Cyclodextrin in Vaccines: Enhancing Efficacy and Stability
Abstract
:1. Introduction
2. Brief History of Cyclodextrins
3. Structure and Physicochemical Properties of Cyclodextrins
4. Cyclodextrin in Vaccine Formulations
4.1. Cyclodextrins in Vaccine Targeting
4.2. Cyclodextrins as Stabilizers
4.3. Cyclodextrins as Immunmodulator
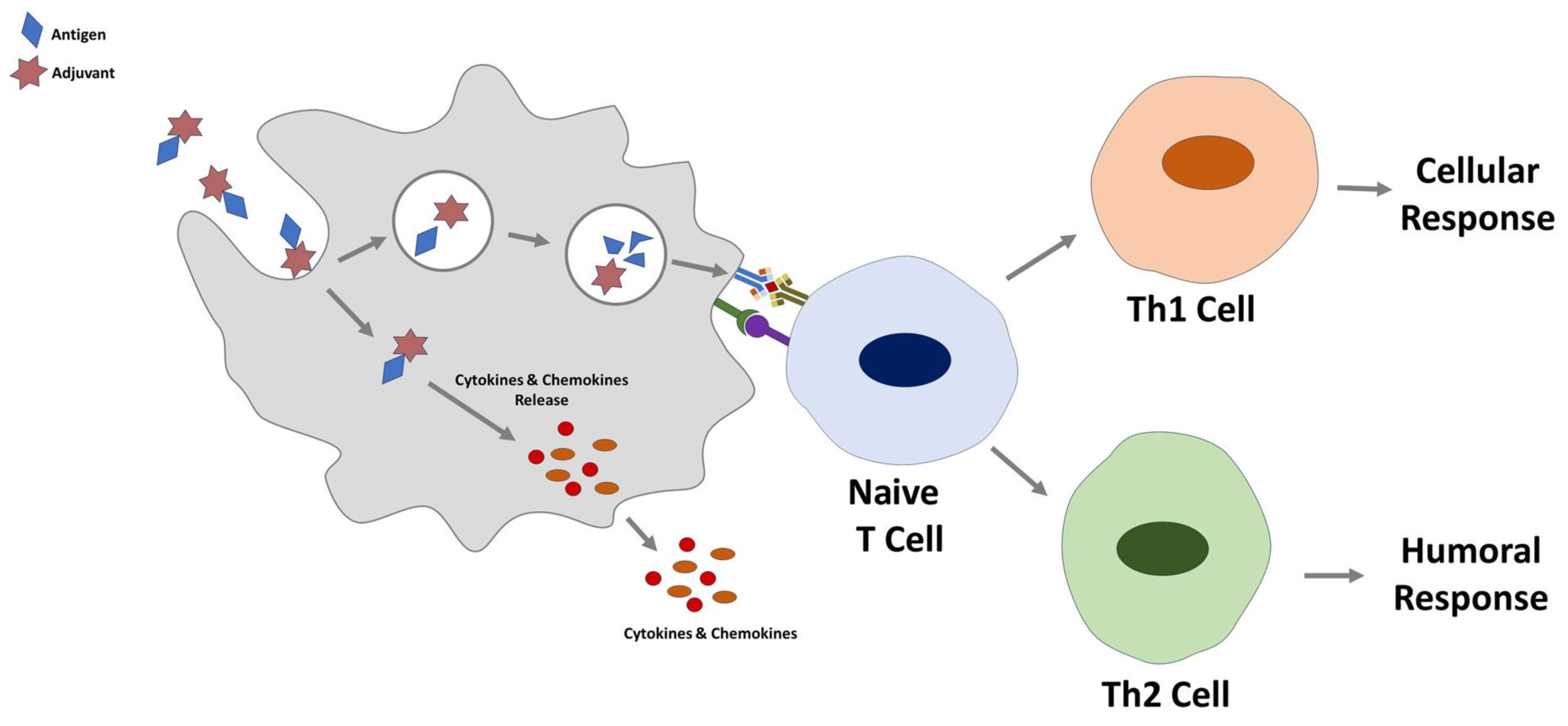
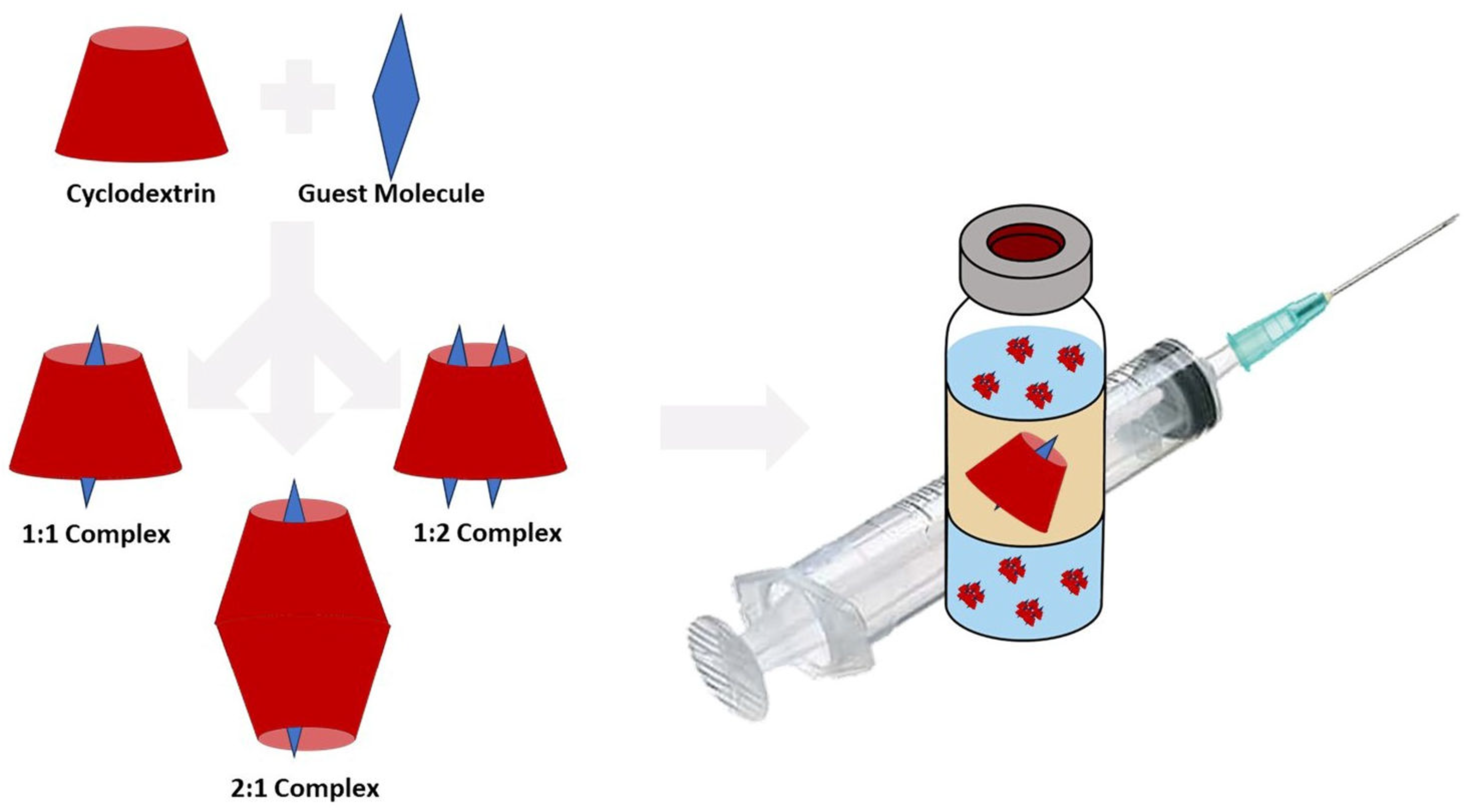
4.4. Cyclodextrins as Nano-Sized Carrier Systems
4.5. Future Directions
5. Conclusions
Funding
Conflicts of Interest
References
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Saylor, K.; Gillam, F.; Lohneis, T.; Zhang, C. Designs of Antigen Structure and Composition for Improved Protein-Based Vaccine Efficacy. Front. Immunol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Braga, S.S.; Barbosa, J.S.; Santos, N.E.; El-Saleh, F.; Paz, F.A.A. Cyclodextrins in Antiviral Therapeutics and Vaccines. Pharmaceutics 2021, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.F.; Calvelo, M.; Blanco-González, A.; Veleiro, U.; Suárez, F.; Conde, D.; Cabezón, A.; Piñeiro, Á.; Garcia-Fandino, R. The Lord of the NanoRings: Cyclodextrins and the battle against SARS-CoV-2. Int. J. Pharm. 2020, 588, 119689. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives-A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef]
- Grego, E.A.; Siddoway, A.C.; Uz, M.; Liu, L.; Christiansen, J.C.; Ross, K.A.; Kelly, S.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Polymeric Nanoparticle-Based Vaccine Adjuvants and Delivery Vehicles. Curr. Top. Microbiol. Immunol. 2021, 433, 29–76. [Google Scholar] [CrossRef]
- Erdoğar, N.; Varan, G.; Varan, C.; Bilensoy, E. Chapter 19—Cyclodextrin-based polymeric nanosystems. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 715–748. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Erdoğar, N.; Hıncal, A.A.; Bilensoy, E. Amphiphilic cyclodextrin nanoparticles. Int. J. Pharm. 2017, 531, 457–469. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Puskás, I.; Szente, L.; Szőcs, L.; Fenyvesi, É. Recent List of Cyclodextrin-Containing Drug Products. Period. Polytech. Chem. Eng. 2023, 67, 11–17. [Google Scholar] [CrossRef]
- Bar, R. Application of Cyclodextrins In Biotechnology. In Proceedings of the Eighth International Symposium on Cyclodextrins; Springer: Dordrecht, The Netherlands, 1996; pp. 521–526. [Google Scholar]
- Singh, M.; Sharma, R.; Banerjee, U.C. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. The cyclodextrins and their applications in biotechnology. Carbohydr. Polym. 1990, 12, 375–392. [Google Scholar] [CrossRef]
