Single Turnover of Transient of Reactants Supports a Complex Interplay of Conformational States in the Mode of Action of Mycobacterium tuberculosis Enoyl Reductase
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant MtInhA Expression and Purification and Enoyl-CoA Substrate Synthesis and Purification
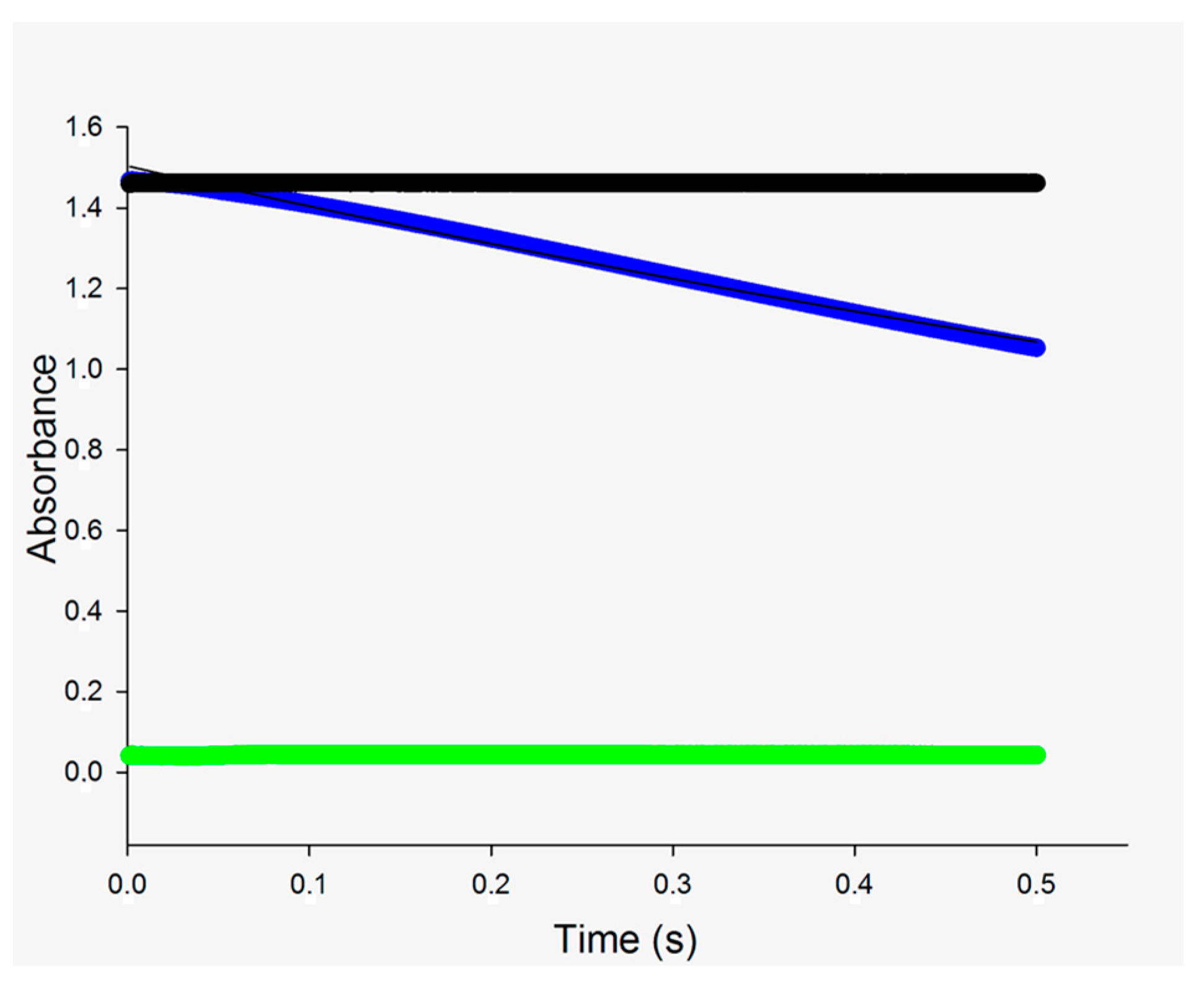
2.2. Single-Turnover Experiments
2.3. Burst
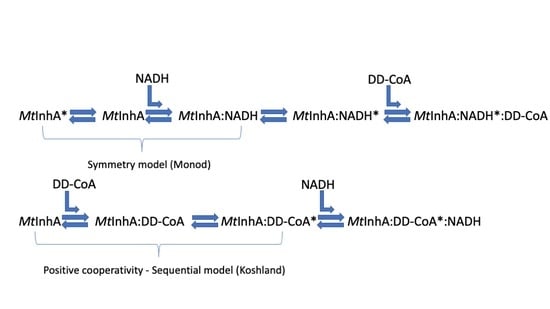
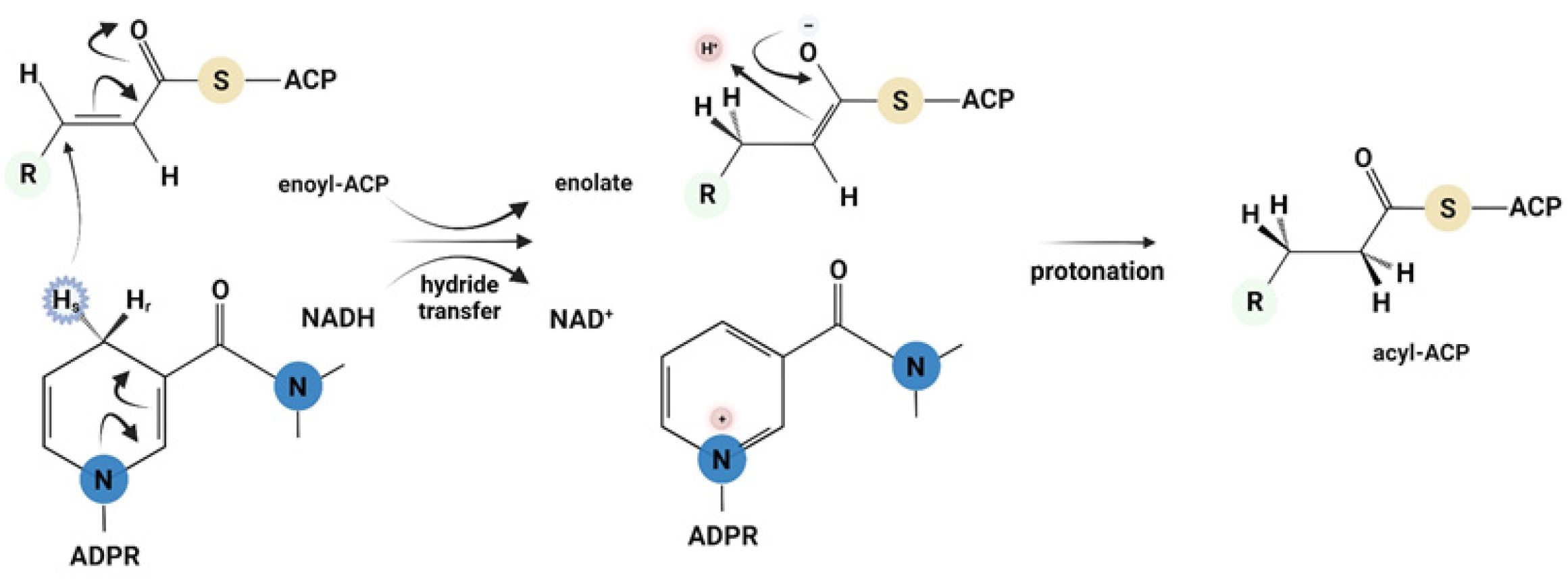
3. Results and Discussion
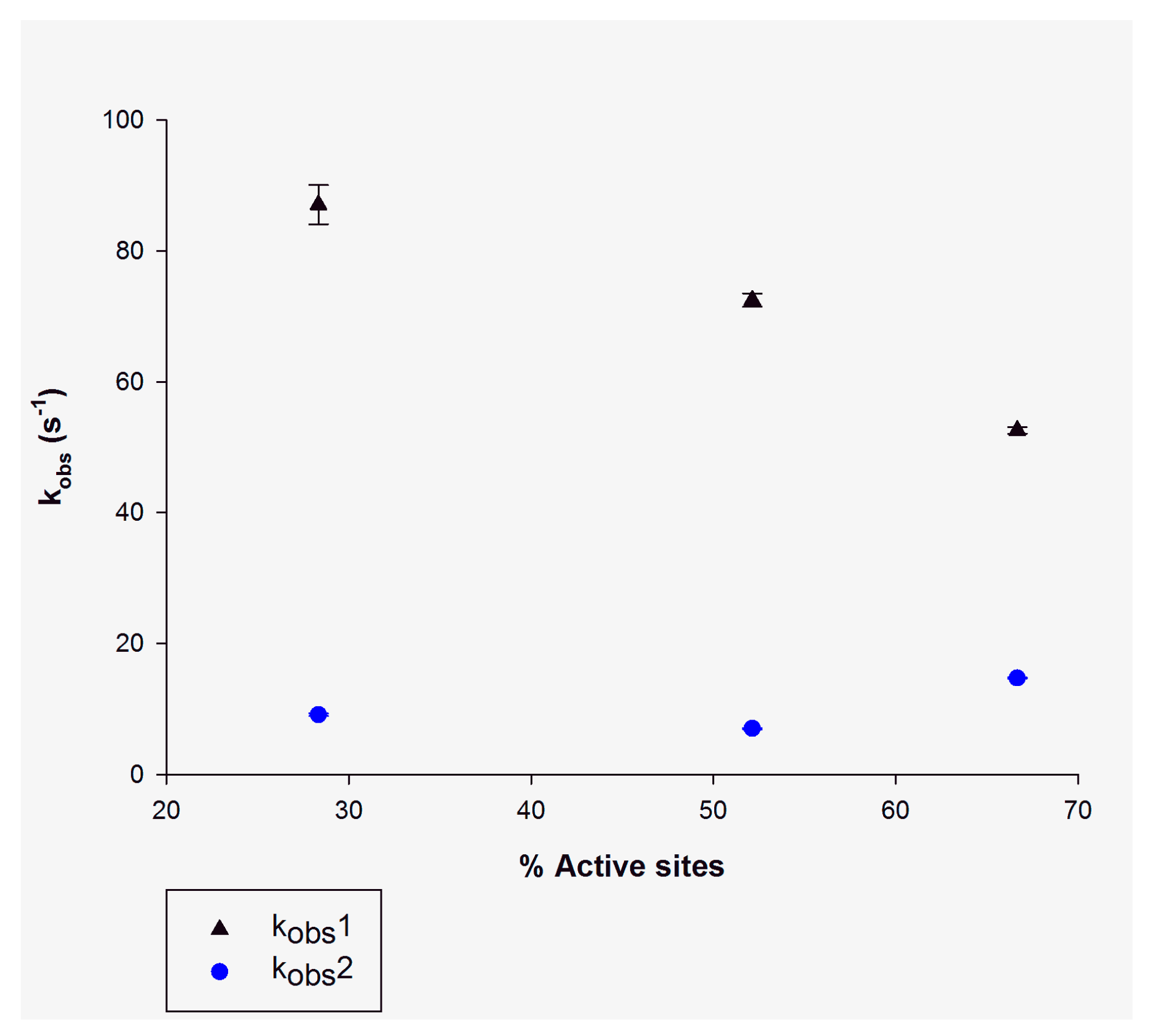
3.1. Single Turnover
3.2. Burst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Lee, A.; Xie, Y.L.; Barry, C.E.; Chen, R.Y. Current and future treatments for tuberculosis. BMJ 2020, 368, m216. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., 3rd; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Yuan, T.; Sampson, N.S. Hit generation in TB drug discovery: From genome to granuloma. Chem. Rev. 2018, 118, 1887–1916. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and challenges in phenotypic drug discovery: An industry perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Werman, J.M.; Sampson, N.S. The pursuit of mechanism of action: Uncovering drug complexity in TB drug discovery. RSC Chem. Biol. 2021, 4, 423. