills, J.; Mouton, J.W.; Paterson, D.L. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, 7th ed.; Taylor and Francis: Abingdon, UK, 2017. [Google Scholar] [CrossRef]
- Dubreuil, L.; Houcke, I.; Mouton, Y.; Rossignol, J.F. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob. Agents Chemother. 1996, 40, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.R.; Curran, M.P. Nitazoxanide. Drugs 2007, 67, 1947–1967. [Google Scholar] [CrossRef]
- Aslam, S.; Musher, D.M. Nitazoxanide: Clinical studies of a broad-spectrum anti-infective agent. Futur. Microbiol. 2007, 2, 583–590. [Google Scholar] [CrossRef]
- Chero, J.C.; Saito, M.; Bustos, J.A.; Blanco, E.M.; Gonzalvez, G.; Garcia, H.H. Hymenolepis nana infection: Symptoms and response to nitazoxanide in field conditions. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Speich, B.; Ame, S.M.; Ali, S.M.; Alles, R.; Hattendorf, J.; Utzinger, J.; Albonico, M.; Keiser, J. Efficacy and Safety of Nitazoxanide, Albendazole, and Nitazoxanide-Albendazole against Trichuris trichiura Infection: A Randomized Controlled Trial. PLoS Negl. Trop. Dis. 2012, 6, e1685. [Google Scholar] [CrossRef]
- Lokhande, A.S.; Devarajan, P.V. A review on possible mechanistic insights of Nitazoxanide for repurposing in COVID-19. Eur. J. Pharmacol. 2021, 891, 173748. [Google Scholar] [CrossRef] [PubMed]
- Jasenosky, L.D.; Cadena, C.; Mire, C.E.; Borisevich, V.; Haridas, V.; Ranjbar, S.; Nambu, A.; Bavari, S.; Soloveva, V.; Sadukhan, S.; et al. The FDA-Approved Oral Drug Nitazoxanide Amplifies Host Antiviral Responses and Inhibits Ebola Virus. iScience 2019, 19, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Hargest, V.; Sharp, B.; Livingston, B.; Cortez, V.; Schultz-Cherry, S. Astrovirus Replication Is Inhibited by Nitazoxanide In Vitro and In Vivo. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xue, F.; Zhang, L.; Zhang, K.; Fei, C.; Zheng, W.; Wang, X.; Wang, M.; Meng, X. The pharmacokinetics of nitazoxanide active metabolite (tizoxanide) in goats and its protein binding ability in vitro. J. Veter-Pharmacol. Ther. 2010, 33, 147–153. [Google Scholar] [CrossRef]
- Stachulski, A.V.; Santoro, M.G.; Piacentini, S.; Belardo, G.; La Frazia, S.; Pidathala, C.; Row, E.C.; Berry, N.G.; Iqbal, M.; Allman, S.A.; et al. Second-generation nitazoxanide derivatives: Thiazolides are effective inhibitors of the influenza A virus. Futur. Med. Chem. 2018, 10, 851–862. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Rajoli, R.K.R.; Pertinez, H.; Arshad, U.; Box, H.; Tatham, L.; Curley, P.; Neary, M.; Sharp, J.; Liptrott, N.J.; Valentijn, A.; et al. Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis. Br. J. Clin. Pharmacol. 2021, 87, 2078–2088. [Google Scholar] [CrossRef]
- Stockis, A.; Allemon, A.-M.; De Bruyn, S.; Gengler, C. Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses. Int. J. Clin. Pharmacol. Ther. 2002, 40, 213–220. [Google Scholar] [CrossRef]
- Stockis, A.; De Bruyn, S.; Gengler, C.; Rosillon, D. Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5 g and 1 g b.i.d. Int. J. Clin. Pharmacol. Ther. 2002, 40, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.E.; FitzGerald, R.; Saunders, G.; Lyon, R.; Fisher, M.; Martin, K.; Eberhart, I.; Woods, C.; Ewings, S.; Hale, C.; et al. An Open Label, Adaptive, Phase 1 Trial of High-Dose Oral Nitazoxanide in Healthy Volunteers: An Antiviral Candidate for SARS-CoV-2. Clin. Pharmacol. Ther. 2022, 111, 585–594. [Google Scholar] [CrossRef]