- Ünal, S.; Bilensoy, E. Oral Administration of Nanoparticles and Approaches for Design, Evaluation, and State of the Art. In Drug Delivery with Targeted Nanoparticles: In Vitro and In Vivo Evaluation Methods; CRC Press: Boca Raton, FL, USA, 2021; pp. 539–568. [Google Scholar]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Cedillo-Flores, O.E.; Rodríguez-Laguna, N.; Hipólito-Nájera, A.R.; Nivón-Ramírez, D.; Gómez-Balderas, R.; Moya-Hernández, R. Effect of the pH on the thermodynamic stability of inclusion complexes of thymol and carvacrol in β-cyclodextrin in water. Food Hydrocoll. 2022, 124, 107307. [Google Scholar] [CrossRef]
- Samuelsen, L.; Holm, R.; Lathuile, A.; Schönbeck, C. Correlation between the stability constant and pH for β-cyclodextrin complexes. Int. J. Pharm. 2019, 568, 118523. [Google Scholar] [CrossRef]
- Albers, E.; Müller, B.W. Cyclodextrin derivatives in pharmaceutics. Crit. Rev. Ther. Drug Carr. Syst. 1995, 12, 311–337. [Google Scholar] [CrossRef]
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. Proceedings 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-B.; Doherty, T.M.; Vetter, V.; Bonanni, P. Response letter Re: The burden of seasonal influenza: Improving vaccination coverage to mitigate morbidity and its impact on healthcare systems. Expert Rev. Vaccines 2023, 22, 528–529. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, C.; Fontana, F.; Cheng, R.; Santos, H.A. Development of vaccine formulations: Past, present, and future. Drug Deliv. Transl. Res. 2021, 11, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Nazari, M. New Generation Vaccines for COVID-19 Based on Peptide, Viral Vector, Artificial Antigen Presenting Cell, DNA or mRNA. Avicenna J. Med. Biotechnol. 2022, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-age vaccine adjuvants, their development, and future perspective. Front. Immunol. 2023, 14, 1043109. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, S.; Rashid, S. Correlation Study of the Most Important Environmental Influencing Factors on the Razi MMR Vaccine. Arch. Razi. Inst. 2021, 76, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, M.; Andreozzi, P.; Paulose, J.; D’Alicarnasso, M.; Cagno, V.; Donalisio, M.; Civra, A.; Broeckel, R.M.; Haese, N.; Jacob Silva, P.; et al. Additives for vaccine storage to improve thermal stability of adenoviruses from hours to months. Nat. Commun. 2016, 7, 13520. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, T.; Ozasa, K.; Kobari, S.; Momota, M.; Kishishita, N.; Kobiyama, K.; Kuroda, E.; Ishii, K.J. Intranasal hydroxypropyl-β-cyclodextrin-adjuvanted influenza vaccine protects against sub-heterologous virus infection. Vaccine 2016, 34, 3191–3198. [Google Scholar] [CrossRef]