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.E.S.; Duque, M.A.; de Freitas, T.F.; Galina, L.; Timmers, L.F.S.M.; Bizarro, C.V.; Machado, P.; Basso, L.A.; Ducati, R.G. Mycobacterium tuberculosis shikimate pathway enzymes as targets for the rational design of anti-tuberculosis drugs. Molecules 2020, 25, 1259. [Google Scholar] [CrossRef]
- Jackson, M.; McNeil, M.R.; Brennan, P.J. Progress in targeting cell envelope biogenesis in Mycobacterium tuberculosis. Future Microbiol. 2013, 8, 855–875. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E.; Rock, C.O. Escherichia coli and Salmonella: Cellular and Molecular Biology; Neidhardt, F.C., Ed.; ASM Press: Washington, DC, USA, 1996; pp. 612–636. [Google Scholar]
- Schroeder, E.K.; de Souza, O.N.; Santos, D.S.; Blanchard, J.S.; Basso, L.A. Drugs that inhibit mycolic acid biosynthesis in Mycobacterium tuberculosis. Curr. Pharm. Biotechnol. 2002, 3, 197–225. [Google Scholar] [CrossRef]
- Banerjee, A.; Sugantino, M.; Sacchettini, J.C.; Jacobs, W.R., Jr. The mabA gene from the inhA operon of Mycobacterium tuberculosis encodes a 3-ketoacyl reductase that fails to confer isoniazid resistance. Microbiology 1998, 144, 2697–2704. [Google Scholar] [CrossRef]
- Quémard, A.; Sacchettini, J.C.; Dessen, A.; Vilchèze, C.; Bittman, R.; Jacobs, W.R., Jr.; Blanchard, J.S. Enzymatic characterization of the target for isoniazid in Mycobacterium tuberculosis. Biochemistry 1995, 34, 8235–8241. [Google Scholar] [CrossRef]