- Walsh, K.F.; McAulay, K.; Lee, M.H.; Vilbrun, S.C.; Mathurin, L.; Francois, D.J.; Zimmerman, M.; Kaya, F.; Zhang, N.; Saito, K.; et al. Early Bactericidal Activity Trial of Nitazoxanide for Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.; Eke, A.C.; Best, B.M.; Mirochnick, M.; Momper, J.D. Pharmacokinetic Enhancement of HIV Antiretroviral Therapy During Pregnancy. J. Clin. Pharmacol. 2020, 60, 1537–1550. [Google Scholar] [CrossRef]
- Larson, K.B.; Wang, K.; Delille, C.; Otofokun, I.; Acosta, E.P. Pharmacokinetic Enhancers in HIV Therapeutics. Clin. Pharmacokinet. 2014, 53, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Westra, N.; Touw, D.; Hooge, M.L.-D.; Kosterink, J.; Munnink, T.O. Pharmacokinetic Boosting of Kinase Inhibitors. Pharmaceutics 2023, 15, 1149. [Google Scholar] [CrossRef] [PubMed]
- van Veelen, A.; Gulikers, J.; Hendriks, L.E.; Dursun, S.; Ippel, J.; Smit, E.F.; Dingemans, A.-M.C.; van Geel, R.; Croes, S. Pharmacokinetic boosting of osimertinib with cobicistat in patients with non-small cell lung cancer: The OSIBOOST trial. Lung Cancer 2022, 171, 97–102. [Google Scholar] [CrossRef]
- Boosman, R.J.; de Gooijer, C.J.; Groenland, S.L.; Burgers, J.A.; Baas, P.; van der Noort, V.; Beijnen, J.H.; Huitema, A.D.; Steeghs, N. Ritonavir-Boosted Exposure of Kinase Inhibitors: An Open Label, Cross-over Pharmacokinetic Proof-of-Concept Trial with Erlotinib. Pharm. Res. 2022, 39, 669–676. [Google Scholar] [CrossRef]
- Zhang, D.; Chando, T.J.; Everett, D.W.; Patten, C.J.; Dehal, S.S.; Humphreys, W.G. In Vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to In Vivo bilirubin glucuronidation. Drug Metab. Dispos. 2005, 33, 1729–1739. [Google Scholar] [CrossRef]
- Neary, M.; Arshad, U.; Tatham, L.; Pertinez, H.; Box, H.; Rajoli, R.K.; Valentijn, A.; Sharp, J.; Rannard, S.P.; Biagini, G.A.; et al. Quantitation of tizoxanide in multiple matrices to support cell culture, animal and human research. J. Chromatogr. B 2023, 1228, 123823. [Google Scholar] [CrossRef]
- FDA. Bioanalytical Method Validation; Guidance for Industry; Availability; Federal Information & News Dispatch, LLC.: Washington, DC, USA, 2018; Volume 83. [Google Scholar]
- FDA. Clinical Drug Interaction Studies: Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions; Guidance for Industry; Centre for Drug Evaluation and Research, FDA: Silver Spring, MY, USA, 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions (accessed on 10 December 2023).
- Li, W.; Tse, F.L.S. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 2010, 24, 49–65. [Google Scholar] [CrossRef]
- Mondal, H.; Lotfollahzadeh, S. Hematocrit. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vets, E.; Rossignol, J.-F.; Jackson, A.S. Effects of nitazoxanide on pharmacokinetics and pharmacodynamics of a single dose of warfarin. Am. J. Health Pharm. 2009, 66, 838–842. [Google Scholar] [CrossRef]
- Stachulski, A.V.; Taujanskas, J.; Pate, S.L.; Rajoli, R.K.R.; Aljayyoussi, G.; Pennington, S.H.; Ward, S.A.; Hong, W.D.; Biagini, G.A.; Owen, A.; et al. Therapeutic Potential of Nitazoxanide: An Appropriate Choice for Repurposing versus SARS-CoV-2? ACS Infect. Dis. 2021, 7, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Blum, V.F.; Cimerman, S.; Hunter, J.R.; Tierno, P.; Lacerda, A.; Soeiro, A.; Cardoso, F.; Bellei, N.C.; Maricato, J.; Mantovani, N.; et al. Nitazoxanide superiority to placebo to treat moderate COVID-19—A Pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine 2021, 37, 100981. [Google Scholar] [CrossRef] [PubMed]