- Lee, A.; Dadhwal, S.; Gamble, A.; Hook, S. Liposomes with cyclodextrin channels and polyethyleneimine (PEI) improves cytoplasmic vaccine delivery and induces anti-cancer immune activity in mice. J. Liposome Res. 2022, 32, 22–31. [Google Scholar] [CrossRef]
- Watanabe, A.; Nishida, S.; Burcu, T.; Shibahara, T.; Kusakabe, T.; Kuroda, E.; Ishii, K.J.; Kumanogoh, A. Safety and immunogenicity of a quadrivalent seasonal influenza vaccine adjuvanted with hydroxypropyl-β-cyclodextrin: A phase 1 clinical trial. Vaccine 2022, 40, 4150–4159. [Google Scholar] [CrossRef]
- Kurosawa, Y.; Goto, S.; Mitsuya, K.; Otsuka, Y.; Yokoyama, H. Interaction mode of hydroxypropyl-β-cyclodextrin with vaccine adjuvant components Tween 80 and Triton X-100 revealed by fluorescence increasing-quenching analysis. Phys. Chem. Chem. Phys. 2023, 25, 6203–6213. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zheng, T.; Li, M.; Zhong, X.; Tang, Y.; Qin, M.; Sun, X. Optimization of an mRNA vaccine assisted with cyclodextrin-polyethyleneimine conjugates. Drug Deliv. Transl. Res. 2020, 10, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Bai, Y.; Dong, X.; Ma, T.; Zhu, D.; Mei, L.; Lv, F. Hydrogel/nanoadjuvant-mediated combined cell vaccines for cancer immunotherapy. Acta Biomater. 2021, 133, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Tan, Z.; Li, M.; Tao, J.; Guan, E.; Du, J.; Hu, Y. Multi-functional nanocomplex codelivery of Trp2 and R837 to activate melanoma-specific immunity. Int. J. Pharm. 2020, 582, 119310. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moreno, A.; Jiménez Blanco, J.L.; Mosher, J.; Swanson, D.R.; García Fernández, J.M.; Sharma, A.; Ceña, V.; Muñoz-Fernández, M.A. Nanoparticle-Delivered HIV Peptides to Dendritic Cells a Promising Approach to Generate a Therapeutic Vaccine. Pharmaceutics 2020, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef]
- Chan, L.; Mehrani, Y.; Minott, J.A.; Bridle, B.W.; Karimi, K. The Potential of Dendritic-Cell-Based Vaccines to Modulate Type 3 Innate Lymphoid Cell Populations. Int. J. Mol. Sci. 2023, 24, 2403. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Hammad, H.; Lambrecht, B.N. Professional and ‘Amateur’ Antigen-Presenting Cells In Type 2 Immunity. Trends Immunol. 2019, 40, 22–34. [Google Scholar] [CrossRef]