- Banerjee, A.; Dubnau, E.; Quémard, A.; Balasubramanian, V.; Um, K.S.; Wilson, T.; Collins, D.; de Lisle, G.; Jacobs, W.R., Jr. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 1994, 263, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Basso, L.A.; Zheng, R.; Musser, J.M.; Jacobs, W.R., Jr.; Blanchard, J.S. Mechanism of isoniazid resistance in Mycobacterium tuberculosis: Enzymatic characterization of enoyl reductase mutants identified in isoniazid-resistant clinical isolates. J. Infect. Dis. 1998, 178, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Wang, F.; Arai, M.; Hazbón, M.H.; Colangeli, R.; Kremer, L.; Weisbrod, T.R.; Alland, D.; Sacchettini, J.C.; Jacobs, W.R., Jr. Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nat. Med. 2006, 12, 1027–1029. [Google Scholar] [CrossRef]
- Parikh, S.; Moynihan, D.P.; Xiao, G.; Tonge, P.J. Roles of tyrosine 158 and lysine 165 in the catalytic mechanism of InhA, the enoyl-ACP reductase from Mycobacterium tuberculosis. Biochemistry 1999, 38, 13623–13634. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.L.; Xiao, G.; Tonge, P.J. Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry 2000, 39, 7645–7650. [Google Scholar] [CrossRef]
- Vasconcelos, I.B.; Basso, L.A.; Santos, D.S. Kinetic and equilibrium mechanisms of substrate binding to Mycobacterium tuberculosis enoyl reductase: Implications to function-based antitubercular agent design. J. Braz. Chem. Soc. 2010, 21, 1503–1508. [Google Scholar] [CrossRef]