- Bobrowski, T.; Chen, L.; Eastman, R.T.; Itkin, Z.; Shinn, P.; Chen, C.Z.; Guo, H.; Zheng, W.; Michael, S.; Simeonov, A.; et al. Synergistic and Antagonistic Drug Combinations against SARS-CoV-2. Mol. Ther. 2021, 29, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Fintelman-Rodrigues, N.; Sacramento, C.Q.; Lima, C.R.; da Silva, F.S.; Ferreira, A.C.; Mattos, M.; de Freitas, C.S.; Soares, V.C.; da Silva Gomes Dias, S.; Temerozo, J.R.; et al. Atazanavir, Alone or in Combination with Ritonavir, Inhibits SARS-CoV-2 Replication and Proinflammatory Cytokine Production. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Chaves, O.A.; Sacramento, C.Q.; Ferreira, A.C.; Mattos, M.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Vazquez, L.; Pinto, D.P.; da Silveira, G.P.E.; da Fonseca, L.B.; et al. Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 Mpro, Impairing Variants Replication In Vitro and In Vivo. Pharmaceuticals 2021, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidance on Regulations for the Transport of Infectious Substances 2013–2014: Applicable as from 1 January 2013; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Olagunju, A.; Amara, A.; Waitt, C.; Else, L.; Penchala, S.D.; Bolaji, O.; Soyinka, J.; Siccardi, M.; Back, D.; Owen, A.; et al. Validation and clinical application of a method to quantify nevirapine in dried blood spots and dried breast-milk spots. J. Antimicrob. Chemother. 2015, 70, 2816–2822. [Google Scholar] [CrossRef]
- Akinloye, A.M.; Eniayewu, O.M.; Adeagbo, B.; Bolaji, O.; Olagunju, A. Validation and Clinical Application of a Liquid Chromatography–Ultraviolet Detection Method to Quantify Dolutegravir in Dried Blood Spots. Ther. Drug Monit. 2022, 44, 430–437. [Google Scholar] [CrossRef] [PubMed]

| Nominal Conc (ng/mL) | Inter-Assay | Intra-Assay | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (ng/mL) | SD | %Accuracy | %CV | Mean (ng/mL) | SD | %Accuracy | %CV | |
| 50 | 46.9 | 3.9 | 84.4 | 8.3 | 45.1 | 0.5 | 90.3 | 1.0 |
| 150 | 154.0 | 9.8 | 102.6 | 6.4 | 162.1 | 3.5 | 108.0 | 2.2 |
| 400 | 410.7 | 33.8 | 92.4 | 8.2 | 448.8 | 16.0 | 112.2 | 3.6 |
| 1000 | 1060.0 | 85.0 | 94.2 | 8.0 | 974.6 | 40.9 | 97.5 | 4.2 |
| 2500 | 2671.6 | 196.2 | 106.9 | 7.3 | 2640.2 | 165.3 | 105.6 | 6.3 |
| 5000 | 5076.0 | 359.9 | 101.5 | 7.1 | 5205.5 | 192.8 | 104.1 | 3.7 |
| 10,000 | 9850.4 | 436.9 | 98.5 | 4.4 | 9797.1 | 416.5 | 98.0 | 4.3 |
| 15,000 | 14,055.0 | 1239.5 | 93.7 | 8.8 | 14,008.4 | 1301.6 | 93.4 | 9.3 |
| 20,000 | 18,729.4 | 1975.5 | 93.6 | 10.5 | 19,647.2 | 1158.0 | 98.2 | 5.9 |
| LLOQ (50 ng/mL) | 47.3 | 3.3 | 94.6 | 7.0 | 46.6 | 4.5 | 93.1 | 9.7 |
| LQC (120 ng/mL) | 116.9 | 10.6 | 97.4 | 9.0 | 125.9 | 9.2 | 104.9 | 7.3 |
| MQC (8600 ng/mL) | 8455.0 | 896.7 | 98.3 | 10.6 | 8934.3 | 697.2 | 103.9 | 7.8 |
| HQC (18,000 ng/mL) | 18,080.8 | 1481.2 | 100.4 | 8.2 | 19,464.7 | 1058.7 | 108.1 | 5.4 |
| Nominal Conc (ng/mL) | Inter-Assay | Intra-Assay | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (ng/mL) | SD | %Accuracy | %CV | Mean (ng/mL) | SD | %Accuracy | %CV | |