- Drouin, M.; Saenz, J.; Chiffoleau, E. C-Type Lectin-Like Receptors: Head or Tail in Cell Death Immunity. Front. Immunol. 2020, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Kamiya, T.; Suabjakyong, P.; Tsuji, N.M. Targeting C-Type Lectin Receptors for Cancer Immunity. Front. Immunol. 2015, 6, 408. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Domingues, C.; Mascarenhas-Melo, F.; Silva, I.; Jarak, I.; Veiga, F.; Figueiras, A. The Role of Cyclodextrins in COVID-19 Therapy-A Literature Review. Int. J. Mol. Sci. 2023, 24, 2974. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Zhou, Z.W.; Zhou, S.F. Cyclodextrin-based targeting strategies for tumor treatment. Drug Deliv. Transl. Res. 2013, 3, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Nizard, M.; Diniz, M.O.; Roussel, H.; Tran, T.; Ferreira, L.C.; Badoual, C.; Tartour, E. Mucosal vaccines: Novel strategies and applications for the control of pathogens and tumors at mucosal sites. Hum. Vaccine Immunother. 2014, 10, 2175–2187. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Nerli, G.; Mennini, N.; D’Ambrosio, M.; Luceri, C.; Mura, P.A. Development of a Cyclodextrin-Based Mucoadhesive-Thermosensitive In Situ Gel for Clonazepam Intranasal Delivery. Pharmaceutics 2021, 13, 969. [Google Scholar] [CrossRef]
- Fürst, A.; Kali, G.; Efiana, N.A.; Akkuş-Dağdeviren, Z.B.; Haddadzadegan, S.; Bernkop-Schnürch, A. Thiolated cyclodextrins: A comparative study of their mucoadhesive properties. Int. J. Pharm. 2023, 635, 122719. [Google Scholar] [CrossRef]
- Grassiri, B.; Cesari, A.; Balzano, F.; Migone, C.; Kali, G.; Bernkop-Schnürch, A.; Uccello-Barretta, G.; Zambito, Y.; Piras, A.M. Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization. Polymers 2022, 14, 3170. [Google Scholar]
- Li, M.; Zhao, M.; Fu, Y.; Li, Y.; Gong, T.; Zhang, Z.; Sun, X. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J. Control. Release 2016, 228, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Z.; Lean, Y.L.; Yeoh, S.F.; Lean, Q.Y.; Lee, K.S.; Suleiman, A.K.; Liew, K.B.; Kassab, Y.W.; Al-Worafi, Y.M.; Ming, L.C. Cold chain time- and temperature-controlled transport of vaccines: A simulated experimental study. Clin. Exp. Vaccine Res. 2020, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Erassa, T.E.; Bachore, B.B.; Faltamo, W.F.; Molla, S.; Bogino, E.A. Vaccine Cold Chain Management and Associated Factors in Public Health Facilities and District Health Offices of Wolaita Zone, Ethiopia. J. Multidiscip. Health 2023, 16, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Pambudi, N.A.; Sarifudin, A.; Gandidi, I.M.; Romadhon, R. Vaccine cold chain management and cold storage technology to address the challenges of vaccination programs. Energy Rep. 2022, 8, 955–972. [Google Scholar] [CrossRef]
- James, E.R. Disrupting vaccine logistics. Int. Health 2021, 13, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, S.; Mathiron, D.; Moufawad, T.; Landy, D.; Djedaini-Pilard, F.; Marçon, F. Cyclodextrin Complexation as a Way of Increasing the Aqueous Solubility and Stability of Carvedilol. Pharmaceutics 2021, 13, 1746. [Google Scholar] [CrossRef]