- Hopf, F.S.M.; Roth, C.D.; de Souza, E.V.; Galina, L.; Czeczot, A.M.; Machado, P.; Basso, L.A.; Bizarro, C.V. Bacterial enoyl-reductases: The ever-growing list of Fabs, their mechanisms and inhibition. Front. Microbiol. 2022, 13, 891610. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Pereira, J.H.; Canduri, F.; Rodrigues, N.C.; de Souza, O.N.; Azevedo, W.F., Jr.; Basso, L.A.; Santos, D.S. Crystallographic and pre-steady-state kinetics studies on binding of NADH to wild-type and isoniazid-resistant enoyl-ACP(CoA) reductase enzymes from Mycobacterium tuberculosis. J. Mol. Biol. 2006, 359, 646–666. [Google Scholar] [CrossRef]
- Monod, J.; Wyman, J.; Changeux, J.P. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965, 12, 88–118. [Google Scholar] [CrossRef]
- Koshland, D.E., Jr.; Némethy, G.; Filmer, D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 1966, 5, 365–385. [Google Scholar] [CrossRef]
- Gutfreund, H. Kinetic analysis of the properties and reactions of enzymes. Prog. Biophys. Mol. Biol. 1975, 29, 161–195. [Google Scholar] [CrossRef] [PubMed]
- Rotta, M.; Timmers, L.F.S.M.; Sequeiros-Borja, C.; Bizarro, C.V.; de Souza, O.N.; Santos, D.S.; Basso, L.A. Observed crowding effects on Mycobacterium tuberculosis 2-trans-enoyl-ACP (CoA) reductase enzyme activity are not due to excluded volume only. Sci. Rep. 2017, 7, 6826. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, B.; King, R.W.; Wheeler, M.R.; Lewis, C.A., Jr.; Goode, S.R.; Dunlap, R.B.; Roberts, G.C. Correction for light absorption in fluorescence studies of protein-ligand interactions. Anal. Biochem. 1983, 132, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Hiromi, K. Kinetics of Fast Enzyme Reactions; Halsted Press: New York, NY, USA, 1979; pp. 187–253. [Google Scholar]
- Motlagh, H.N.; Wrabl, J.O.; Li, J.; Hilser, V.J. The ensemble nature of allostery. Nature 2014, 508, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nussinov, R. Allostery: An overview of its history, concepts, methods, and applications. PLoS Comput. Biol. 2016, 12, e1004966. [Google Scholar] [CrossRef] [PubMed]
- Knoverek, C.R.; Amarasinghe, G.K.; Bowman, G.R. Advanced methods for accessing protein shape-shifting present new therapeutic opportunities. Trends Biochem. Sci. 2019, 44, 351–364. [Google Scholar] [CrossRef]
- Wodak, S.J.; Paci, E.; Dokholyan, N.V.; Berezovsky, I.N.; Horovitz, A.; Li, J.; Hilser, V.J.; Bahar, I.; Karanicolas, J.; Stock, G.; et al. Allostery in its many disguises: From theory to applications. Structure 2019, 27, 566–578. [Google Scholar] [CrossRef]