| 50 | 51.6 | 6.25 | 103.1 | 12.12 | 54.0 | 4.12 | 107.9 | 4.12 |
| 150 | 145.4 | 14.8 | 97.0 | 10.18 | 137.7 | 6.05 | 91.8 | 4.39 |
| 400 | 414.6 | 29.25 | 96.3 | 7.05 | 417.9 | 9.22 | 104.5 | 2.21 |
| 1000 | 998.9 | 86.1 | 99.9 | 8.63 | 987.83 | 68.29 | 98.8 | 6.91 |
| 2500 | 2418.6 | 236.76 | 96.8 | 9.79 | 2487.7 | 93.88 | 99.5 | 3.77 |
| 5000 | 4902.9 | 478.28 | 98.1 | 9.76 | 4660.5 | 235.21 | 93.2 | 5.05 |
| 10,000 | 9829.3 | 773.53 | 98.3 | 7.87 | 9403.2 | 518.96 | 94 | 5.52 |
| 15,000 | 15,510.0 | 720.3 | 103.4 | 4.64 | 14,828.3 | 624.43 | 98.9 | 4.21 |
| 20,000 | 20,058.4 | 673.36 | 100.3 | 3.36 | 19,944.4 | 97.91 | 99.7 | 0.49 |
| LLOQ (50 ng/mL) | 52.9 | 5.7 | 105.7 | 10.8 | 49.5 | 6.5 | 99.1 | 13.1 |
| LQC (120 ng/mL) | 118.3 | 13.5 | 98.6 | 11.4 | 117.5 | 13.1 | 97.9 | 11.2 |
| MQC (8600 ng/mL) | 9085.2 | 729.1 | 105.6 | 8.1 | 9012.3 | 881.7 | 104.8 | 9.8 |
| HQC (18,000 ng/mL) | 17,604.7 | 1793.4 | 97.9 | 10.2 | 19,504.4 | 648.6 | 108.4 | 3.3 |
| Pharmacokinetic Parameters (n = 18) | NTZ Alone | NTZ Plus ATZ/r | GMR (90% CI) | ||
|---|---|---|---|---|---|
| Mean (%CV) | GM (%CV) | Mean (%CV) | GM (%CV) | ||
| AUC0–12 h (ng.h/mL) | 171,135.9 (111.4) | 124,967.7 (6.4) | 289,297.9 (58.9) | 233,984.1 (6.1) | 1872 (1870–1875) |
| Cmax (ng/mL) | 5304.4 (64.1) | 4375.7 (7.8) | 10,495.4 (47.7) | 8882.1 (7.8) | 2029 (1990–2070) |
| Cτ (ng/m L) | 1898.2 (262.2) | 553.8 (22.9) | 3082.8 (74.7) | 1740.7 (29.8) | 3143 (2268–4352) |
| Pharmacokinetic Parameters (n = 18) | NTZ Alone | NTZ Plus ATZ/r | GMR [%, (90% CI)] | ||
|---|---|---|---|---|---|
| Mean (%CV) | GM (%CV) | Mean (%CV) | GM (%CV) | ||
| AUC0–12 h (ng.h/mL) | 16,168.4 (75.8) | 13,646.2 (8.9) | 42,403.0 (24.3) | 41,772.6 (2.3) | 3.1 (1.5–6.2) |
| Cmax (ng/mL) | 3528.3 (94.4) | 2626.5 (14.5) | 8034.7 (7.8) | 8022.5 (0.9) | 3.05 (1.68–10.5) |
| Cτ (ng/m L) | 389.1 (14.2) | 387.1 (2.4) | 699.0 (18.3) | 550.5 (5.8) | 1.42 (1.28–2.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinloye, A.; Oyedeji, T.; Eniayewu, O.; Adeagbo, B.; Bolaji, O.; Rannard, S.; Owen, A.; Olagunju, A. Atazanavir/Ritonavir Increased Tizoxanide Exposure from Oral Nitazoxanide through Pharmacokinetic Interaction in Healthy Volunteers. Future Pharmacol. 2024, 4, 163-172. https://doi.org/10.3390/futurepharmacol4010011
Akinloye A, Oyedeji T, Eniayewu O, Adeagbo B, Bolaji O, Rannard S, Owen A, Olagunju A. Atazanavir/Ritonavir Increased Tizoxanide Exposure from Oral Nitazoxanide through Pharmacokinetic Interaction in Healthy Volunteers. Future Pharmacology. 2024; 4(1):163-172. https://doi.org/10.3390/futurepharmacol4010011
Chicago/Turabian StyleAkinloye, Abdulafeez, Timothy Oyedeji, Oluwasegun Eniayewu, Babatunde Adeagbo, Oluseye Bolaji, Steve Rannard, Andrew Owen, and Adeniyi Olagunju. 2024. "Atazanavir/Ritonavir Increased Tizoxanide Exposure from Oral Nitazoxanide through Pharmacokinetic Interaction in Healthy Volunteers" Future Pharmacology 4, no. 1: 163-172. https://doi.org/10.3390/futurepharmacol4010011
APA StyleAkinloye, A., Oyedeji, T., Eniayewu, O., Adeagbo, B., Bolaji, O., Rannard, S., Owen, A., & Olagunju, A. (2024). Atazanavir/Ritonavir Increased Tizoxanide Exposure from Oral Nitazoxanide through Pharmacokinetic Interaction in Healthy Volunteers. Future Pharmacology, 4(1), 163-172. https://doi.org/10.3390/futurepharmacol4010011