- Su, J.; Chen, J.; Li, L.; Li, B.; Shi, L.; Chen, L.; Xu, Z. Formation of β-cyclodextrin inclusion enhances the stability and aqueous solubility of natural borneol. J. Food Sci. 2012, 77, C658–C664. [Google Scholar] [CrossRef]
- Łagiewka, J.; Girek, T.; Ciesielski, W. Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications. Polymers 2021, 13, 1759. [Google Scholar] [CrossRef]
- Lai, W.F. Cyclodextrins in non-viral gene delivery. Biomaterials 2014, 35, 401–411. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Speiser, D.E.; Bachmann, M.F. COVID-19: Mechanisms of Vaccination and Immunity. Vaccines 2020, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Bezerra, B.B.; Silva, G.; Coelho, S.V.A.; Correa, I.A.; Souza, M.R.M.; Macedo, K.V.G.; Matos, B.M.; Tanuri, A.; Matassoli, F.L.; Costa, L.J.D.; et al. Hydroxypropyl-beta-cyclodextrin (HP-BCD) inhibits SARS-CoV-2 replication and virus-induced inflammatory cytokines. Antivir. Res. 2022, 205, 105373. [Google Scholar] [CrossRef]
- Onishi, M.; Ozasa, K.; Kobiyama, K.; Ohata, K.; Kitano, M.; Taniguchi, K.; Homma, T.; Kobayashi, M.; Sato, A.; Katakai, Y.; et al. Hydroxypropyl-β-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J. Immunol. 2015, 194, 2673–2682. [Google Scholar] [CrossRef]
- Kim, S.K.; Yun, C.H.; Han, S.H. Induction of Dendritic Cell Maturation and Activation by a Potential Adjuvant, 2-Hydroxypropyl-β-Cyclodextrin. Front. Immunol. 2016, 7, 435. [Google Scholar] [CrossRef]
- Javaid, N.; Yasmeen, F.; Choi, S. Toll-Like Receptors and Relevant Emerging Therapeutics with Reference to Delivery Methods. Pharmaceutics 2019, 11, 441. [Google Scholar] [CrossRef]
- Lucia Appleton, S.; Navarro-Orcajada, S.; Martínez-Navarro, F.J.; Caldera, F.; López-Nicolás, J.M.; Trotta, F.; Matencio, A. Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules 2021, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Öncül, S.; Ercan, A.; Benito, J.M.; Ortiz Mellet, C.; Bilensoy, E. Cholesterol-Targeted Anticancer and Apoptotic Effects of Anionic and Polycationic Amphiphilic Cyclodextrin Nanoparticles. J. Pharm. Sci. 2016, 105, 3172–3182. [Google Scholar] [CrossRef]