- Wellington, S.; Nag, P.P.; Michalska, K.; Johnston, S.E.; Jedrzejczak, R.P.; Kaushik, V.K.; Clatworthy, A.E.; Siddiqi, N.; McCarren, P.; Bajrami, B.; et al. A small-molecule allosteric inhibitor of Mycobacterium tuberculosis tryptophan synthase. Nat. Chem. Biol. 2017, 13, 943–950. [Google Scholar] [CrossRef]
- Tarabini, R.F.; Timmers, L.F.S.M.; Sequeiros-Borja, C.E.; Norberto de Souza, O. The importance of the quaternary structure to represent conformational ensembles of the major Mycobacterium tuberculosis drug target. Sci. Rep. 2019, 9, 13683. [Google Scholar] [CrossRef]
- Chitolina, L.D.; de Souza, O.N.; Basso, L.A.; Timmers, L.F.S.M. Rethinking the MtInhA tertiary and quaternary structure flexibility: A molecular dynamics view. J. Mol. Model. 2022, 28, 140. [Google Scholar] [CrossRef]
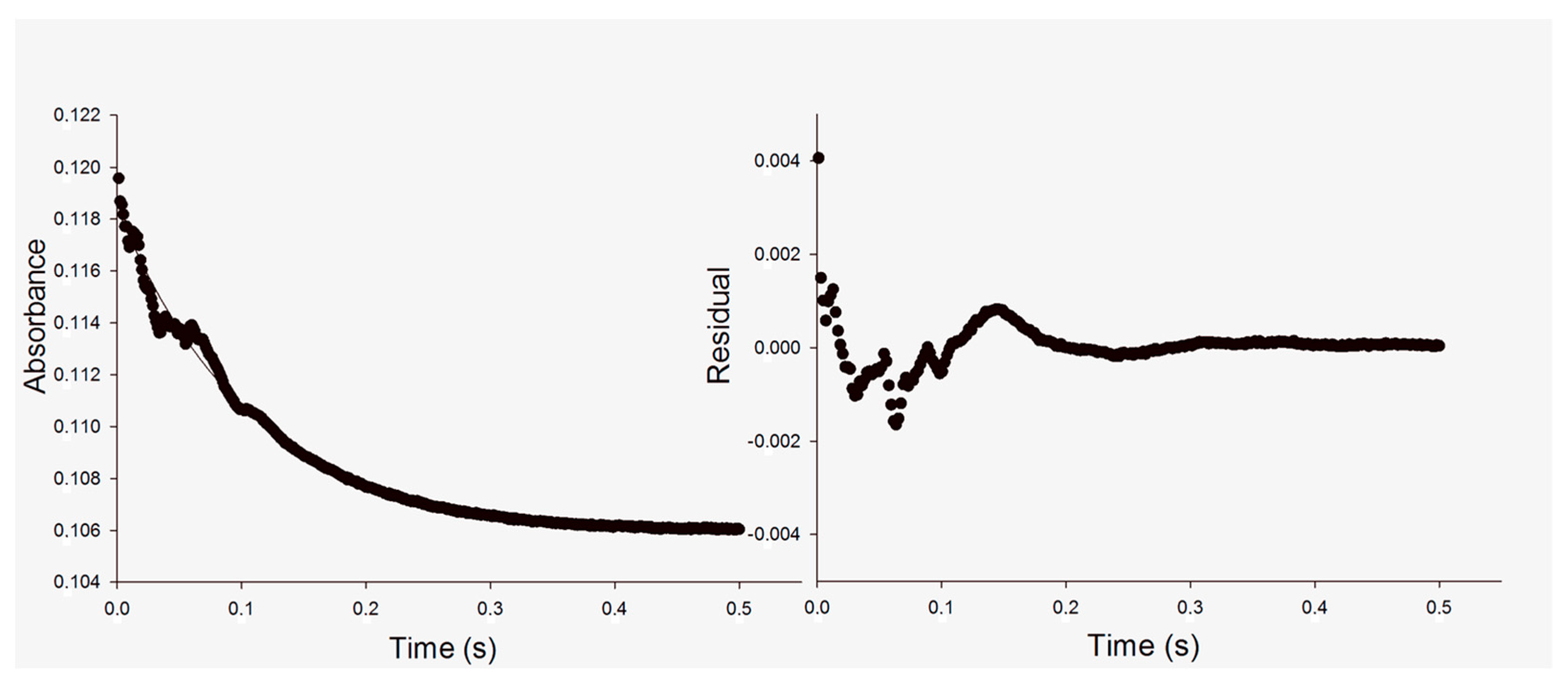

| Single Turnover (No Incubation) | ||
| (NADH) (Active Sites) | kobs | |
| (5 μM) 28% | 9.3 ± 0.1 s−1 | |
| (10 μM) 52% | 10.6 ± 0.03 s−1 | |
| (14 μM) 66% | 11.3 ± 0.01 s−1 | |
| Single turnover (NADH incubation) | ||
| (NADH) (active sites) | kobs1 | kobs2 |
| (5 μM) 28% | 87.2 ± 3.7 s−1 | 9.1 ± 0.2 s−1 |
| (10 μM) 52% | 72.5 ± 1.1 s−1 | 7.0 ± 0.2−1 |
| (14 μM) 66% | 52.6 ± 0.5 s−1 | 14.7 ± 0.1 s−1 |
| Single turnover (DD-CoA incubation) | ||
| (DD-CoA) (active sites) | kobs | |
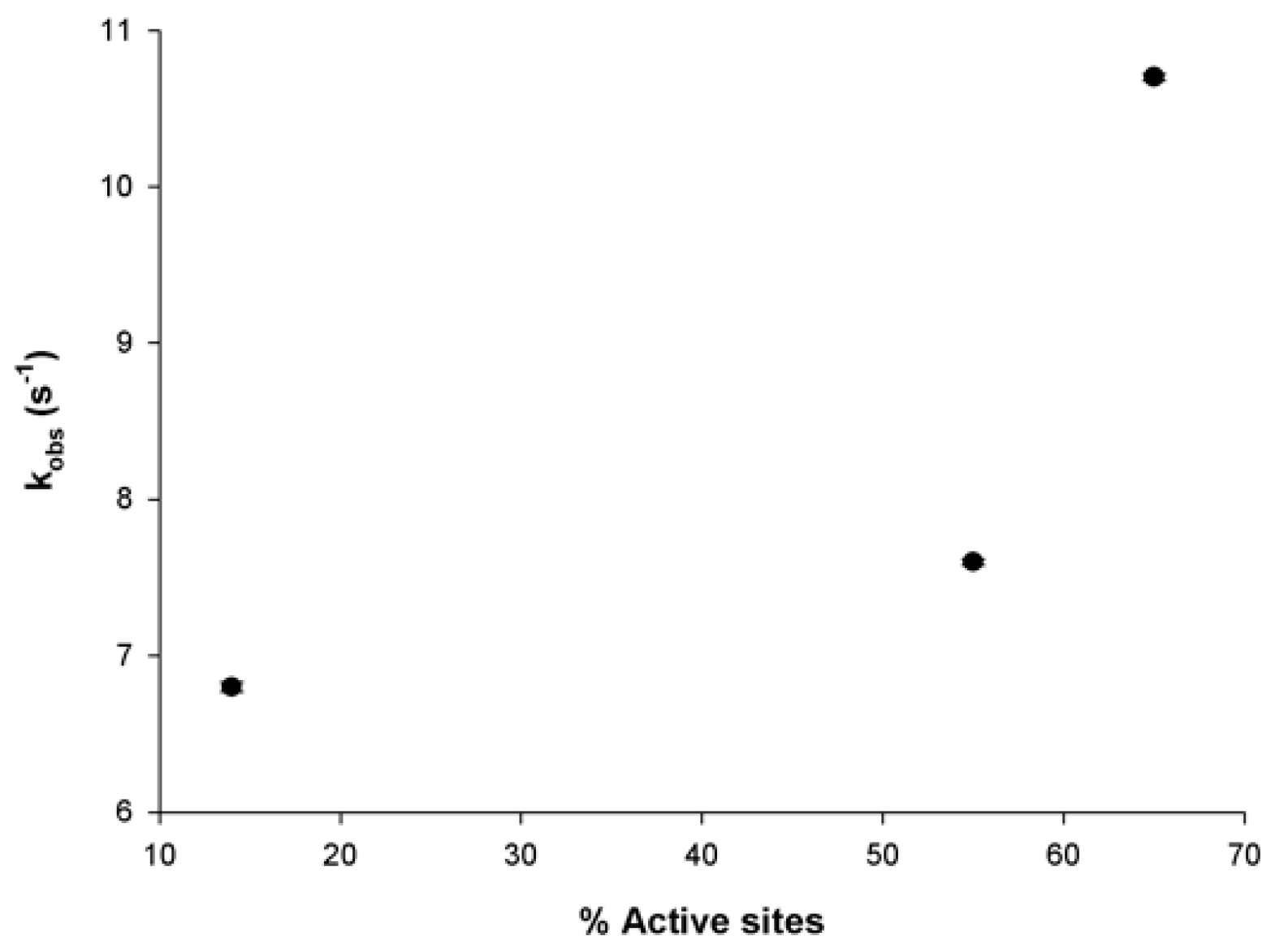
| (14 μM) 14% | 6.8 ± 0.03 s−1 | |
| (30 μM) 55% | 7.6 ± 0.02 s−1 | |