- Kobari, S.; Kusakabe, T.; Momota, M.; Shibahara, T.; Hayashi, T.; Ozasa, K.; Morita, H.; Matsumoto, K.; Saito, H.; Ito, S.; et al. IL-33 Is Essential for Adjuvant Effect of Hydroxypropyl-β-Cyclodexrin on the Protective Intranasal Influenza Vaccination. Front. Immunol. 2020, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Momota, M.; Kuroda, E.; Kusakabe, T.; Kobari, S.; Makisaka, K.; Ohno, Y.; Suzuki, Y.; Nakagawa, F.; Lee, M.S.J.; et al. DAMP-Inducing Adjuvant and PAMP Adjuvants Parallelly Enhance Protective Type-2 and Type-1 Immune Responses to Influenza Split Vaccination. Front. Immunol. 2018, 9, 2619. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.; Magrì, D.; Gioria, S.; Medaglini, D.; Calzolai, L. Characterization of nanoparticles-based vaccines for COVID-19. Nat. Nanotechnol. 2022, 17, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Zhang, A.; Wang, S.; Cheng, H.; Fan, D.; Huang, R.; Wang, Y.; Wan, B.; Zhang, G.; He, H. Splenic-targeting biomimetic nanovaccine for elevating protective immunity against virus infection. J. Nanobiotechnol. 2022, 20, 514. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Z.H.; Li, Y.X.; Xu, H.L.; Fang, W.H.; He, F. Targeted Delivery of Nanovaccine to Dendritic Cells via DC-Binding Peptides Induces Potent Antiviral Immunity in vivo. Int. J. Nanomed. 2022, 17, 1593–1608. [Google Scholar] [CrossRef]
- Rajput, M.K.S.; Kesharwani, S.S.; Kumar, S.; Muley, P.; Narisetty, S.; Tummala, H. Dendritic Cell-Targeted Nanovaccine Delivery System Prepared with an Immune-Active Polymer. ACS Appl. Mater. Interfaces 2018, 10, 27589–27602. [Google Scholar] [CrossRef]
- Xu, P.; Tang, S.; Jiang, L.; Yang, L.; Zhang, D.; Feng, S.; Zhao, T.; Dong, Y.; He, W.; Wang, R.; et al. Nanomaterial-dependent immunoregulation of dendritic cells and its effects on biological activities of contraceptive nanovaccines. J. Control. Release 2016, 225, 252–268. [Google Scholar] [CrossRef]
- Karthic, A.; Roy, A.; Lakkakula, J.; Alghamdi, S.; Shakoori, A.; Babalghith, A.O.; Emran, T.B.; Sharma, R.; Lima, C.M.G.; Kim, B.; et al. Cyclodextrin nanoparticles for diagnosis and potential cancer therapy: A systematic review. Front. Cell Dev. Biol. 2022, 10, 984311. [Google Scholar] [CrossRef]
- Geisshüsler, S.; Schineis, P.; Langer, L.; Wäckerle-Men, Y.; Leroux, J.-C.; Halin, C.; Vogel-Kindgen, S.; Johansen, P.; Gander, B. Amphiphilic Cyclodextrin-Based Nanoparticulate Vaccines Can Trigger T-Cell Immune Responses. Adv. NanoBiomed Res. 2022, 2, 2100082. [Google Scholar] [CrossRef]
- Yu, H.; Lin, H.; Xie, Y.; Qu, M.; Jiang, M.; Shi, J.; Hong, H.; Xu, H.; Li, L.; Liao, G.; et al. MUC1 vaccines using β-cyclodextrin grafted chitosan (CS-g-CD) as carrier via host-guest interaction elicit robust immune responses. Chin. Chem. Lett. 2022, 33, 4882–4885. [Google Scholar] [CrossRef]
| Physicochemical Properties | α-Cyclodextrin | β-Cyclodextrin | γ-Cyclodextrin |
|---|---|---|---|
| Number of glucopyranose unit | 6 | 7 | 8 |
| Formula | C36H60O30 | C42H70O35 | C48H80O40 |
| Water solubility (25 °C, g/L) | 145 | 18.5 | 232 |
| Molar mass (Da) | 972 | 1135 | 1297 |
| Height (Å) | 7.9 | 7.9 | 7.9 |
| Inner volume (Å3) | 174 | 262 | 427 |
| Outer diameter (Å) | 14.6 | 15.4 | 17.5 |
| Inner diameter (Å) | 4.7–5.3 | 6.0–6.5 | 7.5–8.3 |
| Half-life (1M HCL, 60 °C, h) | 6.2 | 5.4 | 3.0 |
| Absorption | 2–3% | 1–2% | 0.1% |
| Type of Cyclodextrin | Vaccine Type and Targeted Disease | Function of Cyclodextrin | Stage of Vaccine Development | References |
|---|---|---|---|---|
| per-fluoroalkyl-β-cyclodextrin | Protein (OVA)-based melanoma vaccine | Cyclodextrin channels to improve stability of liposome | Evaluation of efficacy with model antigen in an in vivo murine melanoma model | [32] |
| Hydroxypropyl-β-cyclodextrin | Protein (HA)-based Seasonal influenza vaccine | Adjuvant | Phase 1 clinical trial (Clinical trial registry: UMIN000028530) | [33] |
| γ-cyclodextrin | Protein (OVA)-based veterinary vaccine | Span 85 modified γ-cyclodextrin metal-organic framework as novel adjuvant | Evaluation of efficacy in immunized mice | [34] |
| β-cyclodextrin | mRNA (encoding OVA) vaccine | Branched PEI conjugated β-cyclodextrin as carrier system | Antigen-specific antibody detection in mice vaccinated subcutaneously, intradermally and intramuscularly | [35] |
| α-cyclodextrin | Combined cell vaccines (tumor whole cells +DCs) for melanoma | CpG adjuvanted-α-cyclodextrin-PEG hydrogel as carrier system | Evaluation of efficacy in an in vivo murine melanoma model | [36] |
| Mannosylated-β-cyclodextrin | Peptide-based and active targeted vaccine for melanoma | Specific delivery of antigen and TLR7 agonists to antigen presenting cells | Evaluation of immune response in immunized mice | [37] |
| Polyanionic amphiphilic β-cyclodextrin | Peptide-based vaccine for HIV | Nanoparticulate carrier system | Determination of cytokine release, DC targeting and immune response by cell culture studies | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varan, G. Cyclodextrin in Vaccines: Enhancing Efficacy and Stability. Future Pharmacol. 2023, 3, 597-611. https://doi.org/10.3390/futurepharmacol3030038
Varan G. Cyclodextrin in Vaccines: Enhancing Efficacy and Stability. Future Pharmacology. 2023; 3(3):597-611. https://doi.org/10.3390/futurepharmacol3030038
Chicago/Turabian StyleVaran, Gamze. 2023. "Cyclodextrin in Vaccines: Enhancing Efficacy and Stability" Future Pharmacology 3, no. 3: 597-611. https://doi.org/10.3390/futurepharmacol3030038
APA StyleVaran, G. (2023). Cyclodextrin in Vaccines: Enhancing Efficacy and Stability. Future Pharmacology, 3(3), 597-611. https://doi.org/10.3390/futurepharmacol3030038