| (39 μM) 65% | 10.7 ± 0.02 s−1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, L.K.B.; Rotta, M.; Bizarro, C.V.; Machado, P.; Basso, L.A. Single Turnover of Transient of Reactants Supports a Complex Interplay of Conformational States in the Mode of Action of Mycobacterium tuberculosis Enoyl Reductase. Future Pharmacol. 2023, 3, 379-391. https://doi.org/10.3390/futurepharmacol3020023
Martinelli LKB, Rotta M, Bizarro CV, Machado P, Basso LA. Single Turnover of Transient of Reactants Supports a Complex Interplay of Conformational States in the Mode of Action of Mycobacterium tuberculosis Enoyl Reductase. Future Pharmacology. 2023; 3(2):379-391. https://doi.org/10.3390/futurepharmacol3020023
Chicago/Turabian StyleMartinelli, Leonardo Kras Borges, Mariane Rotta, Cristiano Valim Bizarro, Pablo Machado, and Luiz Augusto Basso. 2023. "Single Turnover of Transient of Reactants Supports a Complex Interplay of Conformational States in the Mode of Action of Mycobacterium tuberculosis Enoyl Reductase" Future Pharmacology 3, no. 2: 379-391. https://doi.org/10.3390/futurepharmacol3020023
APA StyleMartinelli, L. K. B., Rotta, M., Bizarro, C. V., Machado, P., & Basso, L. A. (2023). Single Turnover of Transient of Reactants Supports a Complex Interplay of Conformational States in the Mode of Action of Mycobacterium tuberculosis Enoyl Reductase. Future Pharmacology, 3(2), 379-391. https://doi.org/10.3390/futurepharmacol3020023